Structural Diversity of Tree Stems of Elephant Camp Natural Forest in Omo Forest Reserve †
Abstract
:1. Introduction
2. Materials and Methods
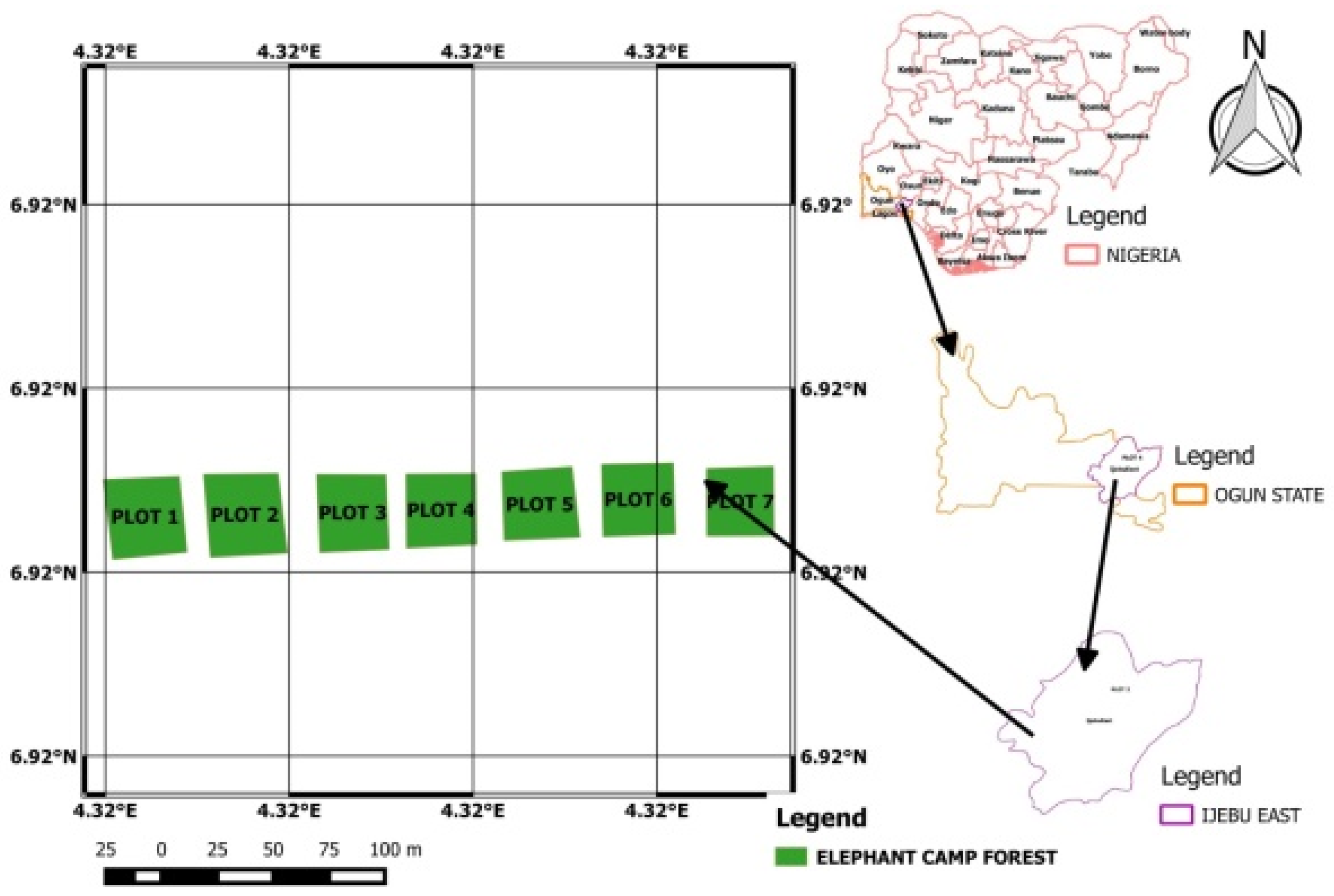
2.1. The Study Area
2.2. Demarcation of Sample Plots and Method of Data Collection
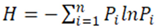
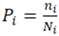
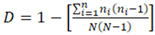
2.3. Stem Diameter Distribution
2.4. Data Analysis
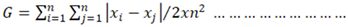
3. Results
3.1. Tree Species Diversity Attributes of Riparian and Old-Growth Forests
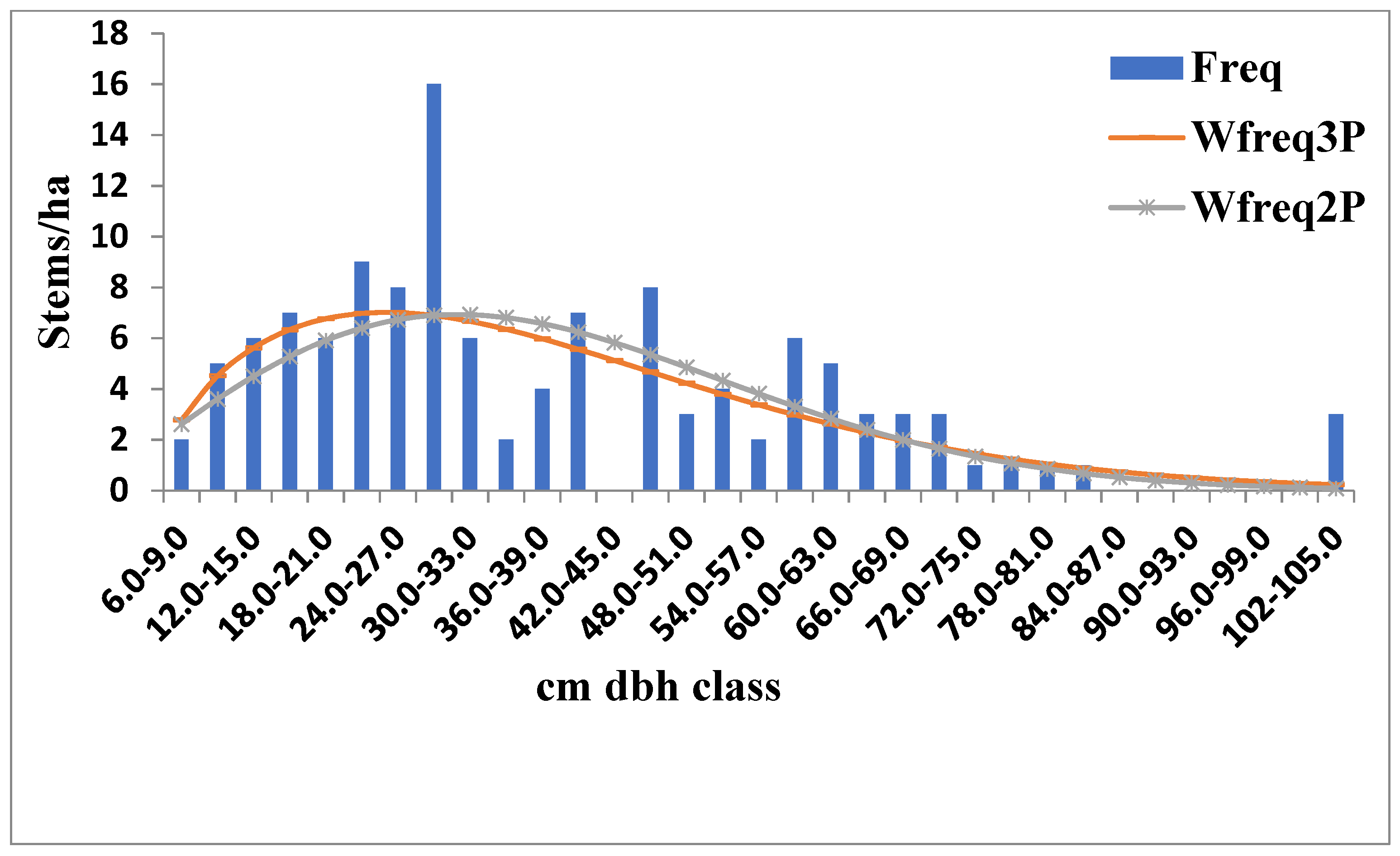
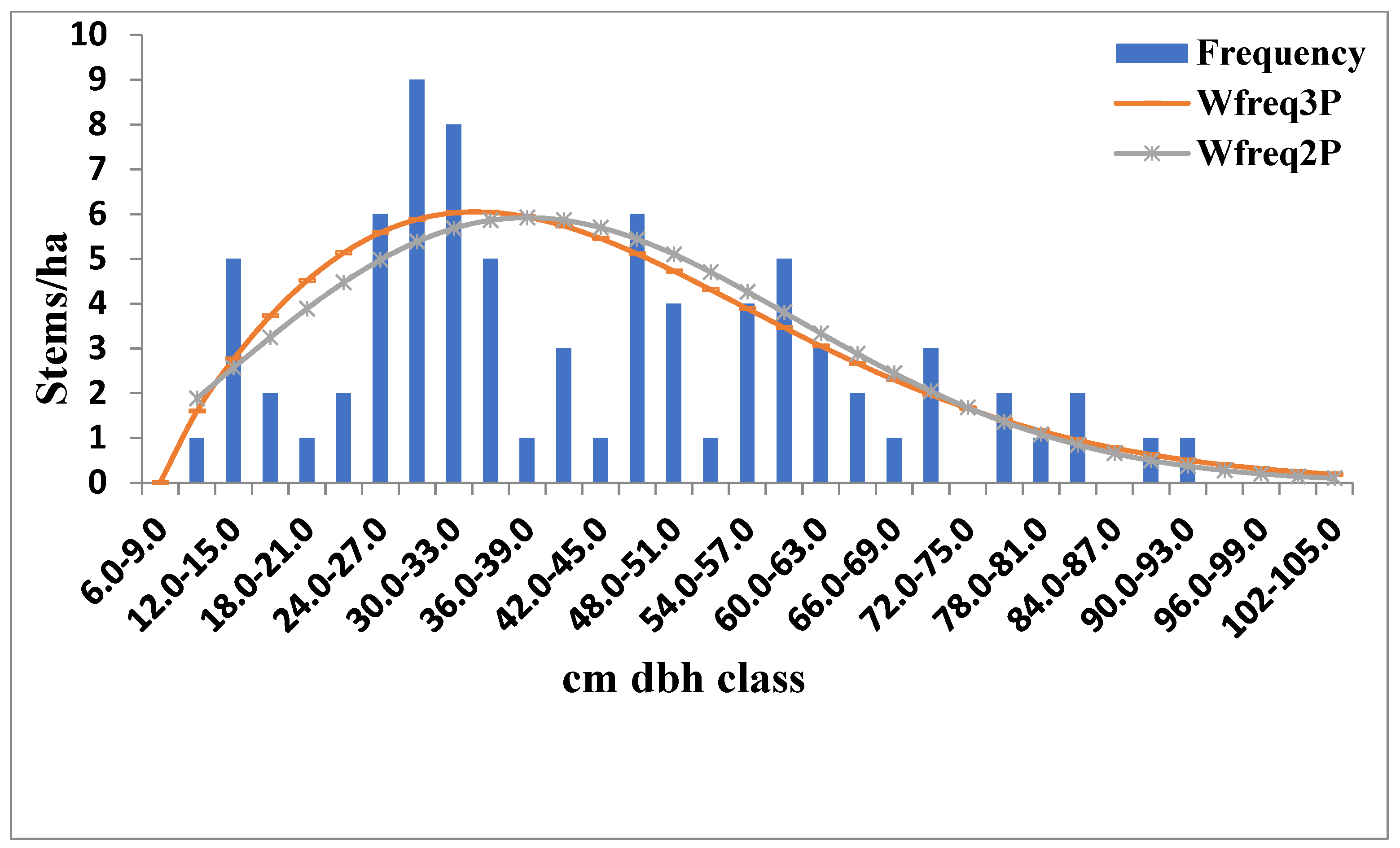
3.2. Diameter Distribution of the Tree Stems
4. Discussion
4.1. Tree Species Diversity and Richness
4.2. Size–Density Distribution of the Elephant Camp Natural Forest
5. Conclusions
Conflicts of Interest
References
- Shaltout, K.H.; Sheded, M.G.; Salem, A.H. Population structure of common shrubs and trees in Wadi Aliaqi Biosphere Reserve, South-East Egypt. Feddes Repert. 2009, 120, 343–354. [Google Scholar] [CrossRef]
- Loetsch, F.; Zohrer, F.; Haller, K.E. Forest Inventory 2; BLV-verlagsgesellschaft: Munique, Germany, 1973. [Google Scholar]
- Podoga, P.; Ochai, W.; Orzel, S. Performance of karnel estimator and Johnson SB function for modeling diameter distribution of Black Alder (Alnus glutinosa (L.). Gaertn.) stands. Forests 2020, 11, 634. [Google Scholar] [CrossRef]
- Pach, M.; Podlaski, R. Tree diameter structural diversity in Central European forests with Abies alba and Fagus sylvatica: Managed versus unmanaged forest stands. Ecol. Res. 2014, 30, 367–384. [Google Scholar] [CrossRef]
- Weiner, J.; Solbrig, O.T. The meaning and measurement of size hierarchies in plant populations. Oecologia 1984, 61, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, A.; Biondi, F.; Di Filippo, A.; Zianco, E.; Piovesan, G. Tree size distribution at increasing spatial scales converges to the rotated sigmoid curve in two old-growth beech stands of the Italin Apennines. For. Ecol. Manag. 2011, 262, 1950–1962. [Google Scholar] [CrossRef]
- Mendez-Alonzo, R.; Hernandez-Trejo, H.; Lopez-Portillo, J. Salinity constrains size inequality and allometry in two contrasting Mangrove habitats in the Gulf of Mexico. J. Trop. Ecol. 2012, 28, 171–179. [Google Scholar] [CrossRef]
- Ojo, L.O. The fate of a tropical rainforest in Nigeria: Abeku sector of Omo Forest Reserve (PDF). Glob. Nest Int. J. 2004, 6, 116–130. [Google Scholar]
- Hutchinson, J.; Datziel, J.M.; Keay, R.W.J.; Hepper, F.N. Folora of West Tropical Africa; Royal Botanical Gardens, Kew: London, UK, 2014; Volume 1 Part 2, 330p. [Google Scholar]
- Hawthorne, W.; Jongkind, C. Woody Lants of Western African Forests; Kew Publishing, Royal Botanical Gardens, Kew: London, UK, 2006; 1023p. [Google Scholar]
- Peet, R.K. Relative diversity indices. Ecology 1975, 56, 496–498. [Google Scholar] [CrossRef]
- Bailey, R.L.; Dell, T.T. Quantifying diameter distributions with the weibull function. For. Sci. 1973, 19, 97–104. [Google Scholar]
- Gilliam, F.S.; Turrill, N.L.; Adams, M.B. Herbaceous layer and overstory species in clear-cut and mature Central Appalachian Hardwood Forests. Ecol. Appl. 1995, 5, 947–955. [Google Scholar] [CrossRef]
- Houchanou, T.D.; Assogbadjo, A.E.; KaKau, R.G.; Kyndt, T.; Houinato, M.; Sinsin, B. How far a protected area contributes to conserve habitat species composition and population structure of endangered African tree species (Benin, West Africa). Ecol. Complex. 2013, 13, 60–68. [Google Scholar] [CrossRef]
- Weiner, J.; Thomas, S.C. Size variability and competition in plant monocultures. Oikos 1986, 47, 211–222. [Google Scholar] [CrossRef]
- Zhang, I.; Packard, K.C.; Liu, C. A comparisons of estimation methods for fitting Weibull and Johnson’s SB distriutions to mixed spruce-fir stands in northeastern North Americ. Can. J. For. Res. 2003, 33, 1340–1347. [Google Scholar] [CrossRef]
- Sharma, K.P.; Bhatta, S.P.; Lamsal, S.K. Species diversity and regeneration status of community-managed hill sal (Shorea robusta) forest in central Nepal. Curr. Sci. 2020, 119, 83–92. [Google Scholar] [CrossRef]
- Bradley, A.F.; Crow, G.F. The flora and vegetation of Timber Island, Lake Winnipesaukee, New Hampshire, U.S.A. Rhodora 2010, 112, 156–190. [Google Scholar] [CrossRef]
- Chivulescu, S.; Ciceu, A.; Leca, S.; Apostol, B.; Popescu, O.; Badea, O. Development phases and structural characterististics of the Penteleu-Viforata virgin forest in the Curvature Carpathians. iForest 2020, 13, 389–395. [Google Scholar] [CrossRef]
- Liu, J.; Burkhart, H.E. Dynamics of size-variable distribution parameters in juvenile loblolly pine (Pinus taeda L.) stands. For. Ecol. Manag. 1993, 58, 321–347. [Google Scholar] [CrossRef]
- Toledo, J.J.; Magnusson, W.E.; Castilho, C.V. Competition, exogenous disturbance and senescence shape tree size distribution in tropical evidence from tree mode of death in Central Amazonia. J. Veg. Sci. 2013, 24, 651–663. [Google Scholar] [CrossRef]
- Rissanen, K.; Martin-Gay, M.; Riopel-Bouvier, A.; Paquette, A. Light interception in experimental forests affected by tree diversity and structural complexity of dominant canopy. Agric. For. Meterol. 2019, 278, 107655. [Google Scholar] [CrossRef]
- Muller-Landau, H.C.; Condit, R.; Harms, K.E.; Marks, C.O.; Thomas, S.C.; Bunyavejchewin, S.; Chuyong, G.; Co, L.; Davies, S.; Foster, R.; et al. Comparing tropical forest tree size distributions with the predictions of metabolic ecology and equilibrium models. Ecol. Lett. 2006, 9, 589–602. [Google Scholar] [CrossRef] [PubMed]
| Riparian Forest | Old-Growth Forest | ||||
|---|---|---|---|---|---|
| Species | Stems/ha | IVI | Species | Stems/ha | IVI |
| Funtumia elastica | 3.0 | 1.97 | Cynnometra megalophylla | 4.0 | 1.24 |
| Baphia nitida | 3.0 | 2.00 | Entandrophragma utile | 4.0 | 2.80 |
| Ficus thonningii | 3.0 | 2.04 | Antiaris africana | 4.0 | 3.22 |
| Detarium macrocarpum | 3.0 | 2.17 | Musanga cecropioides | 4.0 | 3.37 |
| Celtis integrifolia | 3.0 | 2.63 | Hunteria umbellata | 7.0 | 5.87 |
| Macaranga barteri | 3.0 | 2.63 | Dracaena fragrans | 7.0 | 6.59 |
| Musanga cacropioides | 3.0 | 2.76 | Uapaca guinensis | 7.0 | 7.74 |
| Hunteria umbellata | 6.0 | 3.36 | Albizia glaberima | 7.0 | 8.22 |
| Pterigota macrocarpa | 5.0 | 3.46 | Pterigota macrocarpa | 11.0 | 8.31 |
| Okoubaka aubrevillei | 6.0 | 3.59 | Ceiba petandra | 7.0 | 8.76 |
| Ficus exaspirata | 6.0 | 4.75 | Milicia excelsa | 7.0 | 9.40 |
| Nauclea diderrichii | 6.0 | 5.01 | Ficus exasprata | 15.0 | 9.76 |
| Stylochiton hypogaeus | 8.0 | 5.08 | Strombosia pustulata | 11.0 | 10.52 |
| Khaya ivorensis | 6.0 | 5.16 | Nauclea diderrichii | 11.0 | 10.72 |
| Pycnanthus angolensis | 11.0 | 6.98 | Cordia millenii | 11.0 | 11.66 |
| Entandrophragma utile | 11.0 | 7.14 | Funtumia elastica | 15.0 | 11.85 |
| Antiaris africa | 8.0 | 7.42 | Sida acuta | 15.0 | 12.09 |
| Sida acuta | 11.0 | 8.31 | Alstonia boonei | 11.0 | 16.34 |
| Pausinystalia johimbe | 17.0 | 12.95 | Terminalia superba | 15.0 | 17.04 |
| Ceiba petandra | 17.0 | 20.71 | Ficus thonningii | 19.0 | 20.43 |
| Ficu capensis | 25.0 | 21.67 | Gossypium arboreu | 19.0 | 20.94 |
| Irvingia gabonensis | 28.0 | 22.03 | Baphia nitida | 29.0 | 20.35 |
| Albizia glaberima | 28.0 | 25.03 | Khaya ivorensis | 26.0 | 30.19 |
| Dracaena fragrans | 33.0 | 25.19 | Irvingia garbonesis | 37.0 | 36.73 |
| Cordia millenii | 28.0 | 26.93 | |||
| Terminalia superba | 33.0 | 27.86 | |||
| Milicia excelsa | 28.0 | 41.07 | |||
| Diversity Indices | Riparian Forest | Old-Growth Forest |
|---|---|---|
| Tree species richness | 27 | 24 |
| Shannon–Weiner diversity index | 2.963 | 2.98 |
| Simpson diversity index | 0.937 | 0.939 |
| Margalef index | 5.412 | 5.249 |
| Evenness index (H/S) | 0.717 | 0.82 |
| Equitability index | 0.899 | 0.937 |
| Sorensen similarity index | 75.0% | |
| Stem density (stem/ha) | 338.89 ± 9.80 | 296.30 ± 8.92 |
| Forest | N/ha | Mean ± Std (cm) | Minimum (cm) | Maximum (cm) | Skewness | Kurtosis |
|---|---|---|---|---|---|---|
| Riparian | 338.0 | 38.30 ± 21.35 | 6.42 | 104.96 | 0.895 | 0.646 |
| Old growth | 296.0 | 42.87 ± 18.90 | 9.65 | 90.63 | 0.485 | 0.527 |
| Diversity Indices | Riparian Forest | Old-Growth Forest |
|---|---|---|
| Shannon–Weiner (H’) | 3.038 | 3.007 |
| Simpson (1-D’) | 0.942 | 0.940 |
| Margalef index | 5.204 | 5.705 |
| Evenness (e^H’/S) | 0.802 | 0.778 |
| Equitability_J | 0.932 | 0.923 |
| Gini coefficient | 0.825 | 0.915 |
| Coefficient of variation (CV) | 0.557 | 0.44 |
| Dissimilarity coefficient | 0.557 | 0.466 |
| Forest | Distributions | A | β | γ | K-S | A-D | RMSE | Bias | R2 |
|---|---|---|---|---|---|---|---|---|---|
| Riparian | 2-parameter Weibull | - | 2.037 | 42.434 | 0.099 | 0.991 | 2.474 | 1.610 | 0.5064 |
| 3-parameter Weibull | 5.508 | 1.565 | 36.472 | 0.073 | 0.463 | 2.359 | 1.551 | 0.4603 | |
| Old growth | 2-parameter Weibull | - | 2.324 | 47.777 | 0.089 | 0.615 | 1.936 | 1.414 | 0.6651 |
| 3-parameter Weibull | 7.452 | 1.831 | 39.813 | 0.075 | 0.511 | 1.910 | 1.386 | 0.6474 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falade, O.F.; Iheke, J.U. Structural Diversity of Tree Stems of Elephant Camp Natural Forest in Omo Forest Reserve. Environ. Sci. Proc. 2021, 3, 75. https://doi.org/10.3390/IECF2020-08087
Falade OF, Iheke JU. Structural Diversity of Tree Stems of Elephant Camp Natural Forest in Omo Forest Reserve. Environmental Sciences Proceedings. 2021; 3(1):75. https://doi.org/10.3390/IECF2020-08087
Chicago/Turabian StyleFalade, Oladele Fisayo, and Janet Ugochukwu Iheke. 2021. "Structural Diversity of Tree Stems of Elephant Camp Natural Forest in Omo Forest Reserve" Environmental Sciences Proceedings 3, no. 1: 75. https://doi.org/10.3390/IECF2020-08087
APA StyleFalade, O. F., & Iheke, J. U. (2021). Structural Diversity of Tree Stems of Elephant Camp Natural Forest in Omo Forest Reserve. Environmental Sciences Proceedings, 3(1), 75. https://doi.org/10.3390/IECF2020-08087





