Role of Macrophage Polarization in Acute Respiratory Distress Syndrome
Abstract
:1. Introduction
2. Etiology
3. Pathophysiology
4. Biomarkers
5. Based on Anatomical Regions
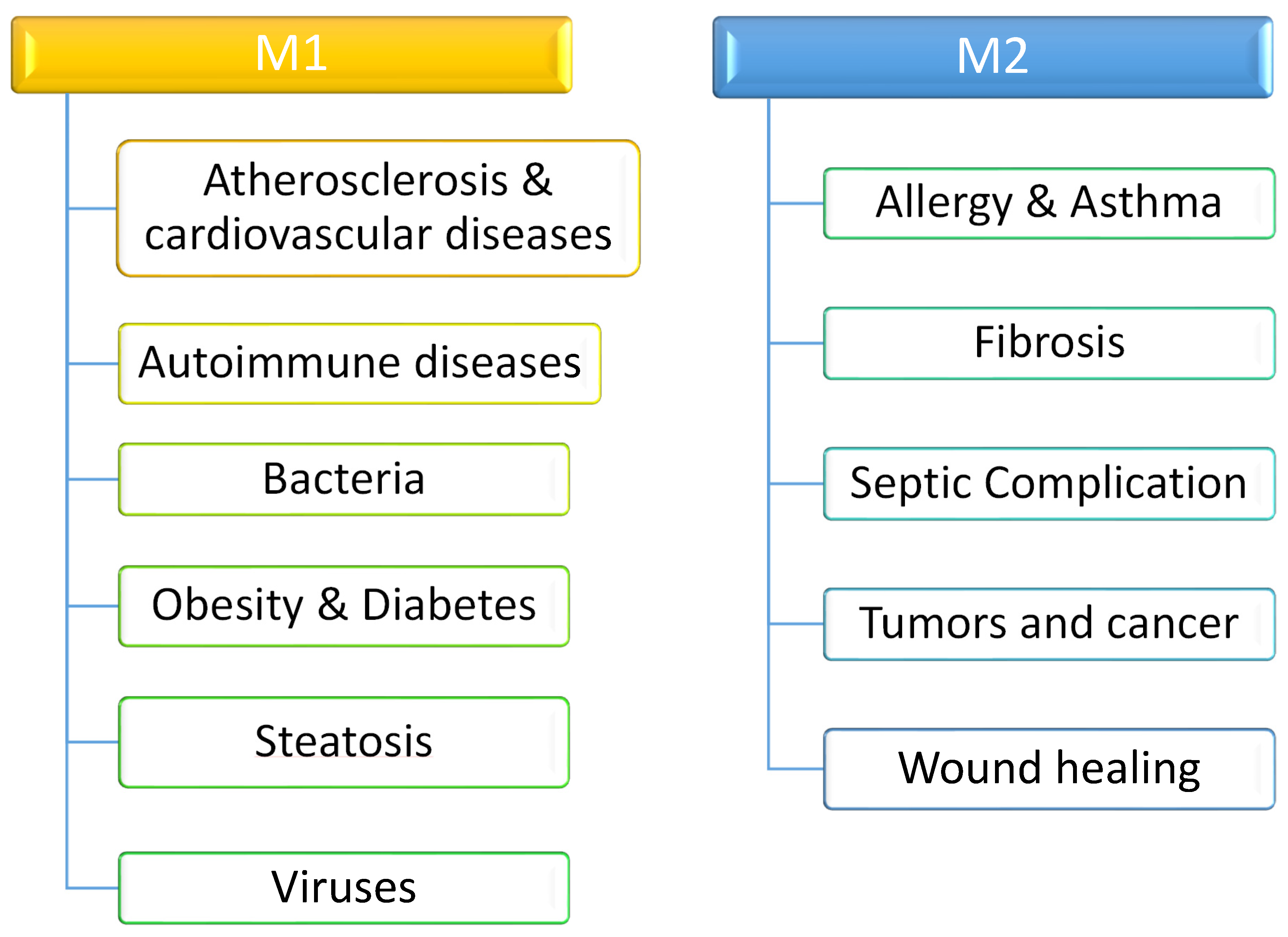
6. Division Depending on Polarization
- (A) M1-type (classically activated macrophages (CAMs) or pro-inflammatory).
- (B) M2-type (alternatively activated macrophages (AAMs) or anti-inflammatory).
- (C) Regulatory Macrophages (Mreg).
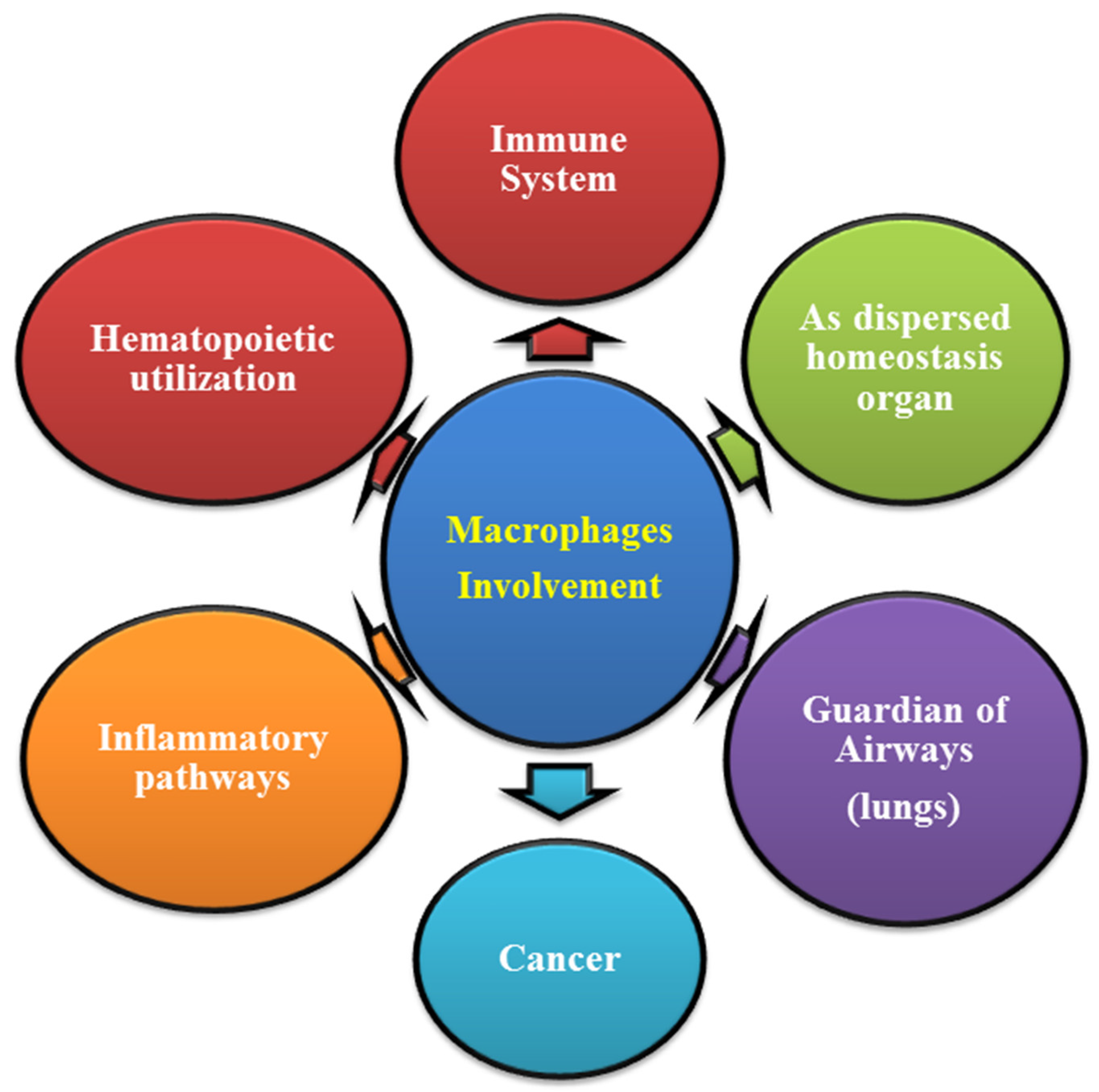
7. Functional Role of Macrophages in Human Physiology
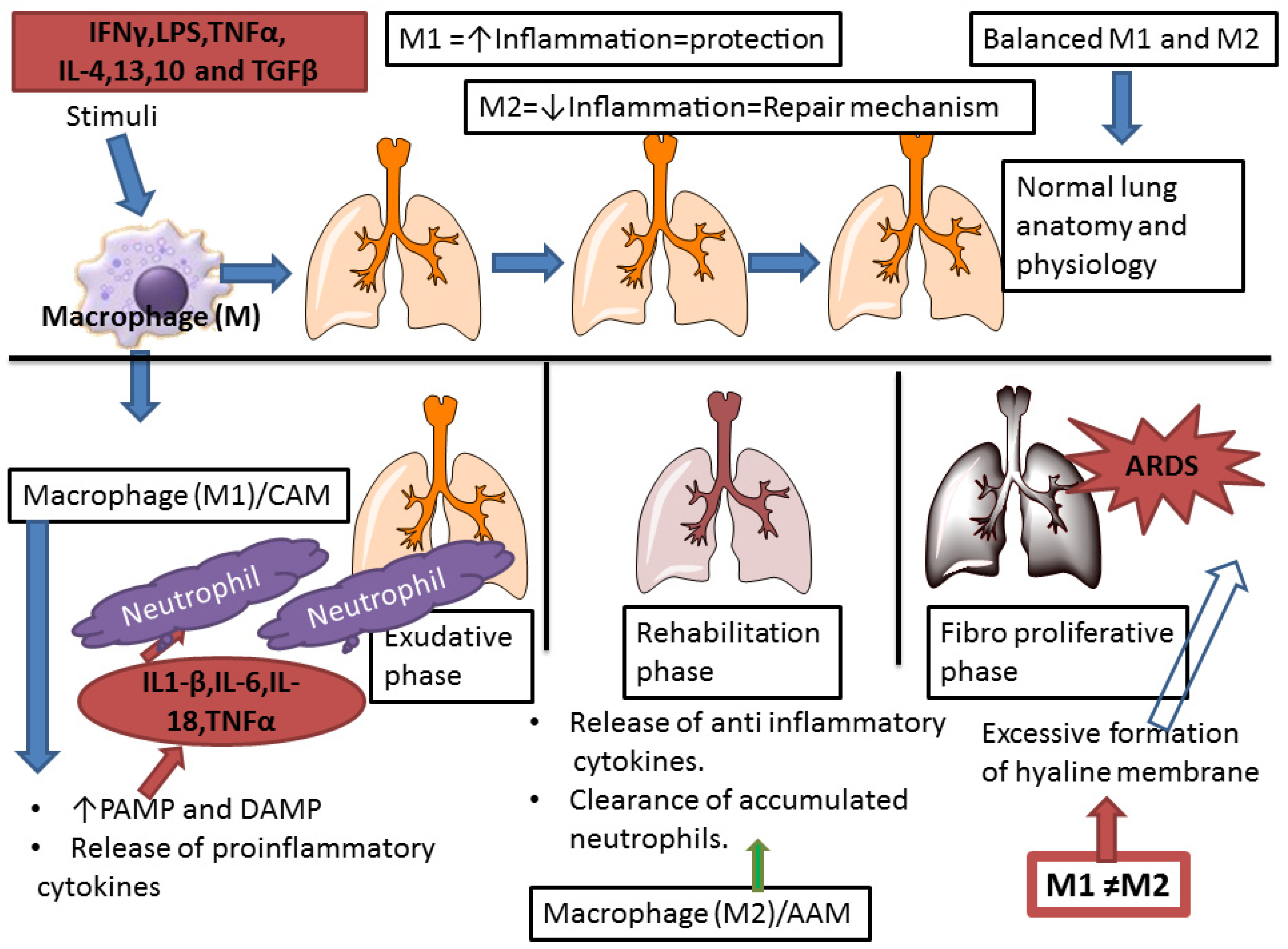
8. Molecular Mechanism of Macrophage in ARDS
9. Discussion
- How do these macrophages decide the level of inflammatory cytokine and neutrophil production?
- How do they interact with localised endothelial cells from airway epithelial progenitors during the regeneration process?
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Ashbaugh, D.G.; Boyd Bigelow, D.; Petty, T.L.; Levine, B.E.; Bigelow, D.B.; Petty, T.L. Acute respiratory distress in adults. Lancet 1967, 290, 319–323. [Google Scholar] [CrossRef]
- Ashbaugh, D.G.; Bigelow, D.B.; Petty, T.L.; Levine, B.E. Acute respiratory distress in adults. The Lancet, Saturday 12 August 1967. Crit. Care Resusc. 2005, 7, 60–61. [Google Scholar]
- Bernard, G.R.; Artigas, A.; Brigham, K.L.; Carlet, J.; Falke, K.; Hudson, L.; Lamy, M.; Legall, J.R.; Morris, A.; Spragg, R.; et al. Report of the American-European consensus conference on ARDS: Definitions, mechanisms, relevant outcomes and clinical trial coordination. Intensive Care Med. 1994, 20, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin definition. JAMA J. Am. Med. Assoc. 2012, 307, 2526–2533. [Google Scholar]
- Rebecca, M.B.; Bruce, D.L. Harrison’s Principles of Internal Medicine; McGraw Hill: New York, NY, USA, 2018. [Google Scholar]
- Bhatia, M.; Moochhala, S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J. Pathol. 2004, 202, 145–156. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Two Types of Macrophages: M1 and M2 Macrophages. Cusabio. Available online: https://www.cusabio.com/c-20938.html (accessed on 18 October 2021).
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [Green Version]
- Kubota, T.; Inoue, M.; Kubota, N.; Takamoto, I.; Mineyama, T.; Iwayama, K.; Tokuyama, K.; Moroi, M.; Ueki, K.; Yamauchi, T.; et al. Downregulation of macrophage Irs2 by hyperinsulinemia impairs IL-4-indeuced M2a-subtype macrophage activation in obesity. Nat. Commun. 2018, 9, 4863. [Google Scholar] [CrossRef]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A thorn of a tangerine tree. British Society for Immunology. Available online: https://www.immunology.org/thorn-tangerine-tree-1882 (accessed on 27 March 2020).
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, M.H.; Abdelwahab, S.F.; Wan, J.; Cai, W.; Huixuan, W.; Jianjun, C.; Kumar, K.D.; Vasudevan, A.; Sadek, A.; Su, Z.; et al. Alternatively activated macrophages; a double-edged sword in allergic asthma. J. Transl. Med. 2020, 18, 1–12. [Google Scholar] [CrossRef]
- Epelman, S.; LaVine, K.J.; Randolph, G.J. Origin and Functions of Tissue Macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Luo, J.; Yang, N.; Wang, S.; Ye, M.; Pan, G. Activation of the IL-1β/KLF2/HSPH1 pathway promotes STAT3 phosphorylation in alveolar macrophages during LPS-induced acute lung injury. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, Y.-S.; Chen, Y.-Q.; Lin, S.-H.; Xie, K.; Wang, C.-J.; Yang, Y.-Z.; Xu, F. Curcumin regulates the differentiation of naïve CD4+T cells and activates IL-10 immune modulation against acute lung injury in mice. Biomed. Pharmacother. 2020, 125, 109946. [Google Scholar] [CrossRef]
- Martin, T.R. Cytokines and the acute respiratory distress syndrome (ARDS): A question of balance. Nat. Med. 1997, 3, 272–273. [Google Scholar] [CrossRef]
- Park, W.Y.; Goodman, R.B.; Steinberg, K.P.; Ruzinski, J.T.; Radella, F.; Park, D.R.; Pugin, J.; Skerrett, S.J.; Hudson, L.D.; Martin, T.R. Cytokine Balance in the Lungs of Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2001, 164, 1896–1903. [Google Scholar] [CrossRef]
- Ariel, A.; Maridonneau-Parini, I.; Rovere-Querini, P.; Levine, J.S.; Mühl, H. Macrophages in inflammation and its resolution. Front. Immunol. 2012, 3, 324. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Zhao, X.; Gao, Y.; Liu, M.; Hou, M.; Jin, H.; Cui, Y. Recombinant human brain natriuretic peptide ameliorates trauma-induced acute lung injury via inhibiting JAK/STAT signaling pathway in rats. J. Trauma Acute Care Surg. 2015, 78, 980–987. [Google Scholar] [CrossRef]
- Kern, K.; Schäfer, S.M.G.; Cohnen, J.; Pierre, S.; Osthues, T.; Tarighi, N.; Hohmann, S.; Ferreiros, N.; Brüne, B.; Weigert, A.; et al. The G2A Receptor Controls Polarization of Macrophage by Determining Their Localization Within the Inflamed Tissue. Front. Immunol. 2018, 9, 2261. [Google Scholar] [CrossRef]
- Yu, Z.-X.; Ji, M.-S.; Yan, J.; Cai, Y.; Liu, J.; Yang, H.-F.; Li, Y.; Jin, Z.-C.; Zheng, J.-X. The ratio of Th17/Treg cells as a risk indicator in early acute respiratory distress syndrome. Crit. Care 2015, 19, 82. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-Y.; Chen, C.-S.; Yiang, G.-T.; Cheng, Y.-L.; Yong, S.-B.; Wu, M.-Y.; Li, C.-J. New Insights into the Immune Molecular Regulation of the Pathogenesis of Acute Respiratory Distress Syndrome. Int. J. Mol. Sci. 2018, 19, 588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, N.R.; King, L.S.; D’Alessio, F.R. Diverse macrophage populations mediate acute lung inflammation and resolution. Am. J. Physiol. Cell. Mol. Physiol. 2014, 306, L709–L725. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Zhou, Z.; Rajasingh, S.; Panda, A.; Sampath, V.; Rajasingh, J. DNMT and HDAC inhibitors together abrogate endotoxemia mediated macrophage death by STAT3-JMJD3 signaling. Int. J. Biochem. Cell Biol. 2018, 102, 117–127. [Google Scholar] [CrossRef]
- Thangavel, J.; Samanta, S.; Rajasingh, S.; Barani, B.; Xuan, Y.-T.; Dawn, B.; Rajasingh, J. Epigenetic modifiers reduce inflammation and modulate macrophage phenotype during endotoxemia-induced acute lung injury. J. Cell Sci. 2015, 128, 3094–3105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, U.; Rajasingh, S.; Samanta, S.; Cao, T.; Dawn, B.; Rajasingh, J. Macrophage polarization in response to epigenetic modifiers during infection and inflammation. Drug Discov. Today 2017, 22, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial transfer from bone-marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef] [Green Version]
- Looney, M.R.; Nguyen, J.X.; Hu, Y.; Van Ziffle, J.A.; Lowell, C.A.; Matthay, M.A. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J. Clin. Investig. 2009, 119, 3450–3461. [Google Scholar] [CrossRef] [Green Version]
- Brodie, D.; Bacchetta, M. Extracorporeal Membrane Oxygenation for ARDS in Adults. New Engl. J. Med. 2011, 365, 1905–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Chu, C.; Wu, Z.; Liu, F.; Xie, J.; Yang, Y.; Qiu, H. IFIH1 Contributes to M1 Macrophage Polarization in ARDS. Front. Immunol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.P.; Cash, M.N.; Santostefano, K.E.; Nakanishi, M.; Terada, N.; Wallet, M.A. CRISPR/Cas9 knockout of USP18 enhances type I IFN responsiveness and restricts HIV-1 infection in macrophages. J. Leukoc. Biol. 2018, 103, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Gerrick, K.Y.; Gerrick, E.R.; Gupta, A.; Wheelan, S.J.; Yegnasubramanian, S.; Jaffee, E.M. Transcriptional profiling identifies novel regulators of macrophage polarization. PLoS ONE 2018, 13, e0208602. [Google Scholar] [CrossRef] [Green Version]

| Features | Ausbugh and Petty 1967 [3] | AECC Definition 1994 [4] | Berlin Definition 2012 [5] |
|---|---|---|---|
| Timing | Acute not specified | Acute, time frame is still missing | Specified timeline-maximum within a week after insult |
| Oxygenation ALI/ARDS | Not specified | <300 for ALI <200 for ARDS PEEP is not considered in this | Mild: 201–300 Moderate: 101–200 Severe: <100 Based on PaO2/FiO2, with PEEP ≥ 5 cm H2O |
| Chest radiograph | Patchy bilateral alveolar infiltrations | Chest X-ray with bilateral pulmonary infiltrations | Chest radiograph criteria clarified Example radiographs created |
| PAWP (Pulmonary Artery Wedge Pressure) | Not mentioned | PAWP 18 mm Hg when measured or no clinical evidence of left atrial hypertension | PAWP requirement removed Hydrostatic oedema is not the primary cause of respiratory failure Clinical vignettes were created to help exclude hydrostatic oedema |
| Risk factors | The initial study included 12 multifactorial patients | Not specified | Included When none is identified, need to objectively rule out hydrostatic oedema |
| Direct Lung Injury/Pulmonary Injury | Indirect Lung Injury/Extra-Pulmonary Injury |
|---|---|
| Common Pneumonia Aspiration of gastric contents Less Common Pulmonary contusion Fat/Amniotic fluid embolism High Altitude Near Drowning Inhalation Injury Reperfusion Injury | Common Sepsis Severe trauma with shock and multiple transfusions Less Common Burns Disseminated intravascular coagulation Cardiopulmonary bypass Drug overdose (heroin, barbiturates) Acute pancreatitis Transfusion of blood products Hypoproteinaemia |
| Macrophage Subtype | Stimuli | Markers | References |
|---|---|---|---|
| M1 | IFN LPS TNFα | CD80, CD86, CD64, CD16 and CD32 iNOS | [9] |
| M2a | IL-4, IL-13 and M-CSF | CD206, Arg1, Ym1, FIZZ1 IL-10, TGF-β | [9,10] |
| M2b | TLR ligands + IL-1R agonist | CD206, IL-1 β, IL-6, TNF-α, IL-12Low IL-10 | [11] |
| M2c | IL-10, Glucocorticoids, TGF-β | CD206, CD163, MerTK IL-10, TGF-β | [12] |
| M2d | TLR + adenosine A2A R ligands, IL-6 | VEGF, IL-10 TGF-β IL-12Low, TNF-αLow | [13] |
| S.No. | Anatomical Location | Types of Macrophages |
|---|---|---|
| 1 | Adipose Tissue | Adipose tissue macrophages |
| 2 | Bone marrow/blood | Monocytes |
| 3 | Liver | Kupffer cells |
| 4 | Lymph nodes | Sinus histocytes |
| 5 | Pulmonary Alveoli of lungs | Alveolar macrophages (dust cells) |
| 6 | Connective tissue | Histiocytes leading to giant cell |
| 7 | Central Nervous System | Microglia |
| 8 | Placenta | Hofbauer cells |
| 9 | Kidney | Intra glomerular mesengial cells |
| 10 | Bone | Osteoclasts |
| 11 | Granulomas | Epitheloid cells |
| 12 | Spleen (Red Pulp) | Red pulp macrophages (sinusoidal) |
| 13 | Peritoneal cavity | Peritoneal macrophages |
| 14 | Peyer’s Patch | Lysomac |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, P.; Pandey, N.; Pandey, R.; Tripathi, Y.B. Role of Macrophage Polarization in Acute Respiratory Distress Syndrome. J. Respir. 2021, 1, 260-272. https://doi.org/10.3390/jor1040024
Mishra P, Pandey N, Pandey R, Tripathi YB. Role of Macrophage Polarization in Acute Respiratory Distress Syndrome. Journal of Respiration. 2021; 1(4):260-272. https://doi.org/10.3390/jor1040024
Chicago/Turabian StyleMishra, Priyanka, Nikhil Pandey, Ratna Pandey, and Yamini B Tripathi. 2021. "Role of Macrophage Polarization in Acute Respiratory Distress Syndrome" Journal of Respiration 1, no. 4: 260-272. https://doi.org/10.3390/jor1040024
APA StyleMishra, P., Pandey, N., Pandey, R., & Tripathi, Y. B. (2021). Role of Macrophage Polarization in Acute Respiratory Distress Syndrome. Journal of Respiration, 1(4), 260-272. https://doi.org/10.3390/jor1040024






