The Effect of Visitors on Zoo Reptile Behaviour during the COVID-19 Pandemic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Low-Resolution Study
2.1.1. Data Collection
2.1.2. Statistical Analysis
2.2. High-Resolution Study
2.2.1. Data Collection
2.2.2. Statistical Analysis
3. Results
3.1. Low-Resolution Study
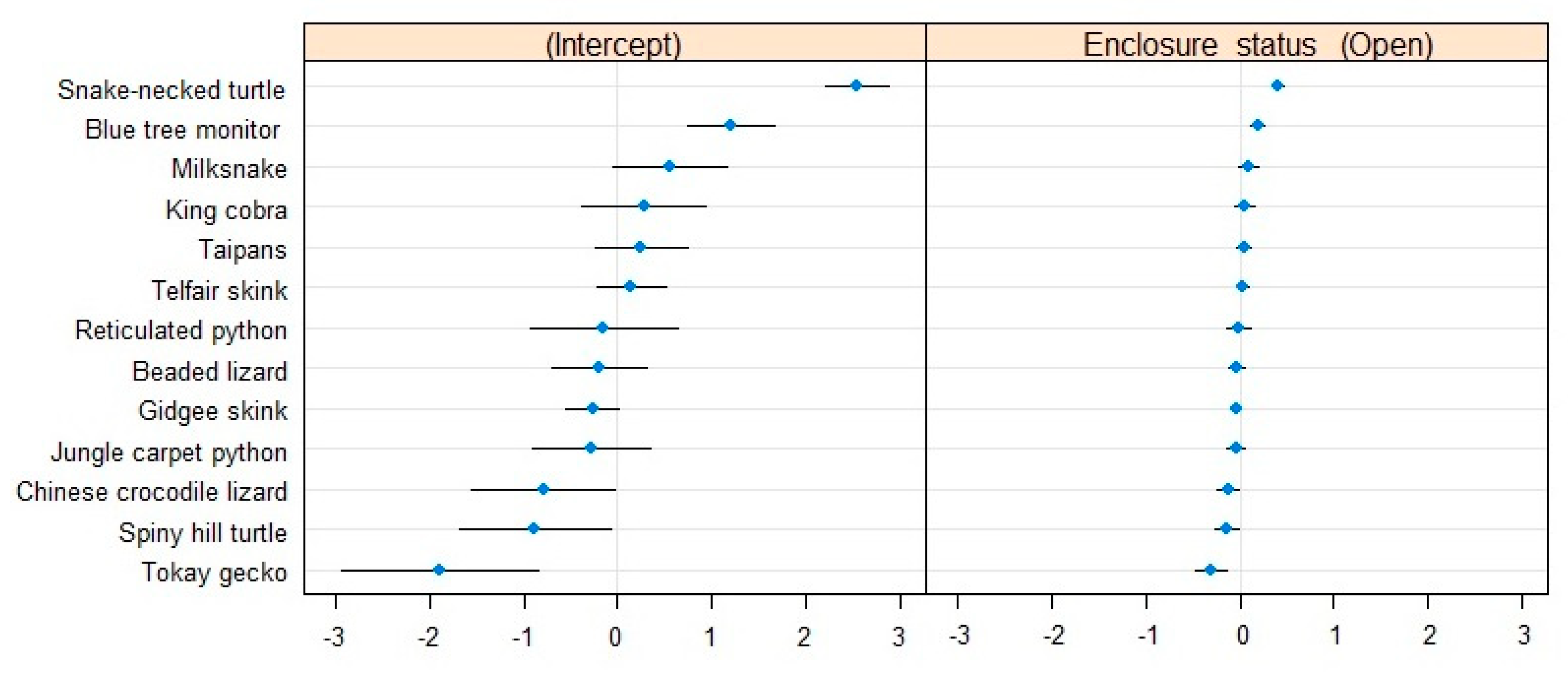
3.1.1. Proportion of Active Individuals
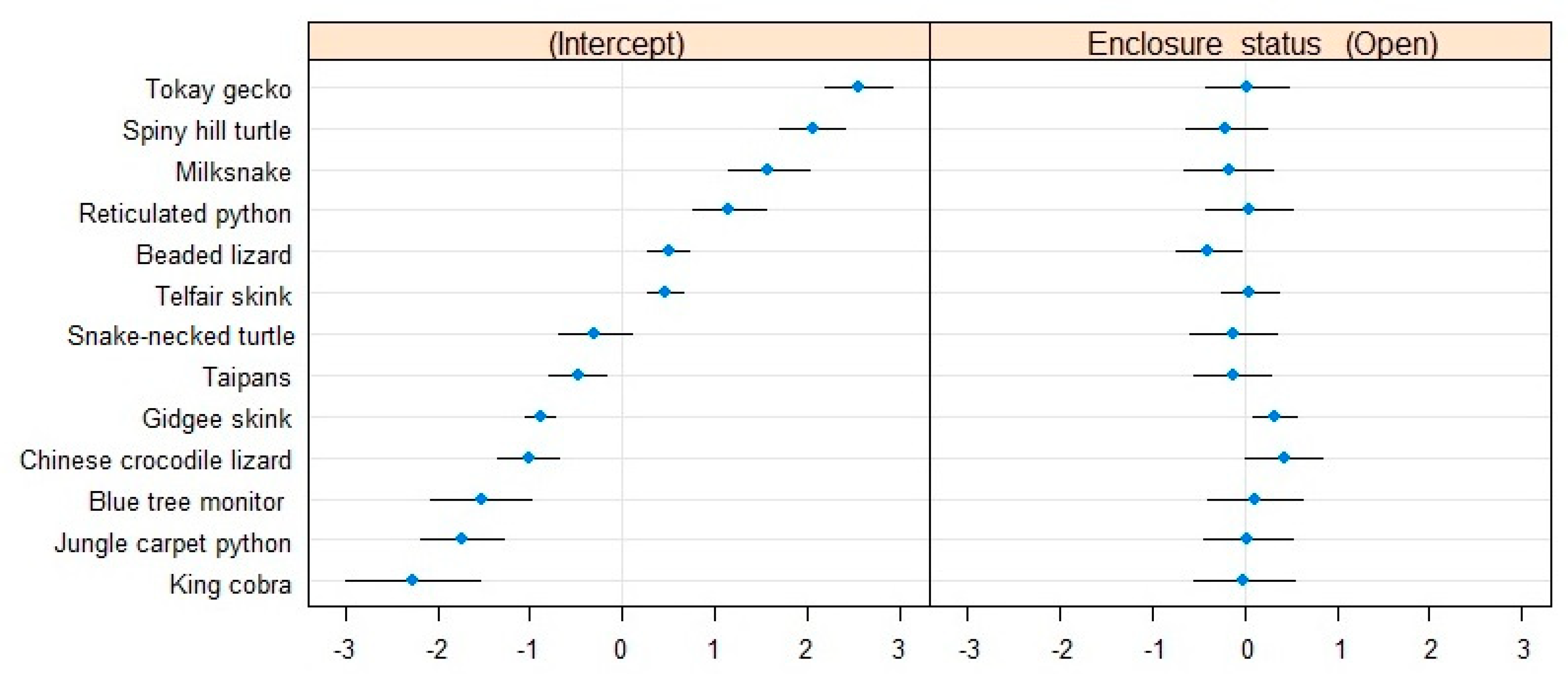
3.1.2. Proportion of Individuals Out-of-Sight
3.2. High-Resolution Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mason, P. Roles of the modern zoo: Conflicting or complementary? Tour. Rev. Int. 2007, 11, 251–263. [Google Scholar] [CrossRef]
- Tribe, A.; Booth, R. Assessing the role of zoos in wildlife conservation. Hum. Dimen. Wildl. 2003, 8, 65–74. [Google Scholar] [CrossRef]
- Carr, N.; Cohen, S. The public face of zoos: Images of entertainment, education and conservation. Anthrozoös 2011, 24, 175–189. [Google Scholar] [CrossRef] [Green Version]
- Sherwen, S.; Hemsworth, P. The visitor effect on zoo animals: Implications and opportunities for zoo animal welfare. Animals 2019, 9, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, S.; Sherwen, S.; Clark, F. Advances in applied zoo animal welfare science. J. Appl. Anim. Welf. Sci. 2018, 21, 23–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broom, D.M. Indicators of poor welfare. Brit. Vet. J. 1986, 142, 524–526. [Google Scholar] [CrossRef]
- Whitham, J.; Wielebnowski, N. New directions for zoo animal welfare science. Appl. Anim. Behav. Sci. 2013, 147, 247–260. [Google Scholar] [CrossRef]
- Barber, J. Programmatic approaches to assessing and improving animal welfare in zoos and aquariums. Zoo Biol. 2009, 28, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.; Broom, D. Measuring zoo animal welfare: Theory and practice. Zoo Biol. 2009, 28, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Learmonth, M.J. Human–Animal Interactions in Zoos: What Can Compassionate Conservation, Conservation Welfare and Duty of Care Tell Us about the Ethics of Interacting, and Avoiding Unintended Consequences? Animals 2020, 10, 2037. [Google Scholar] [CrossRef]
- Hosey, G. A preliminary model of human–animal relationships in the zoo. Appl. Anim. Behav. Sci. 2008, 109, 105–127. [Google Scholar] [CrossRef] [Green Version]
- Sherwen, S.; Magrath, M.; Butler, K.; Hemsworth, P. Little penguins, Eudyptula minor, show increased avoidance, aggression and vigilance in response to zoo visitors. Appl. Anim. Behav. Sci. 2015, 168, 71–76. [Google Scholar] [CrossRef]
- Eltorai, A.; Sussman, R. The “Visitor effect” and captive black-tailed prairie dog. Zool. Gart. 2010, 79, 109–120. [Google Scholar] [CrossRef]
- Todd, P.; Macdonald, C.; Coleman, D. Visitor-associated variation in captive Diana monkey (Cercopithecus diana diana) behaviour. Appl. Anim. Behav. Sci. 2007, 107, 162–165. [Google Scholar] [CrossRef]
- Paré, J.; Lentini, A. Reptile geriatrics. Vet. Clin. Exot. Anim. Pract. 2010, 13, 15–25. [Google Scholar] [CrossRef]
- Benn, A.; McLelland, D.; Whittaker, A. A review of welfare assessment methods in reptiles, and preliminary application of the welfare quality protocol to the pygmy blue-tongue skink, Tiliqua adelaidensis, using animal-based measures. Animals 2019, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Morgan, K.; Tromborg, C. Sources of stress in captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Gillingham, J. Normal behaviour. In Health and Welfare of Captive Reptiles; Warwick, C., Frye, F., Murphy, B., Eds.; Chapman & Hall/Kluwer: London, UK, 2004; pp. 131–164. [Google Scholar]
- Riley, A.; Terry, M.; Freeman, H.; Alba, A.; Soltis, J.; Leeds, A. Evaluating the effect of visitor presence on Nile crocodile (Crocodylus niloticus) behavior. J. Zool. Bot. Gard. 2021, 2, 115–129. [Google Scholar] [CrossRef]
- Kane, D.; Davis, A.C.; Michaels, C.J. Play behaviour by captive tree monitors, Varanus macraei and Varanus prasinus. Herpetol. Bull. 2019, 149, 28–31. [Google Scholar] [CrossRef]
- Boultwood, J.; O’Brien, M.; Rose, P. Bold Frogs or Shy Toads? How did the COVID-19 closure of zoological organisations affect amphibian activity? Animals 2021, 11, 1982. [Google Scholar] [CrossRef]
- Chelodina mccordi. The IUCN Red List of Threatened Species. 2019. Available online: https://www.iucnredlist.org/species/123814489/123814575 (accessed on 9 August 2021).
- Egernia stokesii. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/species/62246/101743684 (accessed on 19 October 2021).
- Gekko gecko. The IUCN Red List of Threatened Species. 2019. Available online: https://www.iucnredlist.org/species/195309/2378260 (accessed on 13 October 2021).
- O’Shea, M. Lizards of the World: A Guide to Every Family; Ivy Press: London, UK, 2021; pp. 200–234. [Google Scholar]
- Goetz, M. Husbandry and breeding of the spiny turtle Heosemys spinosa (GRAY, 1931) at the Durrell Wildlife Conservation Trust. Radiata 2007, 16, 2–15. [Google Scholar]
- Lampropeltis triangulum. The IUCN Red List of Threatened Species. 2019. Available online: https://www.iucnredlist.org/species/197493/2490171 (accessed on 19 October 2021).
- Leiolopisma telfairii. The IUCN Red List of Threatened Species. 2018. Available online: https://www.iucnredlist.org/species/11409/152276731 (accessed on 19 October 2021).
- Malayopython reticulatus. The IUCN Red List of Threatened Species. 2018. Available online: https://www.iucnredlist.org/species/183151/1730027 (accessed on 19 October 2021).
- Morelia spilota. The IUCN Red List of Threatened Species. 2017. Available online: https://www.iucnredlist.org/species/62232/21649539 (accessed on 19 October 2021).
- Ophiophagus hannah. The IUCN Red List of Threatened Species. 2012. Available online: https://www.iucnredlist.org/species/177540/1491874 (accessed on 19 October 2021).
- Oxyuranus microlepidotus. The IUCN Red List of Threatened Species 2018. Available online: https://www.iucnredlist.org/species/42493150/42493160 (accessed on 13 October 2021).
- Huang, H.; Wang, H.; Li, L.; Wu, Z.; Chen, J. Genetic diversity and population demography of the Chinese crocodile lizard (Shinisaurus crocodilurus) in China. PLoS ONE 2014, 9, e91570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, D. International trade in the blue tree monitor lizard Varanus macraei. Biawak J. Varanid Biol. Husb. 2015, 9, 50–57. [Google Scholar]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Millar, R.B.; Anderson, M.J. Remedies for pseudoreplication. Fish. Res. 2004, 70, 397–407. [Google Scholar] [CrossRef]
- Harrison, X.A.; Donaldson, L.; Correa-Cano, M.E.; Evans, J.; Fisher, D.N.; Goodwin, C.E.; Robinson, B.S.; Hodgson, D.J.; Inger, R. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 2018, 6, e4794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, P.C. Extension of Nakagawa & Schielzeth’s R2GLMM to random slopes models. Methods Ecol. Evol. 2014, 5, 944–946. [Google Scholar] [PubMed] [Green Version]
- Raudenbush, S.W.; Yang, M.L.; Yosef, M. Maximum likelihood for generalized linear models with nested random effects via high-order, multivariate Laplace approximation. J. Comput. Graph. Stat. 2000, 9, 141–157. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Soft. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Mu-MIn: Multi-Model Inference. R Package Version 1.43.17. 2020. Available online: http://R-Forge.R-project.org/projects/mumin/ (accessed on 17 June 2021).
- Sarkar, D. Lattice: Multivariate Data Visualization with R; Springer: New York, NY, USA, 2008; Available online: http://lmdvr.r-forge.r-project.org (accessed on 17 June 2021).
- The R Foundation. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.R-project.org/ (accessed on 17 June 2021).
- Dugard, P.; File, P.; Todman, J.; Todman, J.B. Single-Case and Small-n Experimental Designs: A Practical Guide To Randomization Tests; Routledge: New York, NY, USA, 2012; pp. 11–130. [Google Scholar]
- Tanious, R.; Onghena, P. Randomized Single-Case Experimental Designs in Healthcare Research: What, Why, and How? Healthcare 2019, 7, 143–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaels, C.J.; Gini, B.F.; Clifforde, L. A persistent abnormal repetitive behaviour in a false water cobra (Hydrodynastes gigas). Anim. Welf. 2020, 29, 371–378. [Google Scholar] [CrossRef]
- Williams, E.; Carter, A.; Rendle, J.; Ward, S. Impacts of COVID-19 on animals in zoos: A longitudinal multi-species analysis. J. Zool. Bot. Gard. 2021, 2, 130–145. [Google Scholar] [CrossRef]
- Williams, E.; Carter, A.; Rendle, J.; Ward, S. Understanding impacts of zoo visitors: Quantifying behavioural changes of two popular zoo species during COVID-19 closures. Appl. Anim. Behav. Sci. 2021, 236, 105253. [Google Scholar] [CrossRef]
- Stevens, J.; Thyssen, A.; Laevens, H.; Vervaecke, H. The influence of zoo visitor numbers on the behaviour of harbour seals. J. Zoo Aquar. Res. 2013, 1, 31–34. [Google Scholar] [CrossRef]
- Marcellini, D.L.; Jenssen, T.A. Visitor behavior in the National Zoo’s reptile house. Zoo Biol. 1988, 7, 329–338. [Google Scholar] [CrossRef]
- Bonnie, K.E.; Ang, M.Y.; Ross, S.R. Effects of crowd size on exhibit use by and behavior of chimpanzees (Pan troglodytes) and Western lowland gorillas (Gorilla gorilla) at a zoo. Appl. Anim. Behav. Sci. 2016, 178, 102–110. [Google Scholar] [CrossRef]

| Species | Description | No. Individuals (All Co-Housed) | No. Observations Open/Closed |
|---|---|---|---|
| Roti Island Snake-necked turtle (Chelodina mccordi) | Long-necked, carnivorous/piscivorous, freshwater turtle [22]. Diurnal and nocturnal activity. | 1 | 41/88 |
| Gidgee skink (Egernia stokesii) | Social arid land-dwelling omnivorous lizard [23]. Primarily diurnal. | 12 | 41/67 |
| Tokay gecko (Gekko gecko) | Widespread arboreal/scansorial insectivorous lizard from a wide range of natural and anthropogenic habitats [24]. Primarily nocturnal and crepuscular, with some activity (especially basking) diurnally. | 3 | 41/85 |
| Rio Fuerte beaded lizard (Heloderma horridum exasperatum) | Terrestrial/low arboreal, carnivorous and venomous lizard from dry forest [25]. Activity exhibited throughout the day and night. | 3 | 41/84 |
| Spiny hill turtle (Heosemys spinosa) | Forest-dwelling, herbivorous and mainly terrestrial turtle [26]. Primarily crepuscular and nocturnal, with some diurnal activity. | 2 | 41/88 |
| Pueblan milksnake (Lampropeltis triangulum campbelli) | Batesian mimic of venomous coral snakes. Terrestrial and fossorial, carnivorous snake species [27]. Primarily nocturnal activity, with substantial diurnal basking activity. | 1 | 39/83 |
| Telfair’s skink (Leiolopisma telfairii) | Omnivorous arboreal and terrestrial lizard species from grassland and forest [28]. Primarily diurnal. | 4 | 41/88 |
| Reticulated python (Malayopython reticulatus) | Large constrictor snake with wide habitat tolerance, feeding mainly on large mammals and other vertebrates [29]. Primarily nocturnally and crepuscularly active with some diurnal activity. | 1 | 41/87 |
| Jungle carpet python (Morelia spilota cheynei) | Semiarboreal and carnivorous constrictor snake from tropical forest, feeding on medium sized rodents [30]. Primarily nocturnally and crepuscularly active with some diurnal activity. | 2 | 40/83 |
| King cobra (Ophiophagus hannah) | Widely distributed venomous snake from forest and scrub, primarily feeding on other snakes [31]. Primarily diurnally and crepuscularly active. | 1 | 41/87 |
| Inland taipan (Oxyuranus microlepidotus) | Burrow-dwelling, arid land, venomous species feeding predominantly on rodents [32]. Active throughout the day and night. | 2 | 40/85 |
| Chinese crocodile lizard (Shinisaurus crocodilurus): | Cryptic, arboreal and stream-dwelling insectivorous lizard [33]. Primarily diurnal. | 2 | 41/91 |
| Blue tree monitor (Varanus macraei) | Arboreal tropical-forest, carnivorous and insectivorous lizard with high cognitive abilities [34]. Primarily diurnal. | 1 | 41/88 |
| Proportion of Active Individuals | Estimate | Std. Error | z-Value | 2.5% CI | 97.5% CI |
| Parameter estimates | |||||
| Intercept | −3.02163 | 0.32231 | −9.375 | 0.02396 | 0.09467 |
| Enclosure status (Open) | −0.08609 | 0.17863 | −0.482 | 0.61613 | 1.28379 |
| Estimated variance components | |||||
| σ0 | 1.19132 | 1.0915 | |||
| σ1 | 0.03077 | 0.1754 | |||
| R2 marginal | 0.00062 | ||||
| R2 conditional | 0.50910 | ||||
| Dispersion estimate (ĉ) | 0.75 | ||||
| Proportion of Out-of-Sight Individuals | Estimate | Std. Error | z-Value | 2.5% CI | 97.5% CI |
| Parameter estimates | |||||
| Intercept | −0.45238 | 0.41813 | −1.082 | 0.26166 | 1.53633 |
| Enclosure status (Open) | 0.08163 | 0.12849 | 0.635 | 0.81768 | 1.40912 |
| Estimated variance components | |||||
| σ0 | 2.22168 | 1.4905 | |||
| σ1 | 0.09298 | 0.3049 | |||
| R2 marginal | 0.00042 | ||||
| R2 conditional | 0.62870 | ||||
| Dispersion estimate (ĉ) | 1.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carter, K.C.; Keane, I.A.T.; Clifforde, L.M.; Rowden, L.J.; Fieschi-Méric, L.; Michaels, C.J. The Effect of Visitors on Zoo Reptile Behaviour during the COVID-19 Pandemic. J. Zool. Bot. Gard. 2021, 2, 664-676. https://doi.org/10.3390/jzbg2040048
Carter KC, Keane IAT, Clifforde LM, Rowden LJ, Fieschi-Méric L, Michaels CJ. The Effect of Visitors on Zoo Reptile Behaviour during the COVID-19 Pandemic. Journal of Zoological and Botanical Gardens. 2021; 2(4):664-676. https://doi.org/10.3390/jzbg2040048
Chicago/Turabian StyleCarter, Kimberley C., Isabel A. T. Keane, Lisa M. Clifforde, Lewis J. Rowden, Léa Fieschi-Méric, and Christopher J. Michaels. 2021. "The Effect of Visitors on Zoo Reptile Behaviour during the COVID-19 Pandemic" Journal of Zoological and Botanical Gardens 2, no. 4: 664-676. https://doi.org/10.3390/jzbg2040048
APA StyleCarter, K. C., Keane, I. A. T., Clifforde, L. M., Rowden, L. J., Fieschi-Méric, L., & Michaels, C. J. (2021). The Effect of Visitors on Zoo Reptile Behaviour during the COVID-19 Pandemic. Journal of Zoological and Botanical Gardens, 2(4), 664-676. https://doi.org/10.3390/jzbg2040048






