Age-Associated Characteristics of CD4+ T-Cell Composition in Patients with Atherosclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lymphocyte Immunophenotyping
2.2. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.; Robertson, A.-K.L.; Rudling, M.; Hansson, G.K. Lesion development and response to immunization reveal a complex role for CD4 in atherosclerosis. Circ. Res. 2005, 96, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saigusa, R.; Winkels, H.; Ley, K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Ventevogel, M.; Sempowski, G.D. Thymic rejuvenation and aging. Curr. Opin. Immunol. 2013, 25, 516–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaun, R.; Agrawal, D.K.; Thankam, F.G. Treg cells in atherosclerosis. Mol. Biol. Rep. 2021, 48, 4897–4910. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Ley, K. Immunity and inflammation in atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, Y.; Cheng, M.; Ji, Y.; Yang, X.; Liu, Y.; Jia, S.; Yuan, Z. Activation of Th17/Th1 and Th1, but not Th17, is associated with the acute cardiac event in patients with acute coronary syndrome. Atherosclerosis 2011, 217, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D.M.; Rahman, A.H.; Fernandez, N.F.; Chudnovskiy, A.; Amir, E.D.; Amadori, L.; Khan, N.S.; Wong, C.K.; Shamailova, R.; Hill, C.A.; et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 2019, 25, 1576–1588. [Google Scholar] [CrossRef]
- Mor, A.; Luboshits, G.; Planer, D.; Keren, G.; George, J. Altered status of CD4+CD25+ regulatory T cells in patients with acute coronary syndromes. Eur. Heart J. 2006, 27, 2530–2537. [Google Scholar] [CrossRef] [Green Version]
- George, J.; Schwartzenberg, S.; Medvedovsky, D.; Jonas, M.; Charach, G.; Afek, A.; Shamiss, A. Regulatory T cells and IL-10 levels are reduced in patients with vulnerable coronary plaques. Atherosclerosis 2012, 222, 519–523. [Google Scholar] [CrossRef]
- Wigren, M.; Bjorkbacka, H.; Andersson, L.; Ljungcrantz, I.; Fredikson, G.N.; Persson, M.; Bryngelsson, C.; Hedblad, B.; Nilsson, J. Low levels of circulating CD4+FoxP3+ T cells are associated with an increased risk for development of myocardial infarction but not for stroke. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2000–2004. [Google Scholar] [CrossRef] [Green Version]
- Potekhina, A.V.; Pylaeva, E.; Provatorov, S.; Ruleva, N.; Masenko, V.; Noeva, E.; Krasnikova, T.; Arefieva, T. Treg/Th17 balance in stable CAD patients with different stages of coronary atherosclerosis. Atherosclerosis 2015, 238, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, S.; Zeng, Q.T. Role of interleukin-17 and interleukin-17-induced cytokines interleukin-6 and interleukin-8 in unstable coronary artery disease. Coron. Artery Dis. 2006, 17, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yu, X.; Ding, Y.-J.; Fu, Q.-Q.; Xie, J.-J.; Tang, T.-T.; Yao, R.; Chen, Y.; Liao, Y.-H. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin. Immunol. 2008, 127, 89–97. [Google Scholar] [CrossRef]
- Eid, R.E.; Rao, D.A.; Zhou, J.; Lo, S.L.; Ranjbaran, H.; Gallo, A.; Sokol, S.I.; Pfau, S.; Pober, J.S.; Tellides, G. Interleukin-17 and interferon-γ are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation 2009, 119, 1424–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, T.; Taleb, S.; Danchin, N.; Laurans, L.; Rousseau, B.; Cattan, S.; Motely, J.-M.; Dubourg, O.; Tedgui, A.; Kotti, S.; et al. Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur. Heart J. 2013, 34, 570–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boer, O.; der Meer, J.J.; Teeling, P.; van der Loos, C.M.; Idu, M.M.; van Maldegem, F.; Aten, J.; van der Wal, A.C. Differential expression of interleukin-17 family cytokines in intact and complicated human atherosclerotic plaques. J. Pathol. 2010, 220, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Erbel, C.; Dengler, T.J.; Wangler, S.; Lasitschka, F.; Bea, F.; Wambsganss, N.; Hakimi, M.; Bockler, D.; Katus, H.A.; Gleissner, C.A. Expression of IL-17A in human atherosclerotic lesions is associated with increased inflammation and plaque vulnerability. Basic Res. Cardiol. 2011, 106, 125–134. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, J.; Wang, H.; Liu, X.; Shang, F.; Zheng, Q. Increased Th17 cells in coronary artery disease are associated with neutrophilic inflammation. Scand. Cardiovasc. J. 2011, 45, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Filatova, A.Y.; Pylaeva, E.A.; Potekhina, A.V.; Osokina, A.K.; Pogorelova, O.A.; Tripoten, M.I.; Balakhonova, T.V.; Provatorov, S.I.; Noeva, E.A.; Klesareva, E.A.; et al. Subpopulation composition of CD4+ T-lymphocytes as factor contributing to the progression of atherosclerosis of carotid arteries. Kardiologiia 2017, 57, 64–71. [Google Scholar] [CrossRef]
- Filatova, A.Y.; Potekhina, A.V.; Pylaeva, E.A.; Osokina, A.K.; Ruleva, N.Y.; Pogorelova, O.A.; Tripoten, M.I.; Noeva, E.A.; Balakhonova, T.V.; Masenko, V.P.; et al. The severity of internal carotid artery stenosis is associated with the circulatinf Th17 level. Heliyon 2020, 6, e03856. [Google Scholar] [CrossRef]
- Tsanaktsi, A.; Solomou, E.E.; Liossis, S.-N. Th1/17 cells, a subset of Th17, are expanded in patients with active systemic lupus erythematosus. Clin. Immunol. 2018, 195, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ran, H.; Li, Y.; Lu, Y.; Liu, X.; Huang, H.; Yang, W.; Yu, L.; Chen, P.; Huang, X.; et al. Circulating Th1/17 cells serve as a biomarker of disease severity and a target for early intervention in AChR-MG patients. Clin. Immunol. 2020, 218, 108492. [Google Scholar] [CrossRef] [PubMed]
- Gregg, R.; Smith, C.M.; Clark, F.J.; Dunnion, D.; Khan, N.; Chakraverty, R.; Nayak, L.; Moss, P.A. The number of human peripheral blood CD4+CD25high regulatory T cells increases with age. Clin. Exp. Immunol. 2005, 140, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Gottenberg, J.-E.; Lavie, F.; Abbed, K.; Gasnault, J.; Nevot, E.L.; Delfraissy, J.-F.; Taoufik, Y.; Mariette, X. CD4+CD25high regulatory T cells are not impaired in patients with primary Sjögren’s syndrome. J. Autoimmun. 2005, 24, 235–242. [Google Scholar] [CrossRef]
- Lages, C.S.; ISuffia, I.; Velilla, P.A.; Huang, B.; Warshaw, G.; Hildeman, D.A.; Belkaid, Y.; Chougnet, C. Functional Regulatory T Cells Accumulate in Aged Hosts and Promote Chronic Infectious Disease Reactivation. J. Immunol. 2008, 181, 1835–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, K.-A.; Kim, H.-R.; Kang, I. Aging and human CD4+ regulatory T cells. Mech. Ageing Dev. 2009, 130, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Botafogo, V.; Perez-Andres, M.; Jara-Acevedo, M.; Barcena, P.; Grigore, G.; Hernandez-Delgrado, A.; Damasceno, D.; Comans, S.; Blanco, E.; Romero, A.; et al. Age distribution of multiple functionally relevant subsets of CD4+ T cells in human blood using a standardized and validated 14-color euroflow immune monitoring tube. Front. Immunol. 2020, 11, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Carotid Surgery Trialists’ Collaborative Group. MRC European Carotid Surgery Trial: Interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. Lancet 1991, 337, 1235–1243. [Google Scholar] [CrossRef]
- Ferguson, F.G.; Wikby, A.; Maxson, P.; Olsson, J.; Johansson, B. Immune parameters in a longitudinal study of a very old population of Swedish people: A comparison of survivors and nonsurvivors. J. Gerontol. Biol. Sci. 1995, 50, 378–382. [Google Scholar] [CrossRef]
- Wikby, A.; Maxson, P.; Olsson, J.; Johansson, B.; Ferguson, F.G. Changes in CD8 and CD4 lymphocyte subsets, T cell proliferation responses and non-survival in the very old: The Swedich longitudinal OCTO-immune study. Mech. Ageing Dev. 1998, 102, 187–198. [Google Scholar] [CrossRef]
- Zanni, F.; Vescovini, R.; Biasini, C.; Fagnoni, F.; Zanlari, L.; Telera, A.; Di Pede, P.; Passeri, G.; Pedrazzoni, M.; Passeri, M.; et al. Marked increase with age of type 1 cytokines within memory and effector/cytotoxic CD8+ T cells in humans: A contribution to understand the relationship between inflammation and immunosenescence. Exp. Gerontol. 2003, 38, 981–987. [Google Scholar] [CrossRef]
- Huppert, F.A.; Pinto, E.M.; Morgan, K.; Brayene, C. Survival in a population sample is predicted by proportions of lymphocyte subsets. Mech. Ageing Dev. 2003, 124, 449–451. [Google Scholar] [CrossRef]
- Thapa, P.; Farber, D.L. The role of the thymus in the immune response. Thorac. Surg. Clin. 2019, 29, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.S.; Bueno, V. Immunosenescence: Participation of T lymphocytes and myeloid-derives supressor cells in aging-related immune response changes. Einstein 2019, 17, eRB4733. [Google Scholar] [CrossRef] [Green Version]
- Tortorella, C.; Loria, M.P.; Piazzolla, G.; Schulze-Koops, H.; Lipsky, P.E.; Jirillo, E.; Antonaci, S. Age-related impairment of T cell proliferative responses related to the decline of CD28+ T cell subsets. Arch. Gerontol. Geriatr. 1998, 26, 55–70. [Google Scholar] [CrossRef]
- Olivieri, F.; Albertini, M.C.; Orciani, M.; Ceka, A.; Cricca, M.; Procopio, A.D.; Bonafe, M. DNA damage response (DDR) and senescence: Shuttled inflamma-miRNAs on the stage of inflamm-aging. Oncotarget 2015, 6, 35509–35521. [Google Scholar] [CrossRef] [Green Version]
- Amadori, A.; Zamarchi, R.; De Silvestro, G.; Forza, G.; Cavatton, G.; Danieli, G.A.; Clementi, M.; Chieco-Bianchi, L. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat. Med. 1995, 1, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.A.; Rego, D.; Moshkova, M.; Kebir, H.; Chruscinski, A.; Nguyen, H.; Akkermann, R.; Stanczyk, F.Z.; Prat, A.; Steinman, L.; et al. Peroxisome proliferator-activated receptor (PPAR)α and -γ regulate IFNγ and IL-17A production by human T cells in a sex-specific way. Proc. Natl. Acad. Sci. USA 2012, 109, 9505–9510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairweather, D.; Frisancho-Kiss, S.; Rose, N.R. Sex differences in autoimmune disease from a pathological perspective. Am. J. Pathol. 2008, 173, 600–609. [Google Scholar] [CrossRef] [Green Version]
- Miyara, M.; Yoshioka, Y.; Kitoh, A.; Shima, T.; Wing, K.; Niwa, A.; Parizot, C.; Taflin, C.; Heike, T.; Valeyre, D.; et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the Foxp3 transcription factor. Immunity 2009, 30, 899–911. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, J.; Shu, Y.; Tang, T.; Zhu, Z.; Xia, N.; Nie, S.; Liu, J.; Zhou, S.; Li, J.; et al. Impaired thymic export and increased apoptosis account for regulatory T cell defects in patients with non-ST segment elevation acute coronary syndrome. J. Biol. Chem. 2012, 287, 34157–34166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
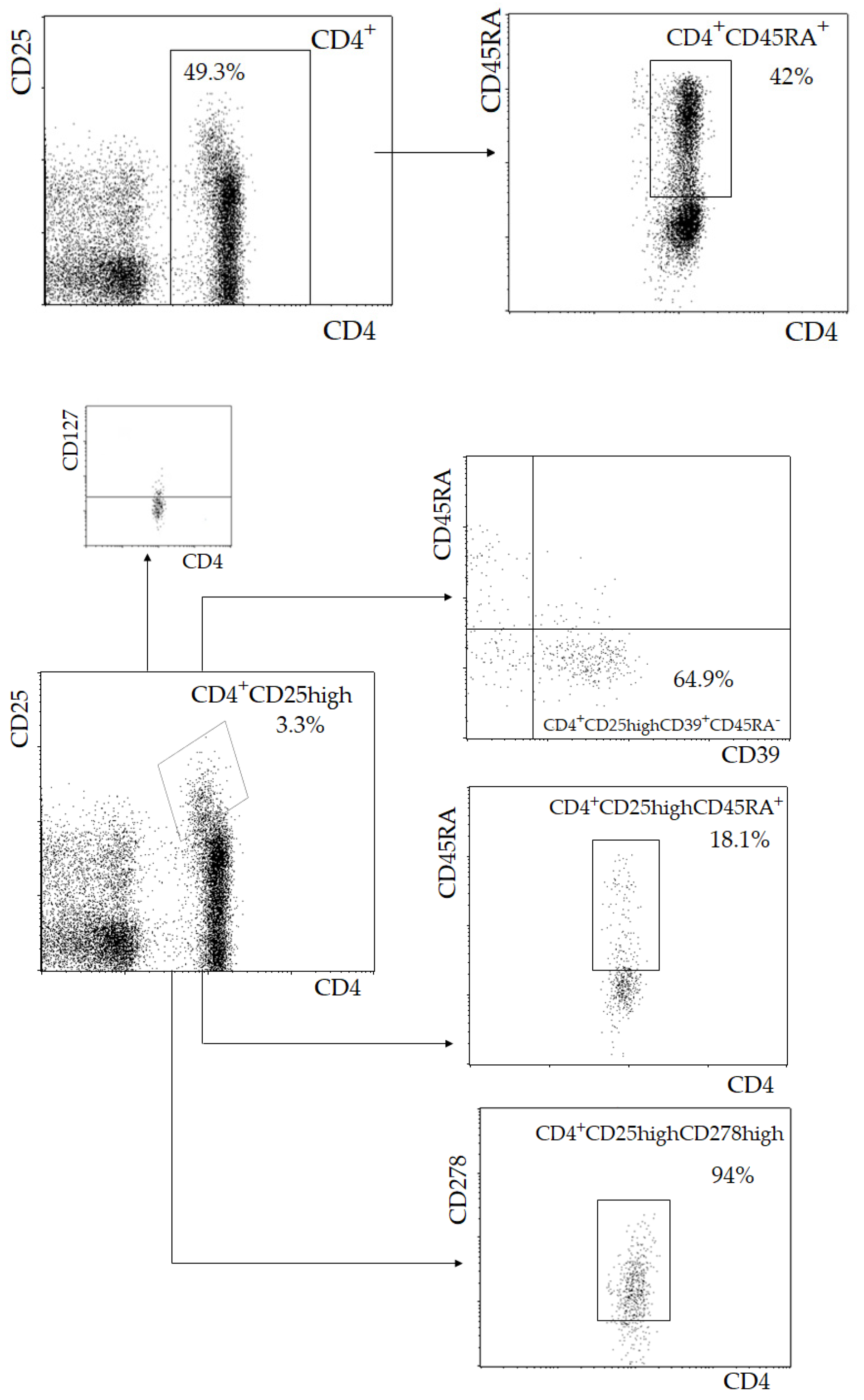
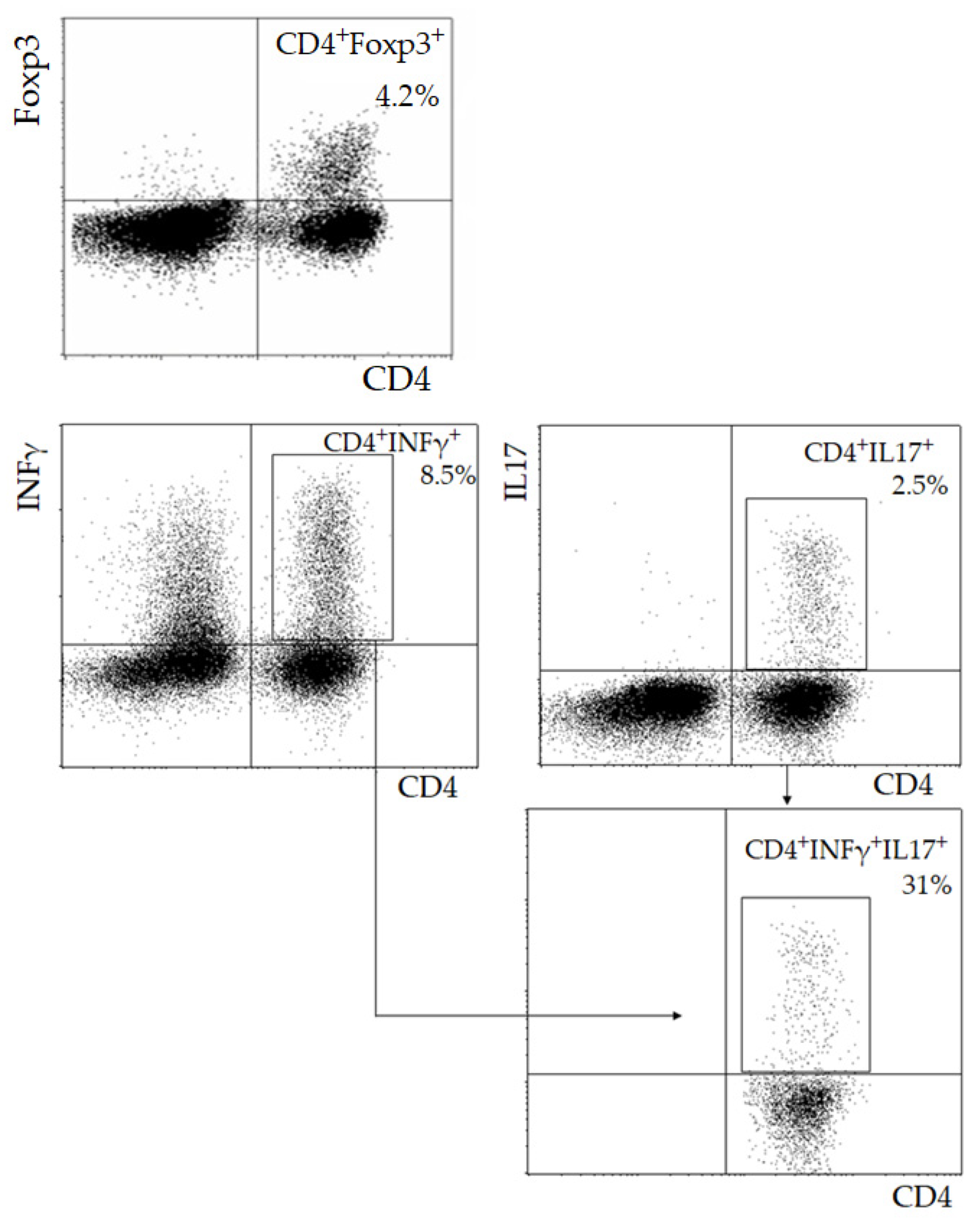
| Parameter | I (<55, n = 23) | II (55–64, n = 42) | III (≥65, n = 46) | p |
|---|---|---|---|---|
| BMI, kg/m2 | 29.0 (28.0; 33.0) | 28.0 (25.0; 30.0) | 27.0 (26.0; 29.0) | 0.07 |
| Arterial hypertension, n (%) | 20 (87%) | 35 (83%) | 41 (89%) | 0.87 |
| Diabetes mellitus, n (%) | 2 (8%) | 1 (2%) | 6 (13%) | 0.18 |
| Previous myocardial infarction, n (%) | 12 (52%) | 25 (59%) | 24 (52%) | 0.75 |
| Coronary AS severity | ||||
| mild, n (%) moderate, n (%) severe, n (%) | 1 (4) 12 (52) 10 (43) | 4 (9) 26 (62) 12 (28) | 1 (2) 23 (50) 22 (48) | 0.28 |
| CCA/CCA bifurcation stenosis, % | 37.5 (30.0; 45.0) | 30.0 (30.0; 40.0) | 40.0 (30.0; 45.0) | 0.21 |
| ICA stenosis, % | 40.0 (35.0; 50.0) | 37.5 (30.0; 50.0) | 40.0 (25.0; 60.0) | 0.62 |
| Severe coronary AS + carotid artery stenosis > 50%, n (%) | 2 (8%) | 0 (0%) | 4 (8%) | 0.41 |
| Total cholesterol, mM | 3.8 (3.6; 4.5) | 4.2 (3.5; 4.8) | 4.0 (3.6; 4.7) | 0.67 |
| Triglycerides, mM | 1.4 (1.1; 2.2) | 1.4 (1.2; 1.8) | 1.5 (1.0; 1.8) | 0.94 |
| LDL, mM | 2.2 (1.8; 2.5) | 2.2 (1.7; 2.9) | 2.2 (1.7; 2.9) | 0.78 |
| HDL, mM | 0.9 (0.8; 1.1) | 1.1 (0.9; 1.2) | 1.0 (0.8; 1.2) | 0.77 |
| Glucose, mM | 5.7 (5.2; 6.1) | 5.4 (5.1; 6.1) | 5.6 (5.0; 6.2) | 0.69 |
| Leukocytes, mln/mL | 7.2 (6.2; 8.4) | 7.4 (5.8; 8.4) | 7.0 (6.0; 8.0) | 0.73 |
| Parameter | I (<55, n = 23) | II (55–64, n = 42) | III (≥65, n = 46) | p |
|---|---|---|---|---|
| Lymphocytes, mln/mL | 2.1 (1.6; 2.6) | 1.9 (1.5; 2.2) | 1.8 (1.4; 2.2) | 0.22 |
| CD4+ T-cells (103/mL) | 903.0 (585.6; 1113.8) | 745.4 (502.2; 924.0) | 646.3 (516.0; 806.4) | 0.03 1 vs. 3 p = 0.008 2 vs. 3 p = 0.06 |
| CD4+CD45RA+ T-cells (103/mL) | 365.0 (262.3; 478.2) | 422.3 (241.1; 553.0) | 197.2 (173.5; 297.5) | 1 vs. 3 p = 0.0008 2 vs. 3 p = 0.0007 |
| CD4+CD45RA+/CD4+CD45RA− T-cells | 0.23 (0.15; 0.33) | 0.23 (0.10; 0.30) | 0.16 (0.09; 0.24) | 1 vs. 3 p = 0.016 2 vs. 3 p = 0.057 |
| CD4+CD25highCD127low Treg (103/mL) | 35.0 (28.7; 54.4) | 31.0 (21.1; 43.6) | 24.2 (18.4; 35.2) | 1 vs. 3 p = 0.008 2 vs. 3 p = 0.03 |
| CD4+CD25highCD39+CD45RA− Treg (% CD25high Treg) | 53.5 (31.4; 64.3) | 56.0 (21.5; 64.5) | 57.4 (45.1; 68.0) | 0.69 |
| CD4+CD25highCD278high Treg (% CD25high Treg) | 86.5 (82.0; 88.5) | 86.5 (82.0; 90.0) | 90.0 (88.0; 93.0) | 0.11 |
| CD4+CD25highCD45RA+Treg (103/mL) | 8.5 (6.1; 14.3) | 6.6 (3.5; 12.7) | 5.7 (2.2; 9.7) | 1 vs. 3 p = 0.01 |
| CD4+Foxp3+ Treg (103/mL) | 74.0 (53.5; 87.2) | 58.0 (35.0; 73.6) | 54.0 (38.4; 73.3) | 0.19 |
| Th17 (103/mL) | 13.4 (8.1; 14.7) | 9.6 (4.8; 15.8) | 11.3 (5.8; 16.6) | 0.66 |
| Th1 (103/mL) | 212.0 (158.0; 258.7) | 142.3 (55.6; 225.0) | 143.5 (98.4; 239.7) | 0.19 |
| Th1/17 (103/mL) | 2.0 (1.4; 4.2) | 2.0 (1.1; 3.7) | 1.4 (0.6; 3.1) | 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filatova, A.Y.; Potekhina, A.V.; Arefieva, T.I. Age-Associated Characteristics of CD4+ T-Cell Composition in Patients with Atherosclerosis. Immuno 2021, 1, 277-284. https://doi.org/10.3390/immuno1030019
Filatova AY, Potekhina AV, Arefieva TI. Age-Associated Characteristics of CD4+ T-Cell Composition in Patients with Atherosclerosis. Immuno. 2021; 1(3):277-284. https://doi.org/10.3390/immuno1030019
Chicago/Turabian StyleFilatova, Anastasiia Yu., Alexandra V. Potekhina, and Tatiana I. Arefieva. 2021. "Age-Associated Characteristics of CD4+ T-Cell Composition in Patients with Atherosclerosis" Immuno 1, no. 3: 277-284. https://doi.org/10.3390/immuno1030019
APA StyleFilatova, A. Y., Potekhina, A. V., & Arefieva, T. I. (2021). Age-Associated Characteristics of CD4+ T-Cell Composition in Patients with Atherosclerosis. Immuno, 1(3), 277-284. https://doi.org/10.3390/immuno1030019






