Abstract
Introduction: In recent years, there has been a growing development of molecularly targeted therapies for various types of solid tumors—in particular, in non-small-cell lung cancer (NSCLC). This has required the need for greater quantities of tissue that is able to support ancillary studies, alongside cyto-histological diagnoses for the assessment of molecular targets. Conventional TBNA (cTBNA) and EBUS-guided TBNA (EBUS-TBNA) have shown a high diagnostic yield for malignant mediastinal and/or hilar lymph node enlargement and peribronchial masses; however, few studies have compared these two procedures. We retrospectively compared TBNA patients (EBUS-TBNA and cTBNA) in order to determine the diagnostic yield and material adequacy for subsequent ancillary analyses. Materials and Methods: We retrospectively evaluated 318 patients with clinical suspicion of lung cancer or with disease recurrence. All of the patients underwent TBNA (either EBUS-TBNA or cTBNA) on enlarged mediastinal and/or hilar lymph nodes and peribronchial masses between January 2017 and June 2021 at the University Hospital of Pisa, Italy. After a definitive diagnosis, molecular analyses and an evaluation of PD-L1 expression were performed in the cases of adenocarcinoma, squamous cell carcinoma, and NSCLC, not otherwise specified (NOS). Results: EBUS-TBNA was performed in 199 patients and cTBNA was performed in 119 patients with 374 and 142 lymph nodes, respectively. The overall diagnostic yield for positive diagnoses was 59% (diagnostic rate of 61% in EBUS-TBNA, and 55% in cTBNA). Adenocarcinoma (ADC) was the most frequent diagnosis in both methods. EBUS-TBNA diagnostic adequacy was 72% for molecular analysis, while it was 55.5% for cTBNA, showing a statistical trend (p = 0.08) towards the significance of EBUS. The average percentage of neoplastic cells was also statistically different between the two methods (p = 0.05), reaching 51.19 ± 22.14 in EBUS-TBNA and 45.25 ± 22.84 in cTBNA. With regard to the PD-L1 protein expression, the percentage of positivity was similar in both procedures (86% in EBUS-TBNA, 85% in cTBNA). Conclusions: Conventional TBNA (cTBNA) and EBUS-guided TBNA (EBUS-TBNA) are minimally invasive diagnostic methods that are associated with a high diagnostic yield. However, EBUS-TBNA has an improved diagnostic adequacy for molecular analysis compared to cTBNA, and is associated with a higher average percentage of neoplastic cells.
1. Introduction
Lung cancer is the second most commonly diagnosed cancer worldwide, and it was the leading cause of cancer death in 2020, with an estimated 2.2 million new cancer cases and 1.8 million deaths. This represents approximately one in ten (11.4%) diagnosed cancers and one in five (18.0%) deaths [1].
Recent developments in drugs targeting the molecular mechanisms of non-small-cell lung cancer (NSCLC), along with the identification of patients that could benefit from a specific drug, have the potential to improve therapy outcomes, while reducing toxic effects [2].
Molecular profiles can provide new treatments and predict responses to therapy, including: the activation of oncogenes by mutations (e.g., EGFR, KRAS, BRAF and ERBB2) and translocations (e.g., ALK, ROS1 and RET); amplifications (e.g., MET and FGFR1) in adenocarcinomas and squamous cell carcinomas. An overexpression of EGFR in NSCLC has been reported alongside poor outcomes; some studies have shown that the EGFR expression in NSCLC is associated with reduced survival, frequent lymph node metastases and poor chemosensitivity [3].
Immunotherapy has emerged as a major new treatment modality for patients with advanced non-small cell lung carcinoma. The main approach is immune checkpoint blockade, with either PD1 or PD-L1 antibodies, that is directed towards the PD1 receptors on the activated T cells, or the PD-L1 receptors on tumor cells and antigens presenting immune cells. PD-L1 protein expression has emerged as a biomarker predicting which patients are more likely to respond to immunotherapy. The prevalence of the PD-L1 expression in the population of patients with NSCLC ranges from 24 to 60%, and patients with a PD-L1 tumor proportion score of ≥50% will likely have a better response to immunotherapy drugs, such as pembrolizumab [4,5].
All of these data indicate a high demand for minimally invasive diagnostic methods that are able to provide sufficient tissue for ancillary studies, including immunohistochemistry and molecular testing [6].
Transbronchial needle aspiration (TBNA) is a diagnostic technique that is characterized by reduced invasiveness and is used for patients that are suffering from mediastinal and hilar diseases. TBNA benefits from endobronchial ultrasound (EBUS), which enables the localization of lymph nodes in real-time during bronchoscopy. The availability of rapid on-site evaluation (ROSE) further enhances the utility of this procedure by ensuring the adequacy of the samples. In this way, if inadequate, the procedure can be repeated immediately, in order to ensure adequacy and to avoid delays in reporting, or unnecessary repetition of the procedure at a later date [7].
Both conventional TBNA (cTBNA) and EBUS-guided TBNA (EBUS-TBNA) are associated with a high diagnostic yield for malignant mediastinal and/or hilar lymph node enlargement (diameter >10 mm) and peribronchial masses; however, few studies have compared these two procedures in terms of material adequacy for molecular profiling [7]. The diagnostic rate for cTBNA (which mostly depends on the operative technique and the type of specimen resulting from needle aspiration) can be improved using EBUS-TBNA. However, the EBUS-TBNA samples can be inadequate, due to the possible presence of cell masses and blood clots within the specimens [8].
We retrospectively compared TBNA patients (EBUS-TBNA and cTBNA) with a clinical suspicion of lung cancer, or with a disease recurrence, and compared the data obtained by cTBNA and EBUS-TBNA, in order to determine the diagnostic yield and material adequacy for subsequent ancillary analyses.
2. Material and Methods
2.1. Patient Selection
In this retrospective study, we reviewed 318 patients that were clinically suspicious of lung cancer or had a disease recurrence. All patients underwent TBNA, either EBUS-TBNA or cTBNA, in enlarged mediastinal and/or hilar lymph nodes and peribronchial masses, between January 2017 and June 2021 at the University Hospital of Pisa, Italy. The ethical code number is 9989 and the date of approval is February 20th 2019 by Ethical Committee Area Vasta Nord Ovest (CEAVNO).
2.2. Performance of Conventional Transbronchial Needle Aspiration
Conventional TBNA was performed in awake patients under local anesthesia. A needle was passed through the working channel of a standard bronchoscope to puncture the airway wall at the site of the target lymph node. The target lymph nodes were preoperatively selected on the basis of chest computed tomography (CT) using contrast media enhancement and Positron Emission Tomography (PET). The possible lymph node stations that could be routinely reached by conventional TBNA were stations 4R and 7, according to the International Association for the Study of Lung Cancer’s (IASLC) lymph node classification [9]. However, the possibility of reaching any other lymph node stations located in the peribronchial area was considered.
A 21-gauge needle (eXelonTM, Boston Scientific, Marlborough, MA, USA) was inserted through the bronchial wall at the level of the selected lymph node station. Negative pressure was applied to the needle, which was quickly moved back and forth (15–20 s for each pass). Then, the aspiration specimen for cytology was collected. Each lymph node station was sampled at least five times. Regarding complications, in our record of cases, we observed only one case of pneumothorax post c-TBNA.
2.3. Performance of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration
All EBUS procedures were performed under general anesthesia (propofol 4–12 mg/kg), with airways secured by a laryngeal mark. A flexible Fuji EBUS-scope (Fujifilm Holdings, Minato, Japan), with an integrated ultrasound convex probe transducer (EB-530US), was introduced through the laryngeal mark in order to visualize and measure the target lymph node stations. This technique enabled real-time ultrasound visualization of the mediastinal structures immediately surrounding the tracheobronchial tree. Once all the lymph node stations had been inspected and measured, a 22-G needle (ViziShot, Olympus Corporation, Tokyo, Japan) was inserted through the working channel of the bronchoscope. Then, under real-time US guidance, the needle was inserted into the target lymph node and, when it appeared to be well-positioned, a negative pressure was applied with a 20-mL syringe.
Thoracic surgeons performed two or three strokes per second for 15–20 s for each pass, by constantly and carefully monitoring the needle motion. At least five passes were obtained for each node station; however, there was reciprocal communication between the cytopathologist and the thoracic surgeon regarding the quality and amount of the sample, making appropriate adjustments in case of a paucicellular, obscured, bloody or clotted sample. The number of needle excursions, and the time spent in the lymph node, may vary during the procedure. In particular, the pass for obtaining cell block material required a higher number of needle excursions, as well as increased time spent in the lymph node (up to 30–40 s). Moreover, the number of needle passes was sometimes established depending on patients’ necessities, on the basis of real-time microscopic assessment [10].
Rapid on-site evaluation (ROSE) was available for every procedure. The EBUS-TBNA material was immediately expressed over labeled glass slides for direct smears. The smears were stained using rapid hematoxylin-eosin stain on wet alcohol fixed smears, and evaluated by the cytopathologist, using light microscopy, for sample adequacy and for a preliminary diagnosis. The needle rinses from the FNA passes were typically collected in 4% buffered formalin, delivered to the Pathology Department and processed as a cell block. If a diagnosis of lung cancer was established on-site, additional passes may have been requested to yield additional sample material for a predictive biomarker assessment [11,12].
2.4. Pathological Analysis
After hematoxylin and eosin staining, four experienced pathologists evaluated the cytological and histological results independently, using standard methods. Immunohistochemistry was performed, when required, for definitive diagnosis.
Samples were selected for molecular analysis, according to the clinicians’ requests, for therapeutic purposes. Both the smears and cell blocks were reviewed to define tumor cellular representativeness, with a percentage of neoplastic cells that varied according to the procedure employed. A minimum of 100 cells is required for evaluation of the PD-L1 expression.
For each case, three 10-μm thick unstained sections from cell blocks, or one Papanicolaou-stained smear, were used for the mutational analysis of EGFR, KRAS, BRAF, PIK3CA, ERBB2 and MET genes.
Before DNA purification, cell block sections underwent xylene/ethanol-based deparaffinization, whereas stained slides were placed in xylene for 48 h to remove the coverslip, and were then rehydrated in a graded ethanol series (99%, 95%, 70% and 50%). Tumor cell enrichment was performed using manual macrodissection, and DNA was purified using the spin column procedure with the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. DNA concentration was assessed using a NanoDrop spectrophotometer (ThermoFisher Scientific, San Francisco, CA, USA). The mutational analysis of EGFR, KRAS, BRAF, ERBB2 and PIK3CA was performed using MADI-TOF mass spectrometry on a Sequenom Mass-Array platform (Agena Bioscience, San Diego, CA, USA) using the Myriapod Lung Status Kit (Diatech Pharmacogenetics, Jesi, Italy), according to the manufacturer’s protocol. MET exon 14 skipping mutations were determined using Sanger sequencing, as previously reported [13].
Two sections (2–4 µm thick formalin-fixed paraffin-embedded tissue sections from cell blocks) were used for the evaluation of ALK, ROS1, RET and MET genetic status using FISH. Alternatively, the analysis was conducted on a destained smear slide.
Immunohistochemistry carried out on the Ventana Medical System (Roche) determined the CD274 molecule (PD-L1) expression and the ALK receptor tyrosine kinase (ALK). The monoclonal primary antibody SP263 clone was used for PD-L1, and the monoclonal primary antibody D5F3 clone was used for ALK. Fluorescence in situ hybridization (FISH) determined the presence of gene fusions regarding ROS proto-oncogene 1, receptor tyrosine kinase (ROS1) (Break Probe ROS1 (6q22) Kreatech—Leica Biosystems, Amsterdam, The Netherlands) and ret proto-oncogene (RET) (Break Probe RET (10q11) Kreatech—Leica Biosystems). The amplification of MET proto-oncogene, receptor tyrosine kinase (MET) (Probes: LSI MET spectrum red and CEP7 spectrum green—Vysis—Abbott, IL, USA), and HER2 (Probes: LSI HER2/neu spectrum orange and CEP17 spectrum green—Vysis, Abbott) was also analyzed using FISH tests.
2.5. PD-L1 Expression Tests
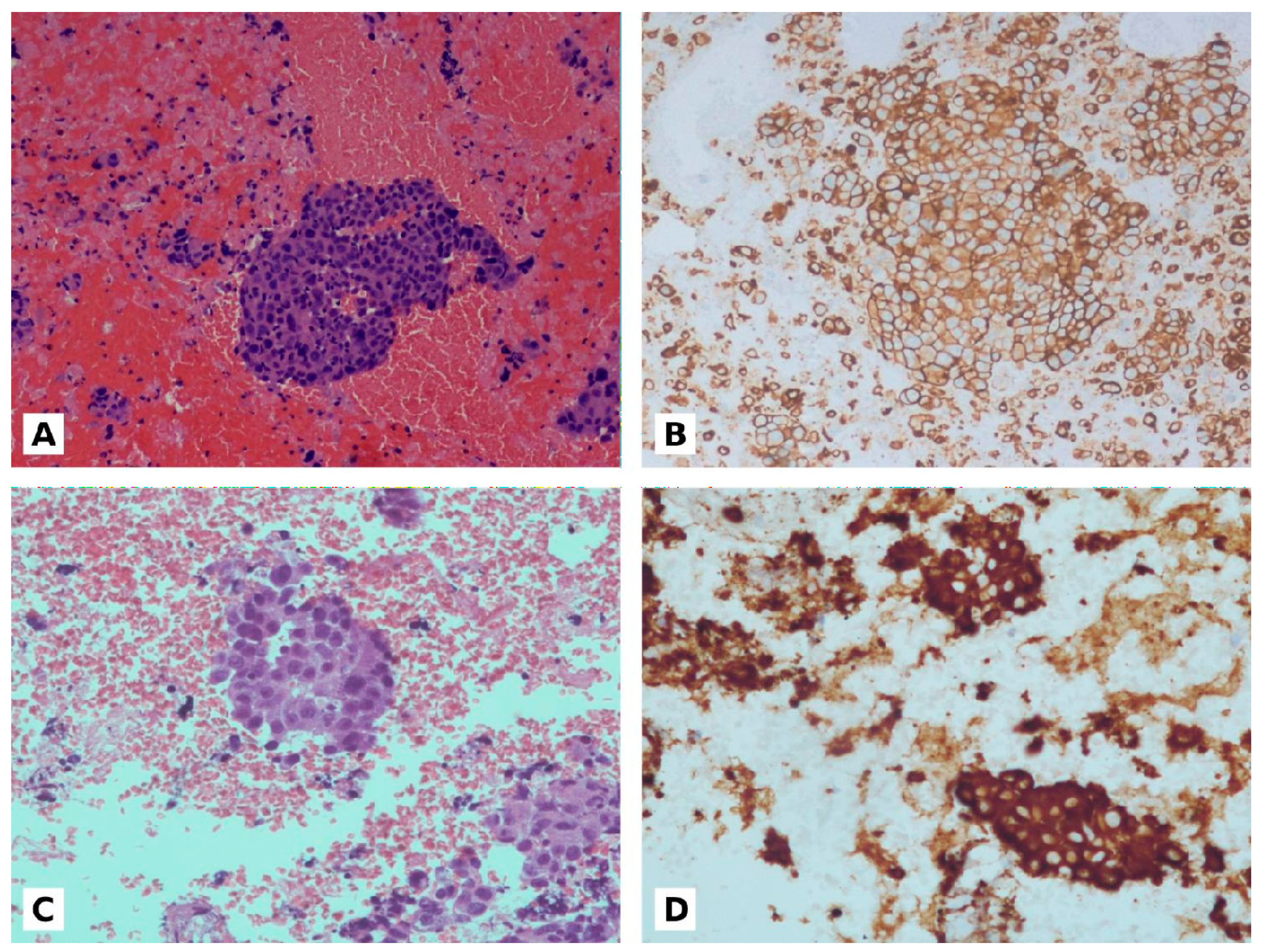
The PD-L1 expression was evaluated on cell block sections using TPS, which is defined as the percentage of viable tumor cells with partial or complete membrane staining of any intensity (≥1+), relative to all viable tumor cells in the examined section (positive and negative). The presence of at least 100 viable tumor cells was necessary for evaluation (Figure 1).
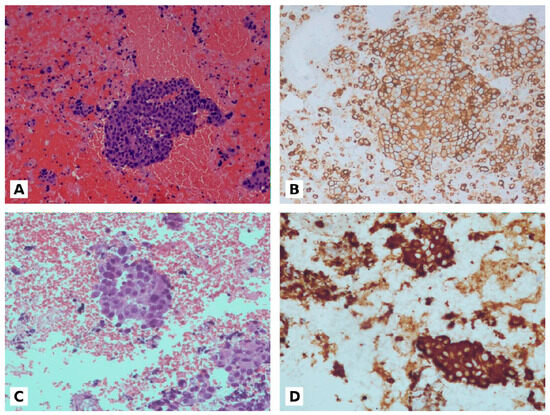
Figure 1.
(A): NSLSC in cell block from 4R lymphnode (20×). (B): PD-L1 expression (90% positive) on cell block (20×). (C): ADC in cell block from 7 lymphnode (20×). (D): ALK positive on cell block (20×).
2.6. Statistical Analysis
Categorical variables were analyzed using Fisher’s exact test. Continuous variables were compared using the Mann–Whitney U test. For all analyses, a two-tailed p-value below 0.05 was considered significant. Analyses were performed in the R environment (https://www.r-project.org/, v.4.0.1, last accessed 8 July 2021).
3. Results
3.1. Patients’ Characteristics
A total of 318 patients (M:F = 230:88) with a suspicious or previous diagnosis of lung cancer were included in the study. Patients’ median age was 68.55 ± 11.11 years. EBUS-TBNA was performed in 199 patients, and cTBNA was performed in 119 patients with 374 and 142 lymph nodes, respectively.
In particular, all mediastinal stations were evaluated using EBUS-TBNA, with a prevalence of station 7 (subcarinal node) (24.33%). Even in cTBNA, station 7 was found to be the most frequently investigated (60%) (Table 1).

Table 1.
Patient demographics and characteristics of lymph nodes.
3.2. Cytopathological Results
The overall diagnostic yield for positive diagnoses was 59% (187/318), with a diagnostic rate of 61% (121/199) in EBUS-TBNA, and 55.46% (66/119) in cTBNA.
The final cytopathological results are presented in Table 2. The most frequent diagnosis was adenocarcinoma for both techniques (30% in EBUS-TBNA and 23% in cTBNA), followed by squamous cell carcinoma (10% in EBUS-TBNA and 9% in cTBNA). In 10% of EBUS-TBNA cases and 11% of cTBNA cases, a final diagnosis of small cell carcinoma was reached. In the remaining cases, the diagnosis included NSCLC, NOS, lymphoma, carcinoid tumors, metastases and lymph nodes negative for neoplasia.

Table 2.
Distribution of cytopathological results.
3.3. Molecular Analysis
Molecular analysis was conducted for the patients with a cyto-histological diagnosis of adenocarcinoma, squamous cell carcinoma and NSCLC, NOS (86 cases in EBUS-TBNA and 45 cases in cTBNA).
Although cytopathological diagnosis was always performed, the specimens with insufficient material for subsequent molecular analyses were excluded (24 cases in EBUS-TBNA and 20 cases in cTBNA). The diagnostic adequacy for molecular analysis was assessed in 62/86 cases in EBUS-TBNA (72%) and 25/45 cases in cTBNA (56%), with an average percentage of neoplastic cells of 51.19 ± 22.14 in EBUS-TBNA, and 45.25 ± 22.84 in cTBNA (Table 3).

Table 3.
Diagnostic accuracy in molecular analysis.
In one EBUS-TBNA case and in two cTBNA cases, the material was not sufficient for PD-L1 mutation analysis, although it was adequate for other molecular analyses. The diagnostic rate of the two procedures was almost comparable, although we observed a statistical tendency (p = 0.08) towards the better performance of EBUS-TBNA versus cTBNA. However, a statistical difference was observed concerning the percentage of neoplastic cells in the cell blocks (p = 0.05), with an average percentage of neoplastic cells that was higher in the EBUS-TBNA samples than in the cTBNA samples.
The molecular status of the patients that were analyzed with the two techniques is shown in Table 4. In particular, the prevalence of the EGFR mutation was 7% in EBUS-TBNA and 4% in cTBNA, while the frequency of the ALK translocations was 2% in EBUS-TBNA and 9% in cTBNA. Finally, the frequency of the KRAS mutations was similar in both procedures (13% in EBUS-TBNA and 13% in cTBNA). Only one case of a BRAF mutation was identified in EBUS-TBNA (1%), and a PIK3CA mutation was only found in cTBNA (2%). The remaining genes (MET, cMET, HER2, HER2/neu, ROS-1 and RET) were all wild-type in both techniques.

Table 4.
Mutation analysis of specimens.
3.4. Evaluation of PD-L1 Expression
The PD-L1 expression was successfully evaluated in 45 samples in EBUS-TBNA, and in 22 samples in cTBNA; however, one test in EBUS-TBNA and two tests in cTBNA were not valuable, owing to an insufficient number of neoplastic cells (although suitable for other molecular analyses).
The cases were classified according to the Tumor Proportion Score (TPS), and they all fell within the three PD-L1 categories. Among the cases with an adequate sample, 38/44 (86%) presented PD-L1 positivity in EBUS-TBNA, with a high positive expression (TPS ≥ 50%) in 18/44 (41%). Additionally, 17/20 (85%) presented PD-L1 positivity in cTBNA, with a high positive expression (TPS ≥ 50%) in 6/20 (30%). PD-L1 was negative in six EBUS-TBNA patients (14%; 6/44) and in three c-TBNA patients (15%; 3/20) (Table 4).
4. Discussion
Lung cancer is a highly malignant carcinoma; approximately 40% of diagnosed lung cancer patients are stage IV and not suitable for surgery [14]. Moreover, the incidence of NSCLC is approximately 85% in all lung cancers. In recent years, the treatment of NSCLC has been changed as a result of new discoveries in immunotherapy and target therapy. In particular, the mutation of EGFR and ALK genes allows the therapy and prognosis of patients to be defined. Although targeted therapy is more expensive than conventional chemotherapy or radiotherapy, it results in better efficacy with fewer side effects [15]. This suggests that molecular testing can bring significant benefits to the treatment and prognosis of advanced lung cancer. In addition, nearly 70% of patients with unresectable lung-cancer are diagnosed via preoperative specimens, which must be handled not only for diagnostic purposes, but also for ancillary tests [16].
In our study, we retrospectively evaluated 318 patients that were clinically suspicious of lung cancer or had a disease recurrence. All of the patients underwent TBNA (either EBUS-TBNA or cTBNA) in enlarged mediastinal and/or hilar lymph nodes and peribronchial masses.
Our focus was to compare the adequacy of small tissue specimens for diagnostic and therapeutic purposes.
Previous studies have shown that EBUS-TBNA and cTBNA are important tools for diagnosing mediastinal and hilar lymphadenopathy, including benign and malignant diseases [17].
EBUS-TBNA is a minimally invasive method that combines the direct visualization of the airways with the ultrasound-guided transbronchial needle aspiration of mediastinal and/or hilar lymphadenopathy, or of extraluminal tumors that are located in direct contact with the airways. In order to improve its diagnostic accuracy, rapid on-site cytologic evaluation (ROSE) can be used to rapidly obtain a preliminary diagnosis, reduce the time of examination and the number of punctures per lymph node, and provide an aid to secure adequate tissue sampling for molecular testing [6].
Moreover, EBUS-TBNA has been observed to be comparable with surgical mediastinoscopy in terms of sensitivity and pathological staging [18,19,20]. The guidelines suggested EBUS-TBNA to stage the mediastinum, on the basis of a sensitivity of 89% for lung cancer. Nevertheless, EBUS-TBNA is not always affordable for hospitals because of the relatively expensive equipment it requires. It is, therefore, necessary to find alternative solutions to EBUS, to reach higher diagnostic efficiency [21]. Conventional transbronchial needle aspiration (cTBNA) has proven to be a minimally invasive, safe and cost-effective technique in establishing the diagnosis of mediastinal lymphadenopathy, which does not require expensive equipment, and only a minimum volume of activity and specific training is necessary [10].
In our series, we evaluated 119 cTBNA patients and 199 EBUS-TBNA patients. We observed a diagnostic yield for positive diagnoses of 58.9%, with a prevalence of adenocarcinomas in both techniques.
Since numerous studies have asserted that molecular analysis on the cytological samples obtained through EBUS-TBNA and conventional TBNA can be routinely performed, we aimed to evaluate and compare the efficiency of the two endoscopic techniques in collecting neoplastic material for molecular analysis.
As previously reported, in the majority of cases, EBUS-TBNA is effective to evaluate the PD-L1 expression status or the genomic phenotype using single-gene testing, rapid techniques (RTPCR, IHC) or direct sequencing; however, it depends largely on the absolute number of tumor cells (preferably > 100), the percentage of tumor cells that are present in the material, the degree of preservation of tumor cells and the type and sensitivity of the molecular test that is utilized. Moreover, the use of ROSE helps to optimize lung cancer genotyping by minimizing non-diagnostic samples and, thereby, preventing the need for a repeat procedure [22].
Eighty-six cases in EBUS-TBNA and 45 cases in cTBNA were processed for molecular analysis, although, for material insufficiency, 24 cases in EBUS-TBNA and 20 cases in cTBNA had to be excluded. Consequently, the diagnostic adequacy for molecular analysis reached the percentage of 72.1% in EBUS-TBNA, compared to 55.6% in cTBNA.
This difference between the two methods reveals that the small samples that are obtained by EBUS-TBNA could be more suitable for molecular analysis than those that are obtained by cTBNA. This may depend on the greater quantity of material obtained through EBUS-TBNA that is assisted by ROSE, and on the better conservation of the tissue that is related to the use of cell blocks rather than smears.
According to a recent meta-analysis, the overall diagnostic yield of intrathoracic adenopathy sampling is higher when EBUS-TBNA is associated with EBUS-IFB (intranodal forceps biopsy) [23].
Another interesting element we identified was the average percentage of neoplastic cells, which were significatively higher in EBUS-TBNA than in cTBNA. This demonstrates the fundamental importance of ROSE, in the application of the endoscopic technique, for the collection of tumor material for molecular analysis. Such data strengthen the results that were previously reported by other influential authors: ROSE permits sufficient tissue to be obtained for molecular testing, with a high rate of success [24,25]. There is no evidence as to why the use of ROSE should be recommended in all of the procedures, although the guidelines suggest that it should be used when molecular testing is needed [26].
Regarding the evaluation of the PD-L1 expression, this study demonstrated that the PD-L1 protein expression can be equally performed in the neoplastic samples that are obtained from the two procedures. The percentage of positivity was similar in both procedures—namely, 86.4% in EBUS-TBNA and 85% in cTBNA. In terms of the diagnostic adequacy for molecular analysis, the consistent percentage of valuable samples may be caused by the use of cell blocks over smears, resulting in a better correlation with tumoral cell content, and, possibly, better conservation of the tissue. With regard to the percentage of positive cases, there is a substantial debate surrounding observer-dependent difficulties in quantifying the PD-L1 expression; however, the conditions of a cell block, which differ from those of a resected specimen, should be taken into account. The cells, organized in blocks, are fragmented and scattered within the clot, with normal tissue fragments and blood embedded within the tumor. Another challenge in quantifying the PD-L1 expression is the varying proportion of tumor and stromal cells in each patient’s tumor [27]. It is widespread opinion that, whenever EBUS-TBNA shows pure tumor upon ROSE, the cell block may have higher tumor cellularity, and, in such cases, PD-L1 staining may be highly reliable [28].
5. Conclusions
Conventional TBNA (cTBNA) and EBUS-guided TBNA (EBUS-TBNA) are minimally invasive diagnostic methods that are associated with an important diagnostic yield for malignant mediastinal and/or hilar lymph node enlargement and peribronchial masses.
Although this study was limited to the application of cyto-assistance for the EBUS technique, it has shown that EBUS-TBNA presented more relevant diagnostic adequacy for molecular analysis, compared to cTBNA, and was associated with a higher average percentage of neoplastic cells and a higher frequency of high PD-L1 expression (TPS ≥ 50%), which is essential for molecular analysis.
Author Contributions
F.S., A.P. (Agnese Proietti), G.A., M.P., A.M.P., R.B. and G.F. conceived and designed the study; F.S., A.P. (Agnese Proietti), G.A., M.P., O.F., A.P. (Alessandro Picchi), A.R., R.B. and A.C. participated in data collection; F.S., A.P. (Agnese Proietti), G.A. and M.P. contributed to the interpretation of the results and in the writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was not supported by any funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by Ethical Committee Area Vasta Nord Ovest (CEAVNO). The ethical code number is 9989 and the date of approval is February 20th 2019.
Informed Consent Statement
Informed consent for participation was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction of the information included in the Institutional Review Board Statement and Informed Consent Statement. This change does not affect the scientific content of the article.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Thomas, A.; Rajan, A.; Lopez-Chavez, A.; Wang, Y.; Giaccone, G. From targets to targeted therapies and molecular profiling in non-small cell lung carcinoma. Ann. Oncol. 2013, 24, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Bethune, G.; Bethune, D.; Ridgway, N.; Xu, Z. Epidermal growth factor receptor (EGFR) in lung cancer: An overview and update. J. Thorac. Dis. 2010, 2, 48–51. [Google Scholar] [PubMed]
- World Health Organization (WHO). Classification of Tumours of Thoracic Tumours, 5th ed.; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Yu, H.; Boyl, T.A.; Caicun, Z.; Rimm, D.L.; Hirsch, F.R. PD-L1 Expression in Lung Cancer. J. Thorac. Oncol. 2016, 11, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Haranguş, A.; Berindan-Neagoe, I.; Toma, L.; Şimon, I.; Pop, O.; Şimon, M. EBUS in optimizing non-small cell lung cancer diagnosis and treatment. Med. Pharm. Rep. 2021, 94, 176–184. [Google Scholar] [CrossRef]
- Anila, K.R.; Nayak, N.; Venugopal, M.; Jayasree, K. Role of Rapid On-site Evaluation in CT-guided Fine Needle Aspiration Cytology of Lung Nodules. J. Cytol. 2018, 35, 229–232. [Google Scholar] [CrossRef]
- Shen, H.; Lou, L.; Chen, T. Comparison of transbronchial needle aspiration with and without ultrasound guidance for diagnosing benign lymph node adenopathy. Diagn. Pathol. 2020, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Rusch, V.W.; Asamura, H.; Watanabe, H.; Giroux, D.J.; Rami-Porta, R.; Goldstraw, P. A Proposal for a New International Lymph Node Map in the Forthcoming Seventh Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2009, 4, 568–577. [Google Scholar] [CrossRef]
- Küpeli, E. Conventional transbronchial needle aspiration in community practice. J. Thorac. Dis. 2015, 7, S256–S265. [Google Scholar]
- Danakas, A.M.; Jones, C.E.; Magguilli, M.; Lada, M.J.; Plavnicky, J.; Parajuli, S.; Wizorek, J.J.; Peyre, C.G.; Ettel, M.; Sweeney, M.; et al. Optimising rapid on-site evaluation-assisted endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal lymph nodes: The real-time cytopathology intervention process. Cytopathology 2021, 32, 318–325. [Google Scholar] [CrossRef]
- Jain, D.; Allen, T.C.; Aisner, D.L.; Beasley, M.B.; Cagle, P.T.; Capelozzi, V.L.; Hariri, L.P.; Lantuejoul, S.; Miller, R.; Mino-Kenudson, M.; et al. Rapid On-Site Evaluation of Endobronchial Ultrasound-Guided Transbronchial Needle Aspirations for the Diagnosis of Lung Cancer: A Perspective from Members of the Pulmonary Pathology Society. Arch. Pathol. Lab. Med. 2018, 142, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Landi, L.; Chiari, R.; Tiseo, M.; D’Incà, F.; Dazzi, C.; Chella, A.; Delmonte, A.; Bonanno, L.; Giannarelli, D.; Cortinovis, D.L.; et al. Crizotinib in MET-Deregulated or ROS1-Rearranged Pretreated Non-Small Cell Lung Cancer (METROS): A Phase II, Prospective, Multicenter, Two-Arms Trial. Clin. Cancer Res. 2019, 25, 7312–7319. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Caglevic, C.; Santarpia, M.; Araujo, A.; Giovannetti, E.; Gallardo, C.D.; Pauwels, P.; Mahave, M. Immunotherapy in NSCLC: A Promising and Revolutionary Weapon. Adv. Exp. Med. Biol. 2017, 995, 97–125. [Google Scholar] [PubMed]
- Xia, Y.; Zhang, B.; Zhang, H.; Li, W.; Wang, K.P.; Shen, H. Evaluation of lymph node metastasis in lung cancer: Who is the chief justice? J. Thorac. Dis. 2015, 7, 231–237. [Google Scholar]
- Um, S.W.; Kim, H.K.; Jung, S.H.; Han, J.; Lee, K.J.; Park, H.Y. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small-cell lung cancer. J. Thorac. Oncol. 2015, 10, 331–337. [Google Scholar] [CrossRef]
- Yasufuku, K.; Pierre, A.; Darling, G.; de Perrot, M.; Waddell, T.; Johnston, M. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J. Thorac. Cardiovasc. Surg. 2011, 142, 1393–1400. [Google Scholar] [CrossRef]
- Xia, Y.; Ma, Y.; Arias, S.; Lee, H.; Wang, K.P. Utilization of the International Association for the Study of Lung Cancer and Wang’s nodal map for the identification of mediastinum and hilar lymph nodes. Thorac. Cancer 2015, 6, 464–468. [Google Scholar] [CrossRef]
- Jurado, J.; Saqi, A.; Maxfield, R.; Newmark, A.; Lavelle, M.; Bacchetta, M.; Gorenstein, L.; Dovidio, F.; Ginsburg, M.E.; Sonett, J. The Efficacy of EBUS-Guided Transbronchial Needle Aspiration for Molecular Testing in Lung Adenocarcinoma. Ann. Thorac. Surg. 2013, 96, 1196–1202. [Google Scholar] [CrossRef]
- Sharma, S.V.; Settleman, J. ErbBs in lung cancer. Exp. Cell Res. 2009, 315, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Ghori, U.; Chaddha, U.; Murgu, S. Combined EBUS-IFB and EBUS-TBNA vs EBUS-TBNA alone for intrathoracic adenopathy: A Meta-analysis. Ann. Thorac. Surg. 2021. [Google Scholar] [CrossRef]
- Casadio, C.; Guarize, J.; Donghi, S. Molecular Testing for Targeted Therapy in Advanced Non-Small Cell Lung Cancer: Suitability of Endobronchial Ultrasound Transbronchial Needle Aspiration. Am. J. Clin. Pathol. 2015, 144, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Trisolini, R.; Cancellieri, A.; Tinelli, C. Randomized Trial of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration with and Without Rapid On-site Evaluation for Lung Cancer Genotyping. Chest 2015, 148, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, E.H.; Casal, R.F.; Trisolini, R. Guideline for the acquisition and preparation of conventional and endobronchial ultrasound-guided transbronchial needle aspiration specimens for the diagnosis and molecular testing of patients with known or suspected lung cancer. Respiration 2014, 88, 500–517. [Google Scholar] [CrossRef]
- Biswas, A.; Leon, M.E.; Drew, P. Clinical performance of endobronchial ultrasound-guided transbronchial needle aspiration for assessing programmed death ligand-1 expression in non-small cell lung cancer. Diagn. Cytopathol. 2018, 46, 378–383. [Google Scholar] [CrossRef]
- Caddha, U.; Hogarth, D.K.; Murgu, S. The role of endobronchial ultrasound transbronchial needle aspiration for programmed death ligand-1 testing and next generation sequencing in advanced non-small cell lung cancer. Ann. Transl. Med. 2019, 7, 351. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).