Cannabinoid Type-2 Receptor Agonist, JWH133 May Be a Possible Candidate for Targeting Infection, Inflammation, and Immunity in COVID-19
Abstract
:1. Introduction
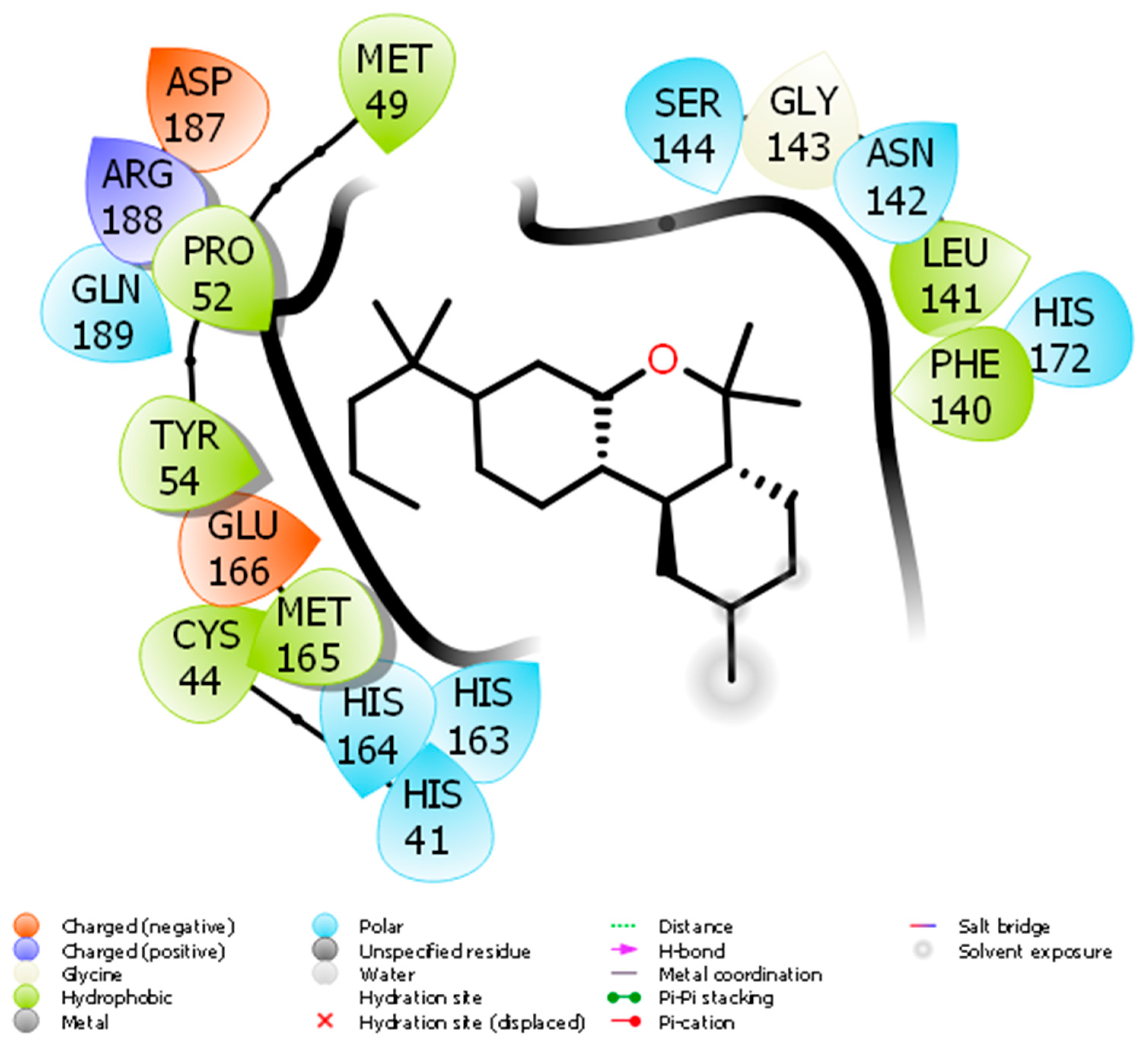
2. Molecular Docking of JWH133 for its Activity on Mpro
3. CB2 Receptors Mediated Anti-Inflammatory Activity of JWH133
4. CB2 Receptors Mediated Immunomodulatory Activity of JWH133
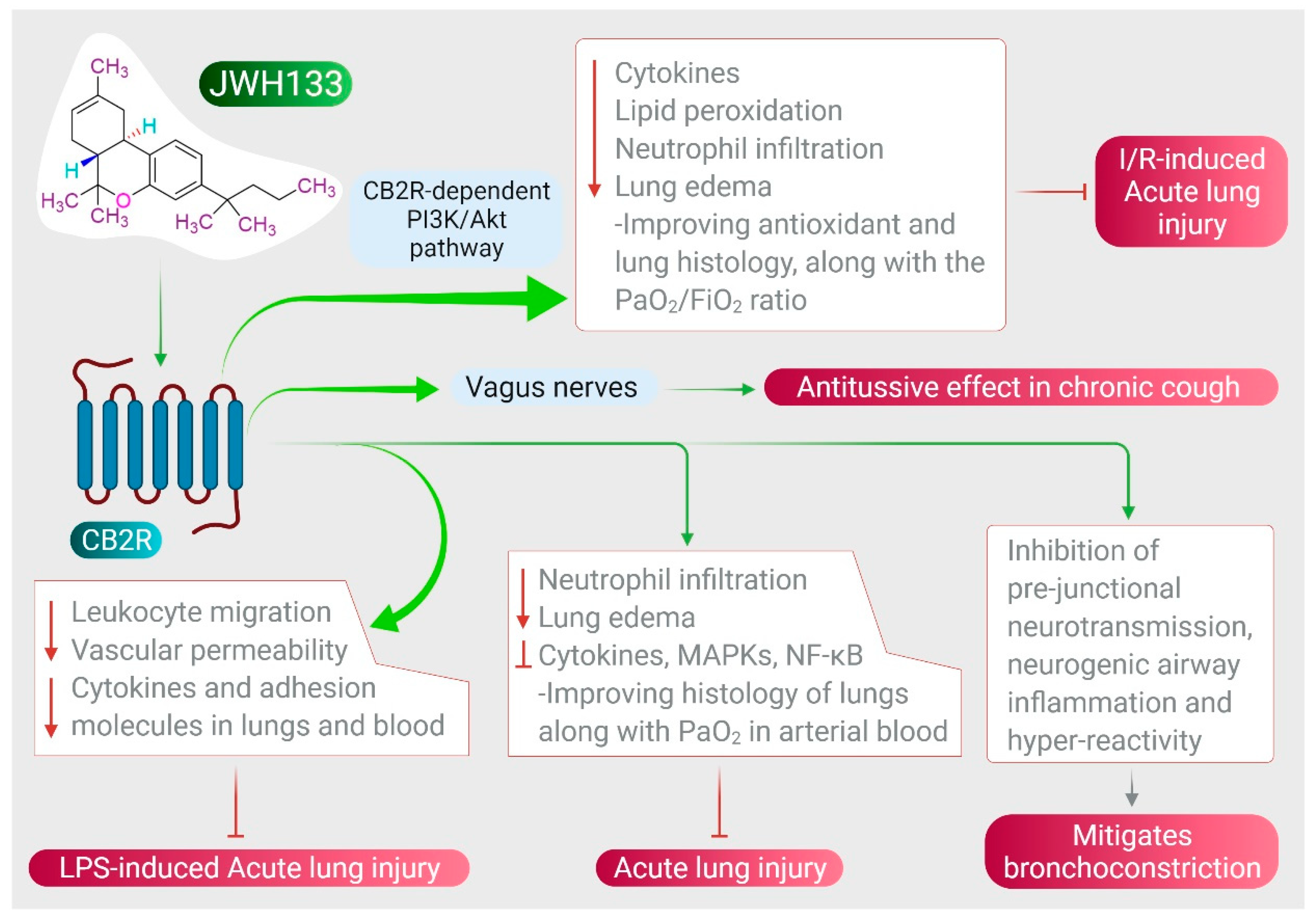
5. CB2 Receptors Mediated Effects of JWH133 on Acute Lung Injury and Airway Activity
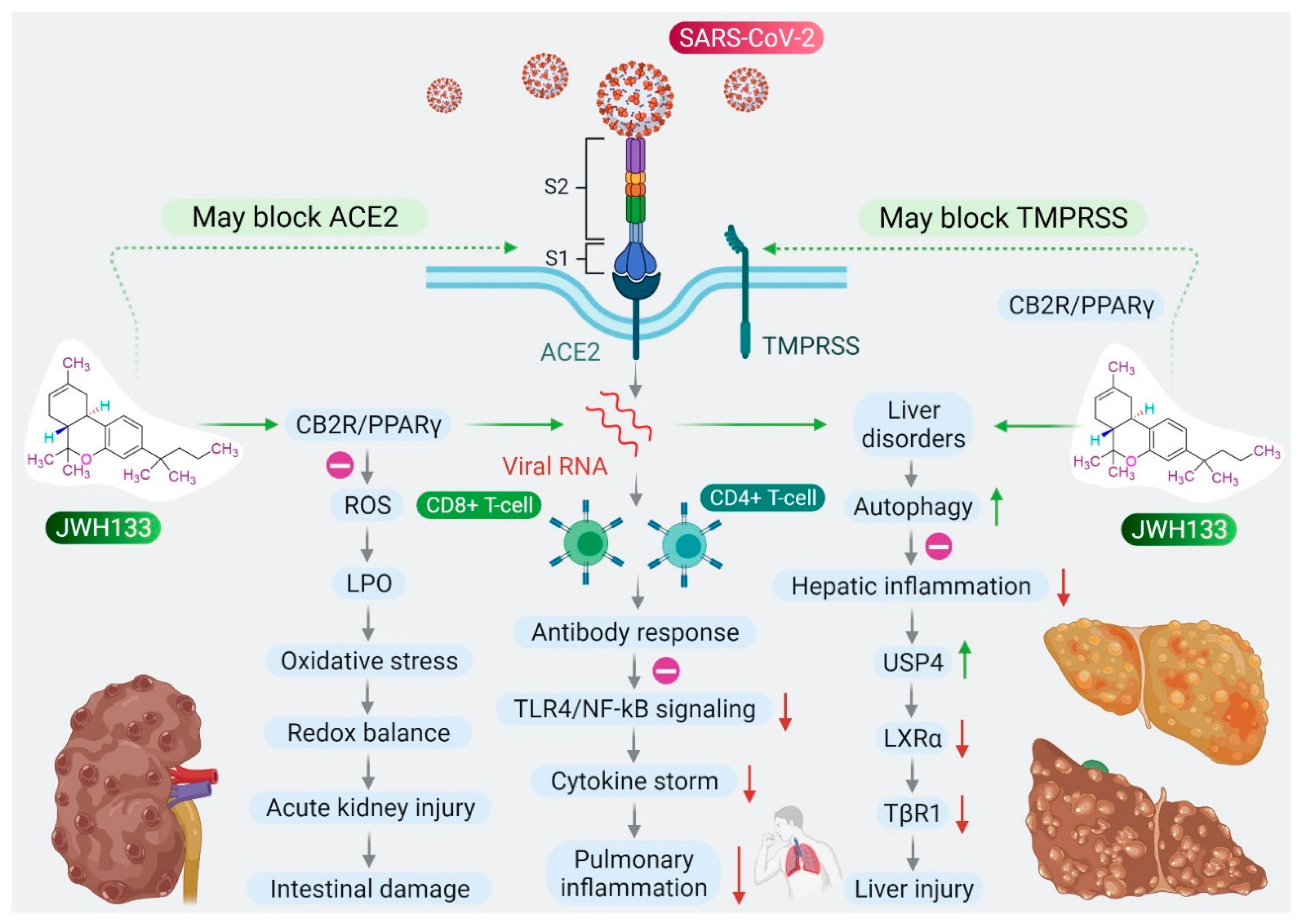
6. CB2 Receptors Mediated Anti-Inflammatory and Antiviral Activity of JWH133
7. CB2 Receptors Mediated Protective Effects of JWH133 in Organ Injuries and Sepsis
8. Limitations on the Proposed Therapeutic Applications of JWH133
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HT2A | 5-hydroxytryptamine receptor 2A |
| ACE-2 | Angiotensin-converting enzyme 2 |
| ALI | Acute lung injury |
| ALT | Alanine amino transaminase |
| ARDS | Acute respiratory distress syndrome |
| AST | Aspartate amino transaminase |
| CB1R | Cannabinoid receptor type 1 |
| CB2R | Cannabinoid receptor type 1 |
| CNS | Central nervous system |
| COVID-19 | Coronavirus disease-2019 |
| COX-2 | Cyclooxygenase-2 |
| ECS | Endocannabinoid system |
| ERK1/2 | Extracellular signal-regulated kinase ½ |
| GPCR | G-protein-coupled receptor |
| HCV | Chronic hepatitis C |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| ITP | Immune thrombocytopenia purpura |
| LPS | Lipopolysaccharide |
| MAPK/JNK | Mitogen-activated protein kinase/c-Jun N-terminal kinase |
| MLKL | Mixed lineage kinase domain like pseudo kinase |
| NF-κB | Nuclear factor-kappa B |
| NK cells | Natural killer cells |
| NLRP# | NOD-like receptor protein 3 |
| NO | Nitric oxide |
| NRF2/Keap1 | Nuclear factor erythroid 2-related factor 2/Kelch-like ECH-associated protein |
| PGCV1-α | PPAR gamma coactivator 1-α |
| PI3K/Akt | Phosphoinositide 3-kinase/protein kinase B |
| RIP | Receptor interacting protein |
| RSV | Respiratory syncytial virus |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| STAT-5 | Signal transducer and activator of transcription 5 |
| TGF | Transforming growth factor |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor |
| TRP | Transient receptor potential |
| USP4 | Ubiquitin-specific peptidase 4 |
References
- Oa, A.; Je, A. Social determinants of health: The role of effective communication in the COVID-19 pandemic in developing countries. Glob. Health Action 2020, 13, 1788263. [Google Scholar]
- Altay, O.; Mohammadi, E.; Lam, S.; Turkez, H.; Boren, J.; Nielsen, J.; Uhlen, M.; Mardinoglu, A. Current Status of COVID-19 Therapies and Drug Repositioning Applications. iScience 2020, 23, 101303. [Google Scholar] [CrossRef]
- Shiyong, F.; Dian, X.; Yanming, W.; Lianqi, L.; Xinbo, Z.; Wu, Z. Research progress on repositioning drugs and specific therapeutic drugs for SARS-CoV-2. Future Med. Chem. 2020, 0158. [Google Scholar] [CrossRef]
- Chiurchiu, V.; Battistini, L.; Maccarrone, M. Endocannabinoid signalling in innate and adaptive immunity. Immunology 2015, 144, 352–364. [Google Scholar] [CrossRef]
- Oláh, A.; Szekanecz, Z.; Bíró, T. Targeting Cannabinoid Signaling in the Immune System: “High”-ly Exciting Questions, Possibilities, and Challenges. Front. Immunol. 2017, 8, 1487. [Google Scholar] [CrossRef] [Green Version]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity-the Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef]
- Stasiulewicz, A.; Znajdek, K.; Grudzień, M.; Pawiński, T.; Sulkowska, A. Guide to Targeting the Endocannabinoid System in Drug Design. Int. J. Mol. Sci. 2020, 21, 2778. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, M.; Tortora, C.; Bellini, G.; Di Paola, A.; Punzo, F.; Rossi, F. The Endocannabinoid System in Pediatric Inflammatory and Immune Diseases. Int. J. Mol. Sci. 2019, 20, 5875. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Xiao, F.; Yuan, Q.; Zhang, J.; Zhan, J.; Zhang, Z. Cannabinoid Receptor 2: A Potential Novel Therapeutic Target for Sepsis? Acta Clin. Belg. 2019, 74, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.T.; Greaves, D.R.; Iqbal, A.J. The Impact of Cannabinoid Receptor 2 Deficiency on Neutrophil Recruitment and Inflammation. DNA Cell Biol. 2019, 38, 1025–1029. [Google Scholar] [CrossRef]
- Huffman, J.W.; Hepburn, S.A.; Lyutenko, N.; Thompson, A.L.; Wiley, J.L.; Selley, D.E.; Martin, B.R. 1-Bromo-3-(1′,1′-dimethylalkyl)-1-deoxy-Δ(8)-tetrahydrocannabinols: New selective ligands for the cannabinoid CB(2) receptor. Bioorganic Med. Chem. 2010, 18, 7809–7815. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.; Wang, L.; Kuo, H.-C.D.; Shannar, A.; Peter, R.; Chou, P.J. An Update on Current Therapeutic Drugs Treating COVID-19. Curr. Pharmacol. Rep. 2020, 6, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Zhang, C.; Fan, X.; Meng, F.P.; Xu, Z.; Xia, P.; Cao, W.J.; Yang, T.; Dai, X.P.; Wang, S.Y.; et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat. Commun. 2020, 11, 3410. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-Cova-Infected Mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Lythgoe, M.P.; Middleton, P. Ongoing Clinical Trials for the Management of the COVID-19 Pandemic. Trends Pharmacol. Sci. 2020, 41, 363–382. [Google Scholar] [CrossRef]
- Allegra, A.; Di Gioacchino, M.; Tonacci, A.; Musolino, C.; Gangemi, S. Immunopathology of SARS-CoV-2 Infection: Immune Cells and Mediators, Prognostic Factors, and Immune-Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 4782. [Google Scholar] [CrossRef]
- Henderson, L.A.; Canna, S.W.; Schulert, G.S.; Volpi, S.; Lee, P.Y.; Kernan, K.F.; Caricchio, R.; Mahmud, S.; Hazen, M.M.; Halyabar, O.; et al. On the Alert for Cytokine Storm: Immunopathology in COVID-19. Arthritis Rheumatol. 2020, 72, 1059–1063. [Google Scholar] [CrossRef] [Green Version]
- Rossi, F.; Tortora, C.; Argenziano, M.; Di Paola, A.; Punzo, F. Cannabinoid Receptor Type 2: A Possible Target in SARS-CoV-2 (CoV-19) Infection? Int. J. Mol. Sci. 2020, 21, 3809. [Google Scholar] [CrossRef]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. Cannabidiol as a Novel Therapeutic for Immune Modulation. ImmunoTargets Ther. 2020, 9, 131–140. [Google Scholar] [CrossRef]
- Rizzo, M.D.; Henriquez, J.E.; Blevins, L.K.; Bach, A.; Crawford, R.B.; Kaminski, N.E. Targeting Cannabinoid Receptor 2 on Peripheral Leukocytes to Attenuate Inflammatory Mechanisms Implicated in HIV-Associated Neurocognitive Disorder. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2020, 15, 780–793. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Sharma, C.; Goyal, S.N.; Kumar, S.; Ojha, S. CB2 receptor-selective agonists as candidates for targeting infection, inflammation, and immunity in SARS-CoV-2 infections. Drug Dev. Res. 2021, 82, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Azhar, E.I.; Kamal, M.A.; Bajrai, L.H.; Dubey, A.; Jha, K.; Yadava, U.; Kang, S.G.; Dwivedi, V.D. SARS-CoV-2 M pro inhibitors: Identification of anti-SARS-CoV-2 M pro compounds from FDA approved drugs. J. Biomol. Struct. Dyn. 2020, 1–16. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Lee, K.E.; Dwivedi, V.D.; Kang, S.G. Computational insights into tetracyclines as inhibitors against SARS-CoV-2 M pro via combinatorial molecular simulation calculations. Life Sci. 2020, 257, 118080. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of M pro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Rostkowski, M.; Olsson, M.H.; Søndergaard, C.R.; Jensen, J.H. Graphical analysis of pH-dependent properties of proteins predicted using PROPKA. BMC Struct. Biol. 2011, 11, 6. [Google Scholar] [CrossRef] [Green Version]
- Muralidharan, N.; Sakthivel, R.; Velmurugan, D.; Gromiha, M.M. Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 protease against COVID-19. J. Biomol. Struct. Dyn. 2020, 39, 2673–2678. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, e26. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Hiyoshi, A.; Montgomery, S. COVID-19 case-fatality rate and demographic and socioeconomic influencers: Worldwide spatial regression analysis based on country-level data. BMJ Open 2020, 10, e043560. [Google Scholar] [CrossRef] [PubMed]
- Kapellos, T.S.; Taylor, L.; Feuerborn, A.; Valaris, S.; Hussain, M.T.; Rainger, G.E.; Greaves, D.R.; Iqbal, A.J. Cannabinoid receptor 2 deficiency exacerbates inflammation and neutrophil recruitment. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 6154–6167. [Google Scholar] [CrossRef] [Green Version]
- Turcotte, C.; Blanchet, M.R.; Laviolette, M.; Flamand, N. The CB 2 receptor and its role as a regulator of inflammation. Cellular Mol. Life Sci. CMLS 2016, 73, 4449–4470. [Google Scholar] [CrossRef] [Green Version]
- van Niekerk, G.; Mabin, T.; Engelbrecht, A.M. Anti-inflammatory mechanisms of cannabinoids: An immunometabolic perspective. Inflammopharmacology 2019, 27, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Li, X.; Cheng, Y.; Ke, B.; Wang, R. Activation of cannabinoid receptor type 2 reduces lung ischemia reperfusion injury through PI3K/Akt pathway. Int. J. Clin. Exp. Pathol. 2019, 12, 4096–4105. [Google Scholar] [PubMed]
- Liu, Z.; Wang, Y.; Zhao, H.; Zheng, Q.; Xiao, L.; Zhao, M. CB2 receptor activation ameliorates the proinflammatory activity in acute lung injury induced by paraquat. BioMed Res. Int. 2014, 2014, 971750. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Jin, G.; Zhang, J.; Wei, W. Selective Activation of Cannabinoid Receptor 2 Attenuates Myocardial Infarction via Suppressing NLRP3 Inflammasome. Inflammation 2019, 42, 904–914. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000. [Google Scholar] [CrossRef]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.L.; Sammani, S.; Kempf, C.; Saadat, L.; Letsiou, E.; Wang, T.; Moreno-Vinasco, L.; Rizzo, A.N.; Fortman, J.D.; Garcia, J.G. Pleiotropic effects of interleukin-6 in a “two-hit” murine model of acute respiratory distress syndrome. Pulm. Circ. 2014, 4, 280–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelaia, C.; Tinello, C.; Vatrella, A.; De Sarro, G.; Pelaia, G. Lung under attack by COVID-19-induced cytokine storm: Pathogenic mechanisms and therapeutic implications. Ther. Adv. Respir. Dis. 2020, 14, 1753466620933508. [Google Scholar] [CrossRef]
- Zhang, S.; Li, L.; Shen, A.; Chen, Y.; Qi, Z. Rational Use of Tocilizumab in the Treatment of Novel Coronavirus Pneumonia. Clin. Drug Investig. 2020, 40, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Staiano, R.I.; Loffredo, S.; Borriello, F.; Iannotti, F.A.; Piscitelli, F.; Orlando, P.; Secondo, A.; Granata, F.; Lepore, M.T.; Fiorelli, A.; et al. Human lung-resident macrophages express CB1 and CB2 receptors whose activation inhibits the release of angiogenic and lymphangiogenic factors. J. Leukoc. Biol. 2016, 99, 531–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Fang, Y.; Zhou, J.; Ding, X.; Ling, G.; Yu, S. Pulmonary fibrosis in critical ill patients recovered from COVID-19 pneumonia: Preliminary experience. Am. J. Emerg. Med. 2020, 38, 2134–2138. [Google Scholar] [CrossRef]
- Servettaz, A.; Kavian, N.; Nicco, C.; Deveaux, V.; Chéreau, C.; Wang, A.; Zimmer, A.; Lotersztajn, S.; Weill, B.; Batteux, F. Targeting the cannabinoid pathway limits the development of fibrosis and autoimmunity in a mouse model of systemic sclerosis. Am. J. Pathol. 2010, 177, 187–196. [Google Scholar] [CrossRef]
- Fu, Q.; Zheng, Y.; Dong, X.; Wang, L.; Jiang, C.G. Activation of cannabinoid receptor type 2 by JWH133 alleviates bleomycin-induced pulmonary fibrosis in mice. Oncotarget 2017, 8, 103486–103498. [Google Scholar] [CrossRef] [Green Version]
- Wawryk-Gawda, E.; Chłapek, K.; Zarobkiewicz, M.K.; Lis-Sochocka, M.; Chylińska-Wrzos, P.; Boguszewska-Czubara, A.; Sławiński, M.A.; Franczak, A.; Jodłowska-Jędrych, B.; Biała, G. CB2R agonist prevents nicotine induced lung fibrosis. Exp. Lung Res. 2018, 44, 344–351. [Google Scholar] [CrossRef]
- Fujii, M.; Sherchan, P.; Soejima, Y.; Doycheva, D.; Zhao, D.; Zhang, J.H. Cannabinoid Receptor Type 2 Agonist Attenuates Acute Neurogenic Pulmonary Edema by Preventing Neutrophil Migration after Subarachnoid Hemorrhage in Rats. Acta Neurochir. Suppl. 2016, 121, 135–139. [Google Scholar]
- Krittanawong, C.; Kumar, A.; Hahn, J.; Wang, Z.; Zhang, H.J.; Sun, T.; Bozkurt, B.; Ballantyne, C.M.; Virani, S.S.; Halperin, J.L.; et al. Cardiovascular risk and complications associated with COVID-19. Am. J. Cardiovasc. Dis. 2020, 10, 479–489. [Google Scholar] [PubMed]
- Capone, V.; Cuomo, V.; Esposito, R.; Canonico, M.E.; Ilardi, F.; Prastaro, M.; Esposito, G.; Santoro, C. Epidemiology, prognosis and clinical manifestation of cardiovascular disease in COVID-19. Expert Rev. Cardiovasc. Ther. 2020, 18, 531–539. [Google Scholar] [CrossRef]
- Shi, H.K.; Guo, H.C.; Liu, H.Y.; Zhang, Z.L.; Hu, M.Y.; Zhang, Y.; Li, Q. Cannabinoid type 2 receptor agonist JWH133 decreases blood pressure of spontaneously hypertensive rats through relieving inflammation in the rostral ventrolateral medulla of the brain. J. Hypertens. 2020, 38, 886–895. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, D.; Dong, X.; Zhu, R.; Ye, Y.; Li, L.; Jiang, Y. Pharmacological activation of CB2 receptor protects against ethanol-induced myocardial injury related to RIP1/RIP3/MLKL-mediated necroptosis. Mol. Cell. Biochem. 2020, 474, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Akinwumi, B.C.; Shao, Z.; Anderson, H.D. Ligand activation of cannabinoid receptors attenuates hypertrophy of neonatal rat cardiomyocytes. J. Cardiovasc. Pharmacol. 2014, 64, 420–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Guo, H.C.; Maslov, L.N.; Qiao, X.W.; Zhou, J.J.; Zhang, Y. Mitochondrial permeability transition pore plays a role in the cardioprotection of CB2 receptor against ischemia-reperfusion injury. Can. J. Physiol. Pharmacol. 2014, 92, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cui, N.; Du, Y.; Ma, H.; Zhang, Y. Anandamide reduces intracellular Ca2+ concentration through suppression of Na+/Ca2+ exchanger current in rat cardiac myocytes. PLoS ONE 2013, 8, e006386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montecucco, F.; Lenglet, S.; Braunersreuther, V.; Burger, F.; Pelli, G.; Bertolotto, M.; Mach, F.; Steffens, S. CB(2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J. Mol. Cell. Cardiol. 2009, 46, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.A.; Abesser, M.; Karcher, J.; Laser, M.; Kunos, G. Coronary vasodilator effects of endogenous cannabinoids in vasopressin-preconstricted unpaced rat isolated hearts. J. Cardiovasc. Pharmacol. 2005, 46, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peng, Z.; Zhou, Y.; Wang, J.; Lin, X.; Dong, X.; Liu, X.; Jiang, J.; Jiang, Y.; Li, L. Quetiapine induces myocardial necroptotic cell death through bidirectional regulation of cannabinoid receptors. Toxicol. Lett. 2019, 313, 77–90. [Google Scholar] [CrossRef]
- Li, L.; Dong, X.; Tu, C.; Li, X.; Peng, Z.; Zhou, Y.; Zhang, D.; Jiang, J.; Burke, A.; Zhao, Z.; et al. Opposite effects of cannabinoid CB 1 and CB 2 receptors on antipsychotic clozapine-induced cardiotoxicity. Br. J. Pharmacol. 2019, 176, 890–905. [Google Scholar] [CrossRef] [PubMed]
- Gąsecka, A.; Borovac, J.A.; Guerreiro, R.A.; Giustozzi, M.; Parker, W.; Caldeira, D.; Chiva-Blanch, G. Thrombotic Complications in Patients with COVID-19: Pathophysiological Mechanisms, Diagnosis, and Treatment. Cardiovasc. Drugs Ther. 2020, 35, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tecson, K.M.; McCullough, P.A. Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev. Cardiovasc. Med. 2020, 21, 315–319. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Haskó, G.; Liaudet, L.; Huffman, J.W.; Csiszar, A.; Ungvari, Z.; Mackie, K.; Chatterjee, S.; et al. CB2-receptor stimulation attenuates TNF-alpha-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2210–H2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netherland, C.D.; Pickle, T.G.; Bales, A.; Thewke, D.P. Cannabinoid receptor type 2 (CB2) deficiency alters atherosclerotic lesion formation in hyperlipidemic Ldlr-null mice. Atherosclerosis 2010, 213, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Yuan, Z.; Liu, Y.; Xue, J.; Tian, Y.; Liu, W.; Zhang, W.; Shen, Y.; Xu, W.; Liang, X.; et al. Activation of cannabinoid CB2 receptor ameliorates atherosclerosis associated with suppression of adhesion molecules. J. Cardiovasc. Pharmacol. 2010, 55, 292–298. [Google Scholar] [CrossRef]
- Molica, F.; Matter, C.M.; Burger, F.; Pelli, G.; Lenglet, S.; Zimmer, A.; Pacher, P.; Steffens, S. Cannabinoid receptor CB2 protects against balloon-induced neointima formation. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1064–H1074. [Google Scholar] [CrossRef] [Green Version]
- Montecucco, F.; Di Marzo, V.; da Silva, R.F.; Vuilleumier, N.; Capettini, L.; Lenglet, S.; Pagano, S.; Piscitelli, F.; Quintao, S.; Bertolotto, M.; et al. The activation of the cannabinoid receptor type 2 reduces neutrophilic protease-mediated vulnerability in atherosclerotic plaques. Eur. Heart J. 2012, 33, 846–856. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, S.H.; Haskó, J.; Skuba, A.; Fan, S.; Dykstra, H.; McCormick, R.; Reichenbach, N.; Krizbai, I.; Mahadevan, A.; Zhang, M.; et al. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 4004–4016. [Google Scholar] [CrossRef]
- Feng, G.; Zheng, K.I.; Yan, Q.Q.; Rios, R.S.; Targher, G.; Byrne, C.D.; Poucke, S.V.; Liu, W.Y.; Zheng, M.H. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J. Clin. Transl. Hepatol. 2020, 8, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Luque, J.; Ros, J.; Fernández-Varo, G.; Tugues, S.; Morales-Ruiz, M.; Alvarez, C.E.; Friedman, S.L.; Arroyo, V.; Jiménez, W. Regression of fibrosis after chronic stimulation of cannabinoid CB2 receptor in cirrhotic rats. J. Pharmacol. Exp. Ther. 2008, 324, 475–483. [Google Scholar] [CrossRef] [Green Version]
- Guillot, A.; Hamdaoui, N.; Bizy, A.; Zoltani, K.; Souktani, R.; Zafrani, E.S.; Mallat, A.; Lotersztajn, S.; Lafdil, F. Cannabinoid receptor 2 counteracts interleukin-17-induced immune and fibrogenic responses in mouse liver. Hepatology 2014, 59, 296–306. [Google Scholar] [CrossRef]
- Rossi, F.; Bellini, G.; Nobili, B.; Maione, S.; Perrone, L.; del Giudice, E.M. Association of the cannabinoid receptor 2 (CB2) Gln63Arg polymorphism with indices of liver damage in obese children: An alternative way to highlight the CB2 hepatoprotective properties. Hepatology 2011, 54, 1102–1103. [Google Scholar] [CrossRef] [PubMed]
- Denaës, T.; Lodder, J.; Chobert, M.N.; Ruiz, I.; Pawlotsky, J.M.; Lotersztajn, S.; Teixeira-Clerc, F. The Cannabinoid Receptor 2 Protects Against Alcoholic Liver Disease Via a Macrophage Autophagy-Dependent Pathway. Sci. Rep. 2016, 6, 28806. [Google Scholar] [CrossRef] [Green Version]
- Tomar, S.; Zumbrun, E.E.; Nagarkatti, M.; Nagarkatti, P.S. Protective role of cannabinoid receptor 2 activation in galactosamine/lipopolysaccharide-induced acute liver failure through regulation of macrophage polarization and microRNAs. J. Pharmacol. Exp. Ther. 2015, 353, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louvet, A.; Teixeira-Clerc, F.; Chobert, M.N.; Deveaux, V.; Pavoine, C.; Zimmer, A.; Pecker, F.; Mallat, A.; Lotersztajn, S. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology. 2011, 54, 1217–1226. [Google Scholar] [CrossRef]
- Çakır, M.; Tekin, S.; Okan, A.; Çakan, P.; Doğanyiğit, Z. The ameliorating effect of cannabinoid type 2 receptor activation on brain, lung, liver and heart damage in cecal ligation and puncture-induced sepsis model in rats. Int. Immunopharmacol. 2020, 78, 105978. [Google Scholar] [CrossRef]
- Bátkai, S.; Osei-Hyiaman, D.; Pan, H.; El-Assal, O.; Rajesh, M.; Mukhopadhyay, P.; Hong, F.; Harvey-White, J.; Jafri, A.; Haskó, G.; et al. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 1788–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.Y.; Hsieh, S.L.; Lee, P.C.; Yeh, Y.C.; Lee, K.C.; Hsieh, Y.C.; Wang, Y.W.; Lee, T.Y.; Huang, Y.H.; Chan, C.C.; et al. Long-term cannabinoid type 2 receptor agonist therapy decreases bacterial translocation in rats with cirrhosis and ascites. J. Hepatol. 2014, 61, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Hashiesh, H.M.; Sharma, C.; Goyal, S.N.; Jha, N.K.; Ojha, S. Pharmacological Properties, Therapeutic Potential and Molecular Mechanisms of JWH133, a CB2 Receptor-Selective Agonist. Front Pharm. 2021, 12, 702675. [Google Scholar] [CrossRef]
- Reifart, J.; Rentsch, M.; Mende, K.; Coletti, R.; Sobocan, M.; Thasler, W.E.; Khandoga, A. Modulating CD4+ T cell migration in the postischemic liver: Hepatic stellate cells as new therapeutic target? Transplantation 2015, 99, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.C.; Wang, S.S.; Hsin, I.F.; Chang, C.C.; Lee, F.Y.; Lin, H.C.; Chuang, C.L.; Lee, J.Y.; Hsieh, H.G.; Lee, S.D. Cannabinoid receptor 2 agonist ameliorates mesenteric angiogenesis and portosystemic collaterals in cirrhotic rats. Hepatology 2012, 56, 248–258. [Google Scholar] [CrossRef]
- Steib, C.J.; Gmelin, L.; Pfeiler, S.; Schewe, J.; Brand, S.; Göke, B.; Gerbes, A.L. Functional relevance of the cannabinoid receptor 2-heme oxygenase pathway: A novel target for the attenuation of portal hypertension. Life Sci. 2013, 93, 543–551. [Google Scholar] [CrossRef]
- Wu, H.M.; Kim, T.H.; Kim, A.; Koo, J.H.; Joo, M.S.; Kim, S.G. Liver X Receptor α-Induced Cannabinoid Receptor 2 Inhibits Ubiquitin-Specific Peptidase 4 Through miR-27b, Protecting Hepatocytes From TGF-β. Hepatol. Commun. 2019, 3, 1373–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farouk, S.S.; Fiaccadori, E.; Cravedi, P.; Campbell, K.N. COVID-19 and the kidney: What we think we know so far and what we don’t. J. Nephrol. 2020, 33, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Çakır, M.; Tekin, S.; Doğanyiğit, Z.; Çakan, P.; Kaymak, E. The protective effect of cannabinoid type 2 receptor activation on renal ischemia-reperfusion injury. Mol. Cell. Biochem. 2019, 462, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Feizi, A.; Jafari, M.R.; Hamedivafa, F.; Tabrizian, P.; Djahanguiri, B. The preventive effect of cannabinoids on reperfusion-induced ischemia of mouse kidney. Exp. Toxicol. Pathol. Official J. Ges. Toxikol. Pathol. 2008, 60, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Royce, S.G.; Lubel, J.S. Letter: Intestinal inflammation, COVID-19 and gastrointestinal ACE2-exploring RAS inhibitors. Aliment. Pharmacol. Ther. 2020, 52, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Smid, S.D. Gastrointestinal endocannabinoid system: Multifaceted roles in the healthy and inflamed intestine. Clinical Exp. Pharmacol. Physiol. 2008, 35, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Neish, A.S. Acute inflammation: Endogenous cannabinoids mellow the harsh proinflammatory environment. J. Clin. Investig. 2018, 128, 3750–3751. [Google Scholar] [CrossRef]
- Izzo, A.A. The cannabinoid CB(2) receptor: A good friend in the gut. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2007, 19, 704–708. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, N.P.; Singh, B.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S. Cannabinoid receptor-2 (CB2) agonist ameliorates colitis in IL-10(-/-) mice by attenuating the activation of T cells and promoting their apoptosis. Toxicol. Appl. Pharmacol. 2012, 258, 256–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.Y.; Li, Y.N.; Ni, J.B.; Chen, C.J.; Lv, S.; Chai, S.Y.; Wu, R.H.; Yüce, B.; Storr, M. Involvement of cannabinoid-1 and cannabinoid-2 receptors in septic ileus. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2010, 22, 350–388. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.; Mouihate, A.; Mackie, K.; Keenan, C.M.; Buckley, N.E.; Davison, J.S.; Patel, K.D.; Pittman, Q.J.; Sharkey, K.A. Cannabinoid CB2 receptors in the enteric nervous system modulate gastrointestinal contractility in lipopolysaccharide-treated rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimball, E.S.; Schneider, C.R.; Wallace, N.H.; Hornby, P.J. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am. J. Physiol. Gastrointest. and Liver Physiol. 2006, 291, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Kimball, E.S.; Wallace, N.H.; Schneider, C.R.; D’Andrea, M.R.; Hornby, P.J. Small intestinal cannabinoid receptor changes following a single colonic insult with oil of mustard in mice. Front. Pharmacol. 2010, 1, 132. [Google Scholar] [CrossRef] [Green Version]
- Ojo, A.S.; Balogun, S.A.; Idowu, A.O. Acute Ischemic Stroke in COVID-19: Putative Mechanisms, Clinical Characteristics, and Management. Neurol. Res. Int. 2020, 2020, 7397480. [Google Scholar] [CrossRef]
- Tang, J.; Tao, Y.; Tan, L.; Yang, L.; Niu, Y.; Chen, Q.; Yang, Y.; Feng, H.; Chen, Z.; Zhu, G. Cannabinoid receptor 2 attenuates microglial accumulation and brain injury following germinal matrix hemorrhage via ERK dephosphorylation in vivo and in vitro. Neuropharmacology 2015, 95, 424–433. [Google Scholar] [CrossRef]
- Li, L.; Tao, Y.; Tang, J.; Chen, Q.; Yang, Y.; Feng, Z.; Chen, Y.; Yang, L.; Yang, Y.; Zhu, G.; et al. A Cannabinoid Receptor 2 Agonist Prevents Thrombin-Induced Blood-Brain Barrier Damage via the Inhibition of Microglial Activation and Matrix Metalloproteinase Expression in Rats. Transl. Stroke Res. 2015, 6, 467–477. [Google Scholar] [CrossRef]
- Rajkumar, R.P. COVID-19 and mental health: A review of the existing literature. Asian J. Psychiatry 2020, 52, 102066. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.; Zachariae, R.; Bovbjerg, D.H. Influence of psychological stress on upper respiratory infection-a meta-analysis of prospective studies. Psychosom. Med. 2010, 72, 823–832. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Liu, S.; Wang, T.; Guan, J.; Jia, J. Role of hypothalamic cannabinoid receptors in post-stroke depression in rats. Brain Res. Bull. 2016, 121, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Zoppi, S.; Madrigal, J.L.; Caso, J.R.; García-Gutiérrez, M.S.; Manzanares, J.; Leza, J.C.; García-Bueno, B. Regulatory role of the cannabinoid CB2 receptor in stress-induced neuroinflammation in mice. Br. J. Pharmacol. 2014, 171, 2814–2826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruk-Slomka, M.; Michalak, A.; Biala, G. Antidepressant-like effects of the cannabinoid receptor ligands in the forced swimming test in mice: Mechanism of action and possible interactions with cholinergic system. Behav. Brain Res. 2015, 284, 24–36. [Google Scholar] [CrossRef]
- Franklin, J.M.; Vasiljevik, T.; Prisinzano, T.E.; Carrasco, G.A. Cannabinoid 2 receptor- and beta Arrestin 2-dependent upregulation of serotonin 2A receptors. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2013, 23, 760–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, W.S.; Chauhan, P.; Hu, S.; Prasad, S.; Lokensgard, J.R. Antiallodynic Effects of Cannabinoid Receptor 2 (CB 2 R) Agonists on Retrovirus Infection-Induced Neuropathic Pain. Pain Res. Manag. 2019, 2019, 1260353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Almeida, A.A.; Silva, R.O.; Nicolau, L.A.; de Brito, T.V.; de Sousa, D.P.; Barbosa, A.L.; de Freitas, R.M.; Lopes, L.D.; Medeiros, J.R.; Ferreira, P.M. Physio-pharmacological Investigations About the Anti-inflammatory and Antinociceptive Efficacy of (+)-Limonene Epoxide. Inflammation 2017, 40, 511–522. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Yuan, M.; Kiertscher, S.M.; Cheng, Q.; Zoumalan, R.; Tashkin, D.P.; Roth, M.D. Delta 9-Tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J. Neuroimmunol. 2002, 133, 124–131. [Google Scholar] [CrossRef]
- Klein, T.W.; Lane, B.; Newton, C.A.; Friedman, H. The cannabinoid system and cytokine network. Proc. Soc. Exp. Biol. Med. 2000, 225, 1–8. [Google Scholar] [CrossRef]
- Do, Y.; McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-kappaB-dependent apoptosis: Novel role for endogenous and exogenous cannabinoids in immunoregulation. J. Immunol. 2004, 173, 2373–2382. [Google Scholar] [CrossRef] [Green Version]
- Leleu-Chavain, N.; Desreumaux, P.; Chavatte, P.; Millet, R. Therapeutical potential of CB2 receptors in immune-related diseases. Curr. Mol. Pharmacol. 2013, 6, 183–203. [Google Scholar] [CrossRef]
- Rossi, F.; Tortora, C.; Palumbo, G.; Punzo, F.; Argenziano, M.; Casale, M.; Di Paola, A.; Locatelli, F.; Perrotta, S. CB2 Receptor Stimulation and Dexamethasone Restore the Anti-Inflammatory and Immune-Regulatory Properties of Mesenchymal Stromal Cells of Children with Immune Thrombocytopenia. Int. J. Mol. Sci. 2019, 20, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa, F.; Mestre, L.; Docagne, F.; Guaza, C. Activation of cannabinoid CB2 receptor negatively regulates IL-12p40 production in murine macrophages: Role of IL-10 and ERK1/2 kinase signaling. Br. J. Pharmacol. 2005, 145, 441–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.P.; Yuan, Q.H.; Zhang, B.; Yang, L.; He, Q.W.; Chen, K.; Liu, Q.S.; Li, Z.; Zhan, J. Cannabinoid Receptor 2 Activation Alleviates Septic Lung Injury by Promoting Autophagy via Inhibition of Inflammatory Mediator Release. Cell. Signal. 2020, 69, 109556. [Google Scholar] [CrossRef] [PubMed]
- Costola-de-Souza, C.; Ribeiro, A.; Ferraz-de-Paula, V.; Calefi, A.S.; Aloia, T.P.; Gimenes-Júnior, J.A.; de Almeida, V.I.; Pinheiro, M.L.; Palermo-Neto, J. Monoacylglycerol lipase (MAGL) inhibition attenuates acute lung injury in mice. PLoS ONE 2013, 8, e77706. [Google Scholar] [CrossRef]
- Patel, H.J.; Birrell, M.A.; Crispino, N.; Hele, D.J.; Venkatesan, P.; Barnes, P.J.; Yacoub, M.H.; Belvisi, M.G. Inhibition of guinea-pig and human sensory nerve activity and the cough reflex in guinea-pigs by cannabinoid (CB2) receptor activation. Br. J. Pharmacol. 2003, 140, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Bozkurt, T.E.; Kaya, Y.; Durlu-Kandilci, N.T.; Onder, S.; Sahin-Erdemli, I. The effect of cannabinoids on dinitrofluorobenzene-induced experimental asthma in mice. Respir. Physiol. Neurobiol. 2016, 231, 7–13. [Google Scholar] [CrossRef]
- Oka, S.; Ikeda, S.; Kishimoto, S.; Gokoh, M.; Yanagimoto, S.; Waku, K.; Sugiura, T. 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces the migration of EoL-1 human eosinophilic leukemia cells and human peripheral blood eosinophils. J. Leukoc. Biol. 2004, 76, 1002–1009. [Google Scholar] [CrossRef] [Green Version]
- McCoy, K.L.; Matveyeva, M.; Carlisle, S.J.; Cabral, G.A. Cannabinoid inhibition of the processing of intact lysozyme by macrophages: Evidence for CB2 receptor participation. J. Pharmacol. Exp. Ther. 1999, 289, 1620–1625. [Google Scholar]
- Carayon, P.; Marchand, J.; Dussossoy, D.; Derocq, J.M.; Jbilo, O.; Bord, A.; Bouaboula, M.; Galiègue, S.; Mondière, P.; Pénarier, G.; et al. Modulation and functional involvement of CB2 peripheral cannabinoid receptors during B-cell differentiation. Blood 1998, 92, 3605–3615. [Google Scholar] [CrossRef]
- Karmaus, P.W.; Chen, W.; Kaplan, B.L.; Kaminski, N.E. Δ9-tetrahydrocannabinol suppresses cytotoxic T lymphocyte function independent of CB1 and CB 2, disrupting early activation events. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2012, 7, 843–855. [Google Scholar] [CrossRef] [Green Version]
- Buchweitz, J.P.; Karmaus, P.W.; Williams, K.J.; Harkema, J.R.; Kaminski, N.E. Targeted deletion of cannabinoid receptors CB1 and CB2 produced enhanced inflammatory responses to influenza A/PR/8/34 in the absence and presence of Delta9-tetrahydrocannabinol. J. Leukoc. Biol. 2008, 83, 785–796. [Google Scholar] [CrossRef] [Green Version]
- Tahamtan, A.; Samieipoor, Y.; Nayeri, F.S.; Rahbarimanesh, A.A.; Izadi, A.; Rashidi-Nezhad, A.; Tavakoli-Yaraki, M.; Farahmand, M.; Bont, L.; Shokri, F.; et al. Effects of Cannabinoid Receptor Type 2 in Respiratory Syncytial Virus Infection in Human Subjects and Mice. Virulence 2018, 9, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Coppola, N.; Zampino, R.; Bellini, G.; Macera, M.; Marrone, A.; Pisaturo, M.; Boemio, A.; Nobili, B.; Pasquale, G.; Maione, S.; et al. Association between a polymorphism in cannabinoid receptor 2 and severe necroinflammation in patients with chronic hepatitis C. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2014, 12, 334–340. [Google Scholar] [CrossRef]
- Rossi, F.; Mancusi, S.; Bellini, G.; Roberti, D.; Punzo, F.; Vetrella, S.; Matarese, S.M.; Nobili, B.; Maione, S.; Perrotta, S. CNR2 functional variant (Q63R) influences childhood immune thrombocytopenic purpura. Haematologica 2011, 96, 1883–1885. [Google Scholar] [CrossRef] [Green Version]
- Sagnelli, C.; Uberti-Foppa, C.; Hasson, H.; Bellini, G.; Minichini, C.; Salpietro, S.; Messina, E.; Barbanotti, D.; Merli, M.; Punzo, F.; et al. Cannabinoid receptor 2–63 RR variant is independently associated with severe necroinflammation in HIV/HCV coinfected patients. PLoS ONE 2017, 12, e0181890. [Google Scholar] [CrossRef] [Green Version]
- Murikinati, S.; Jüttler, E.; Keinert, T.; Ridder, D.A.; Muhammad, S.; Waibler, Z.; Ledent, C.; Zimmer, A.; Kalinke, U.; Schwaninger, M. Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 788–798. [Google Scholar] [CrossRef] [Green Version]
- Fraga, D.; Raborn, E.S.; Ferreira, G.A.; Cabral, G.A. Cannabinoids inhibit migration of microglial-like cells to the HIV protein Tat. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2011, 6, 566–577. [Google Scholar] [CrossRef]
- Rock, R.B.; Gekker, G.; Hu, S.; Sheng, W.S.; Cabral, G.A.; Martin, B.R.; Peterson, P.K. WIN55,212–2-mediated inhibition of HIV-1 expression in microglial cells: Involvement of cannabinoid receptors. J. Neuroimmune Pharmacol. Off. J. Soc. on NeuroImmune Pharmacol. 2007, 2, 178–183. [Google Scholar] [CrossRef]
- Peterson, P.K.; Gekker, G.; Hu, S.; Cabral, G.; Lokensgard, J.R. Cannabinoids and morphine differentially affect HIV-1 expression in CD4(+) lymphocyte and microglial cell cultures. J. Neuroimmunol. 2004, 147, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Costantino, C.M.; Gupta, A.; Yewdall, A.W.; Dale, B.M.; Devi, L.A.; Chen, B.K. Cannabinoid Receptor 2-mediated Attenuation of CXCR4-tropic HIV Infection in Primary CD4+ T Cells. PLoS ONE 2012, 7, e33961. [Google Scholar]
- Hu, S.; Sheng, W.S.; Rock, R.B. Immunomodulatory properties of kappa opioids and synthetic cannabinoids in HIV-1 neuropathogenesis. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2011, 6, 528–539. [Google Scholar] [CrossRef]
- Gamal, M.; Moawad, J.; Rashed, L.; El-Eraky, W.; Saleh, D.; Lehmann, C.; Sharawy, N. Evaluation of the effects of Eserine and JWH-133 on brain dysfunction associated with experimental endotoxemia. J. Neuroimmunol. 2015, 281, 9–16. [Google Scholar] [CrossRef]
- Zhou, J.; Burkovskiy, I.; Yang, H.; Sardinha, J.; Lehmann, C. CB2 and GPR55 Receptors as Therapeutic Targets for Systemic Immune Dysregulation. Front. Pharmacol. 2016, 7, 264. [Google Scholar] [CrossRef] [Green Version]
- Plastina, P.; Apriantini, A.; Meijerink, J.; Witkamp, R.; Gabriele, B.; Fazio, A. In Vitro Anti-Inflammatory and Radical Scavenging Properties of Chinotto (Citrus myrtifolia Raf.). Essent. Oils. Nutr. 2018, 10, 783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Hsouna, A.; Gargouri, M.; Dhifi, W.; Ben Saad, R.; Sayahi, N.; Mnif, W.; Saibi, W. Potential anti-inflammatory and antioxidant effects of Citrus aurantium essential oil against carbon tetrachloride-mediated hepatotoxicity: A biochemical, molecular and histopathological changes in adult rats. Environ. Toxicol. 2019, 34, 388–400. [Google Scholar] [CrossRef]
- Lone, J.; Yun, J.W. Monoterpene limonene induces brown fat-like phenotype in 3T3-L1 white adipocytes. Life Sci. 2016, 153, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Pesce, M.; Seguella, L.; Sanseverino, W.; Lu, J.; Corpetti, C.; Sarnelli, G. The potential of cannabidiol in the COVID-19 pandemic. Br. J. Pharmacol. 2020, 177, 4967–4970. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jha, N.K.; Sharma, C.; Meeran, M.F.N.; Jha, S.K.; Dwivedi, V.D.; Gupta, P.K.; Dey, A.; Kesari, K.K.; Ojha, S. Cannabinoid Type-2 Receptor Agonist, JWH133 May Be a Possible Candidate for Targeting Infection, Inflammation, and Immunity in COVID-19. Immuno 2021, 1, 285-304. https://doi.org/10.3390/immuno1030020
Jha NK, Sharma C, Meeran MFN, Jha SK, Dwivedi VD, Gupta PK, Dey A, Kesari KK, Ojha S. Cannabinoid Type-2 Receptor Agonist, JWH133 May Be a Possible Candidate for Targeting Infection, Inflammation, and Immunity in COVID-19. Immuno. 2021; 1(3):285-304. https://doi.org/10.3390/immuno1030020
Chicago/Turabian StyleJha, Niraj Kumar, Charu Sharma, Mohamed Fizur Nagoor Meeran, Saurabh Kumar Jha, Vivek Dhar Dwivedi, Piyush Kumar Gupta, Abhijit Dey, Kavindra Kumar Kesari, and Shreesh Ojha. 2021. "Cannabinoid Type-2 Receptor Agonist, JWH133 May Be a Possible Candidate for Targeting Infection, Inflammation, and Immunity in COVID-19" Immuno 1, no. 3: 285-304. https://doi.org/10.3390/immuno1030020
APA StyleJha, N. K., Sharma, C., Meeran, M. F. N., Jha, S. K., Dwivedi, V. D., Gupta, P. K., Dey, A., Kesari, K. K., & Ojha, S. (2021). Cannabinoid Type-2 Receptor Agonist, JWH133 May Be a Possible Candidate for Targeting Infection, Inflammation, and Immunity in COVID-19. Immuno, 1(3), 285-304. https://doi.org/10.3390/immuno1030020











