Fine-Needle Aspiration Is Suitable for Breast Cancer BRCA Molecular Assessment: A Case Report
Abstract
:1. Introduction
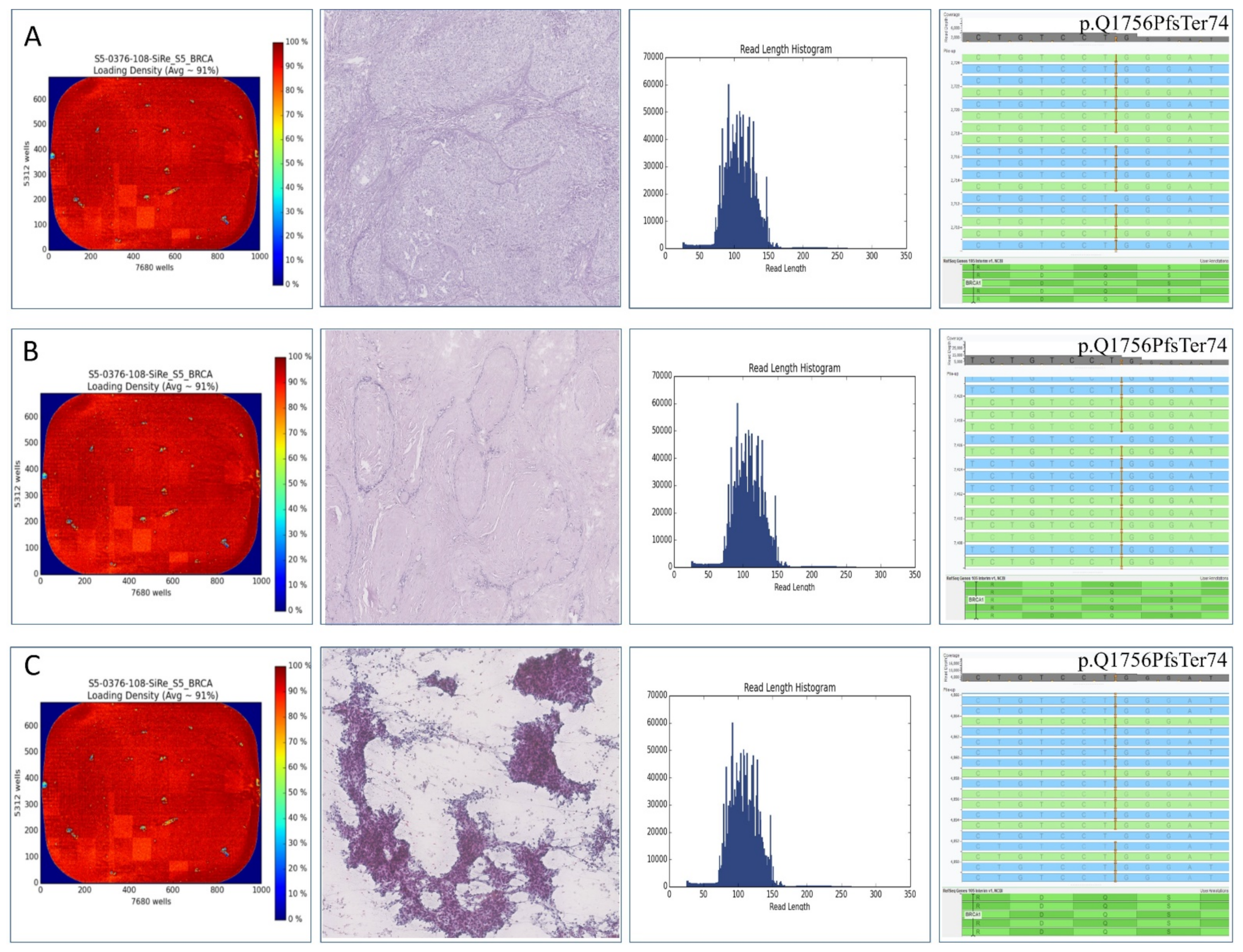
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef]
- Thill, M.; Jackisch, C.; Janni, W.; Müller, V.; Albert, U.S.; Bauerfeind, I.; Blohmer, J.; Budach, W.; Dall, P.; Diel, I.; et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Locally Advanced and Metastatic Breast Cancer: Update 2019. Breast Care 2019, 14, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, L.; Rugo, H.S.; Jackisch, C. An Overview of PARP Inhibitors for the Treatment of Breast Cancer. Target Oncol. 2021, 16, 255–282. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves Olaparib for Germline BRCA-Mutated Metastatic Breast Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-germline-brca-mutated-metastatic-breast-cancer (accessed on 1 June 2021).
- Lynparza Approved in EU for the Treatment of Germline BRCA-Mutated HER2-Negative Advanced Breast Cancer. Available online: https://www.astrazeneca.com/media-centre/press-releases/2019/lynparza-approved-in-eu-for-the-treatment-of-germline-brca-mutated-her2-negative-advanced-breast-cancer-10042019.html# (accessed on 1 June 2021).
- FDA Approves Talazoparib for gBRCAm HER2-Negative Locally Advanced or Metastatic Breast Cancer. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-gbrcam-her2-negative-locally-advanced-or-metastatic-breast-cancer (accessed on 1 June 2021).
- European Commission Approves TALZENNA® (Talazoparib) for Patients with Inherited (Germline) BRCA-Mutated Locally Advanced or Metastatic Breast Cancer. Available online: https://www.pfizer.com/news/press-release/press-release-detail/european_commission_approves_talzenna_talazoparib_for_patients_with_inherited_germline_brca_mutated_locally_advanced_or_metastatic_breast_cancer (accessed on 1 June 2021).
- Field, A.S.; Raymond, W.A.; Schmitt, F. The International Academy of Cytology Yokohama System for Reporting Breast Fine Needle Aspiration Biopsy Cytopathology: Recent research findings and the future. Cancer Cytopathol. 2021. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, F.; Migliatico, I.; Vigliar, E.; Salatiello, M.; Pisapia, P.; Iaccarino, A.; Russo, D.; Insabato, L.; Accurso, A.; Arpino, G.; et al. The continuing role of breast fine-needle aspiration biopsy after the introduction of the IAC Yokohama System For Reporting Breast Fine Needle Aspiration Biopsy Cytopathology. Diagn. Cytopathol. 2020, 48, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Beca, F.; Schmitt, F.C. Ancillary Tests in Breast Cytology: A Practical Guide. Acta Cytol. 2019, 63, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Concolino, P.; Gelli, G.; Rizza, R.; Costella, A.; Scambia, G.; Capoluongo, E. BRCA1 and BRCA2 Testing through Next Generation Sequencing in a Small Cohort of Italian Breast/Ovarian Cancer Patients: Novel Pathogenic and Unknown Clinical Significance Variants. Int. J. Mol. Sci. 2019, 20, 3442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, A.S.; Schmitt, F.; Vielh, P. IAC Standardized Reporting of Breast Fine-Needle Aspiration Biopsy Cytology. Acta Cytol. 2017, 61, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Field, A.S.; Raymond, W.A.; Rickard, M.; Arnold, L.; Brachtel, E.F.; Chaiwun, B.; Chen, L.; Di Bonito, L.; Kurtycz, D.F.I.; Lee, A.H.S. The International Academy of Cytology Yokohama System for Reporting Breast Fine-Needle Aspiration Biopsy Cytopathology. Acta Cytol. 2019, 63, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Pisapia, P.; Pepe, F.; Iaccarino, A.; Sgariglia, R.; Nacchio, M.; Conticelli, F.; Salatiello, M.; Tufano, R.; Russo, G.; Gragnano, G.; et al. Next Generation Sequencing in Cytopathology: Focus on Non-Small Cell Lung Cancer. Front. Med. 2021, 8, 633923. [Google Scholar] [CrossRef] [PubMed]
- Pisapia, P.; Pepe, F.; Sgariglia, R.; Nacchio, M.; Russo, G.; Conticelli, F.; Girolami, I.; Eccher, A.; Bellevicine, C.; Vigliar, E.; et al. Next generation sequencing in cytology. Cytopathology 2021. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Sedmak, D.; Jewell, S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am. J. Pathol. 2002, 161, 1961–1971. [Google Scholar] [CrossRef] [Green Version]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J. OlympiA Clinical Trial Steering Committee and Investigators. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Vinayak, S.; Tolaney, S.M.; Schwartzberg, L.; Mita, M.; McCann, G.; Tan, A.R.; Wahner-Hendrickson, A.E.; Forero, A.; Anders, C.; Wulf, G.M.; et al. Open-label Clinical Trial of Niraparib Combined with Pembrolizumab for Treatment of Advanced or Metastatic Triple-Negative Breast Cancer. JAMA Oncol. 2019, 5, 1132–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domchek, S.M.; Postel-Vinay, S.; Im, S.A.; Park, Y.H.; Delord, J.P.; Italiano, A.; Alexandre, J.; You, B.; Bastian, S.; Krebs, M.G. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): An open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020, 21, 1155–1164. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepe, F.; Pisapia, P.; Russo, G.; Nacchio, M.; Vigliar, E.; Giuliano, M.; Malapelle, U.; Troncone, G.; Bellevicine, C. Fine-Needle Aspiration Is Suitable for Breast Cancer BRCA Molecular Assessment: A Case Report. J. Mol. Pathol. 2021, 2, 319-324. https://doi.org/10.3390/jmp2040028
Pepe F, Pisapia P, Russo G, Nacchio M, Vigliar E, Giuliano M, Malapelle U, Troncone G, Bellevicine C. Fine-Needle Aspiration Is Suitable for Breast Cancer BRCA Molecular Assessment: A Case Report. Journal of Molecular Pathology. 2021; 2(4):319-324. https://doi.org/10.3390/jmp2040028
Chicago/Turabian StylePepe, Francesco, Pasquale Pisapia, Gianluca Russo, Mariantonia Nacchio, Elena Vigliar, Mario Giuliano, Umberto Malapelle, Giancarlo Troncone, and Claudio Bellevicine. 2021. "Fine-Needle Aspiration Is Suitable for Breast Cancer BRCA Molecular Assessment: A Case Report" Journal of Molecular Pathology 2, no. 4: 319-324. https://doi.org/10.3390/jmp2040028
APA StylePepe, F., Pisapia, P., Russo, G., Nacchio, M., Vigliar, E., Giuliano, M., Malapelle, U., Troncone, G., & Bellevicine, C. (2021). Fine-Needle Aspiration Is Suitable for Breast Cancer BRCA Molecular Assessment: A Case Report. Journal of Molecular Pathology, 2(4), 319-324. https://doi.org/10.3390/jmp2040028











