Abstract
Glaciers are significant sources of fresh water on planet Earth. The Hindukush–Karakoram–Himalayan (HKH) glaciers provide the water supply to more than half of the human population of the globe, for agricultural activities, biodiversity survival, and ecosystem services. In recent years, the loss of glacial ice has been forecasted to cause problems such as sea level rise, changes in water availability, and release of contaminants that reside in the surfaces of glaciers or within them. In this regard, mineralogical sediments play a significant role in the geochemistry of glaciers and element cycling. This study analyzed elemental pollutants found in the glaciers of Pakistan and investigated the diverse bacterial communities residing therein. Samples of ice and sediments were collected from the Gilgit, Hunza, and Swat glaciers in northern Pakistan. Nine elements, including co-factors, heavy metals, and nutrients, were assessed using atomic absorption spectrophotometry. The research findings indicate higher concentrations of the elements K, Fe, Cu, and Cr in Hunza glacier ice (Hgi) and Ni, Zn, As, and Cd in Gilgit glacier ice (Ggi). In terms of glacier sediments, Swat (Sgs), Gilgit (Ggs), and Hunza (Hgs) samples showed the highest concentrations of K, Cu, Ni, Zn, As, Pb, Cd, and, respectively, of Fe, and Cr. The amount of Cu and Cr is the same in Swat glacier ice and Swat glacier foot. However, the concentration of some elements (As, K, Pb, Zn) is higher in Swat glacier ice, while the amount of some elements (Cd, Ni) is greater in Swat glacier foot. Furthermore, microbial cultivation techniques revealed diverse bacterial communities inhabiting the sampled glaciers. Phylogenetic analysis of the bacterial isolates, based on 16S rRNA gene sequences, showed high homology (99–100%) with previously reported species. The resultant phylogenetic tree grouped the bacterial isolates, such as Serratia marcescens, Cupriavidus sp., and Bacillus cereus, with closely related species known for their roles in nutrient cycling, environmental resilience, and metal tolerance. These findings highlight the ecological significance and adaptive potential of microbial communities in glacier environments, emphasizing their role in elemental cycling and environmental resilience.
1. Introduction
Glaciers are land-based ice masses ranging from small cirque glaciers to massive ice sheets like those in Greenland and Antarctica [1]. Glacier-covered mountain-ranges act as water towers by storing a significant portion of Earth’s fresh water [2]. For more than half of the global population, the seasonal thawing of ice and snow is a source of fresh water [3]. Unfortunately, global warming is causing loss of the mountain cryosphere, with direct impacts on the lives of people living in the vicinity and an indirect impact to those living downstream [4]. Glacial ice loss contributes to sea level rise, altered water availability, and the release of previously trapped contaminants [5].
The ice loss is changing the physicochemical properties of glacier-fed rivers and their aquatic communities [6]. Recent studies have highlighted how glacial microbial diversity varies by region, yet glaciers in Pakistan remain underexplored in this context. The richness, abundance, and diversity of microbial populations are greatly affected due to glacier melting and surface exposures [7,8]. Glacier surfaces contain mineralogical sediments that play a significant role in glacial geochemistry, carbon cycling, and associated glacial retreat [9]. A study examined the microbial population of the cryoconite holes (i.e., aqueous ecosystem) present on glacial surfaces in the Tibetan Plateau and found that bacterial communities residing therein varied according to their geographical locations [10,11]. Moreover, significant biological activity was also reported within such glacial environments [12], while on the ice surface of glaciers, organic and inorganic impurities, regardless of their nature, provide nutrients for the growth of snow and ice algae and heterotrophic bacteria [13].
Glacial sediments, aquatic ecosystems, and cryoconite holes are home to algae, prokaryotic photoautotrophs, heterotrophs, and viruses [14]. From various experiments, it has been shown that there is a relationship between microbial activity and the presence of sediments [15]. For instance, for subglacial microorganisms, the sediment is a supplier of carbon, while weathered or eroded sediments provide aqueous nutrients [16]. The deposits create nutrient-rich micro-zones for microbes. There is evidence that in these micro-zones, bacteria are attached to the sediments [17]. Between 1 × 1017 and 1 × 1021 viable microbes are released due to glaciers melting globally each year [18]. In glacial ecosystems, food webs [19] represent the simple trophic structures present in snowpacks, while respiration and photosynthesis are carried out by a few groups of microorganisms thriving therein. The most complexity of microorganisms is seen in cryoconite holes. Microbes utilize ions for metabolism from a network of veins which have a micron-size diameter [20]. These veins are formed because of the impenetrability of ionic impurities in the crystal shape of ice [21]. Moreover, when ice interacts with mineral surfaces, it develops a thick film or layer of unfrozen water, and this nanometer-thick film provides a habitat for microorganisms that gain energy from oxidation–reduction reactions between ions in the film or ions (acetate, ammonia, sulphate, etc.) in the mineral structure even at great depths [22].
The release of pollutants and greenhouse gases affects the glacial environment by increasing soil temperatures, extending the melting season, and altering microbial communities and nutrient cycling [23]. The melting of glaciers and ice caps reveals forefield soils containing microbes and biogeochemical cycles [24,25]. As a result of the melting, forefield soils are exposed to and are inhabited by varied microbes. The microbial activity in these newly exposed soils carries out the carbon and nutrient cycling [26]. For instance, the heterotrophic microbes and their capability to degrade dissolved organic carbon were studied in different sites in the Himalayan and Antarctic regions [27,28]. From microcosm experiments, it was found that 13–60% of the dissolved organic carbon within the cryoconite holes was bio-available for microorganisms therein. From substrate utilization tests, it was observed that Antarctic microbial communities consumed complex molecules, while Himalayan microbes consumed compounds like carbohydrates, carboxylic acids, amino acids, amines/amides, and polymers [10,29]. Studies in Arctic and Alpine regions have revealed similar microbial adaptations and metal accumulation patterns, underscoring the need for comparative assessments across diverse glacial environments [5,30].
Environmental pollution and associated microbiota are thus the main contributors in this whole scenario, while very little is known about glaciers in Pakistan. This study investigates elemental pollutants and culturable microbial communities in glacial ice and sediments from three glaciers in northern Pakistan. The aim is to contribute baseline data on contaminant presence, bacterial diversity, and their ecological significance within these vulnerable cryosphere ecosystems.
2. Materials and Methods
2.1. Study Sites
From northern areas of Pakistan, three different glaciers were sampled to report this work. Sampling sites, locations and geographical details are presented in Figure 1. Specifically, the samples (ice and sediments) were collected from Ghamurbam glacier, Ghizer—Gilgit (Latitude and Longitude: 35°51′66″ N, 74°63′36″ E), Matiltan glacier, Yasin Darkut—Batin Chorat, Swat (36°44′42″ N, 73°25′59″ E) and Shispare glacier, Hunza (36°26.4′ N, 74°40.9′ E). The entire area, i.e., Himalaya, Karakoram, and Hindukush (HKH)—dominated by high mountains and glaciers—is a part of the world’s third pole, representing a source of water to the most populated region (south Asia) of the globe. Our sampling sites—from Karakoram, and Hindukush parts of HKH—represent the catchments and tributaries for the Swat River and Indus River. Particularly, the river Indus has a total drainage area of more than a million km2, with an estimated annual flow of about 243 km3, making it among the 50 largest rivers in the world. Beginning in a mountain spring and fed with glaciers and rivers from HKH ranges, the Indus river supports the varied ecosystems of temperate forests, plains, and arid countryside in Pakistan.
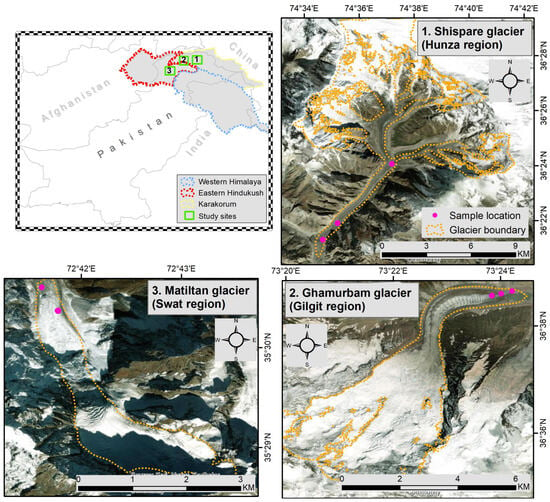
Figure 1.
Geographical map of the sampling locations from HKH region in Pakistan. The numbers, i.e., 1, 2 and 3, represent three different glaciers sampled in this work. Pink dots represent the sampling locations in the glaciers’ surfaces. For panel ‘3. Matiltan glacier (Swat region)’, each dot represents three different locations which were geographically indistinguishable (though 10–15 m away from each other).
2.2. Sample Collection
A total of 80 glacial samples (ice and sediments) were collected from three sites, i.e., Ghamurbam glacier (Gilgit), Matiltan glacier (Swat), and Shispare glacier (Hunza). The sampling was conducted during the summer months of July and August 2021, when access to glacier surfaces was feasible. Seven different treatments of this study include Gilgit glacial ice (Ggi), Swat glacial ice (Sgi), Swat glacial ice front (Sgif), Hunza glacial ice (Hgi), Gilgit glacial sediments (Ggs), Swat glacial sediments (Sgs) and Hunza glacial sediments (Hgs). At each site, 3–5 random samples were taken from the surface (0–10 cm depth). Deeper core sampling was not feasible due to field constraints and the absence of mechanized drilling equipment. Therefore, the results primarily reflect surface-layer conditions of the sampled glaciers. The random samples were combined to form one biological replicate.
For Matiltan glacier in Swat, glacial ice was sampled from two distinct elevations (higher and lower), approximately 500 m apart. These were marked as Sgi (higher elevation) and Sgif (glacier foot, the lower elevation). Sgif represents the melting edge where the glacier begins to retreat. Due to site limitations, only one sampling location was possible for the other two glaciers, i.e., Ghamurbam (Gilgit) and Shispare (Hunza).
Glacial ice samples were collected by digging the surface (0–10 cm depth), while glacial sediments included the mineral debris therein and also the solid particles suspended in melted ice. All samples were collected aseptically using sterile gloves and pre-cleaned tools to prevent contamination. All digging tools were plastic or Teflon-coated to avoid metal contamination during the ice sampling. Samples were stored in sterile, zip-lock ultra-clean plastic bags and transported on ice packs to the laboratory at CUI-Abbottabad. Upon arrival, melted ice samples were immediately filtered to minimize the contamination. Filtration was performed using 0.22 µm pore size syringe filters (Millipore, Burlington, MA, USA) to ensure bacterial separation and minimize particulate matter.
2.3. Sample Preparation and Atomic Absorption Spectrophotometry
The filtered samples were properly marked and labelled (as glacial ice water) for appropriate subsequent analyses. The glacial sediments were passed through pre-cleaned stainless-steel sieve #10 (2.0 mm pore size) and covered with sterilized aluminum foil. Hydrochloric acid and nitric acid were added to respective glacial sediment samples in a ratio of 1:3 with glass pipettes and mixed manually for one minute. The samples were placed on a hotplate in a fume hood at 100 °C for 10 min. The acid evaporated while leaving behind the particles with brown color. The samples were then allowed to cool. Afterwards, 15 mL distilled water was added into these flasks and shaken well. Filtration was carried out by using filter paper, funnel and flasks, while the final filtrates were transferred to glass tubes for subsequent analyses. All these samples were subjected to Atomic Absorption Spectrophotometry using a ZA3000 AAS system (Hitachi, Tokyo, Japan) for elemental analyses. The instrument was calibrated using standard solutions, and analytical accuracy was evaluated using standard reference material SRM 1640a (National Institute of Standards and Technology, NIST, Gaithersburg, MD, USA).
2.4. Microbiological Analyses
Bacterial nutrient media (broth as well as agar) were prepared with distilled water, according to the manufacturer’s instructions. For instance, the nutrient agar (Jinan Biotech, Jinan, China) was prepared with 2.0 g/L yeast extract, 5.0 g/L peptone, 5.0 g/L sodium chloride, and 15.0 g/L agar at pH 7. Subsequently, the media were autoclaved at 121 °C for 20 min. Afterwards, the agar medium was poured into the Petri dishes (20–25 mL in each), while the broth was distributed into glass test tubes (6–8 mL each) for colony counts and optical density assessment, respectively. For this purpose, 50 µL suspension—for each sample—was spread equally onto the agar plates or mixed with nutrient broth.
The samples were serially diluted (up to 10−6) for the microbial inoculation, as mentioned above. After appropriate inoculation, samples (agar media plates and broth test tubes) were placed for 24 h at two temperatures, i.e., one in a refrigerator at 4–8 °C (for potential Psychrophiles) and the other at 12–18 °C (for potential Psychrotolerants). Growth was observed in both sets of samples. All test tubes with a liquid medium showed turbidity that was evaluated via optical density (Spectrophotometry), while the colony forming units (CFUs) were assessed visually under a colony counter. The bacterial colonies were evaluated based on their morphology, such as color, shape, and edge of the colony. Moreover, to obtain a pure bacterial culture, single colonies were streaked on nutrient agar plates to obtain a uniform colony morphology. For long-term storage and further use in different microbiological analyses, the fresh overnight-grown bacterial cultures were mixed 1:1 with sterilized glycerol (50% w/v—Dae Jung Chemicals, Siheung-si, Republic of Korea) as a −80 °C stock [31].
2.5. Molecular Identification of Glacial Bacteria and Phylogenetic Analysis
16S rRNA gene of the bacterial isolates (n = 9) was amplified using universal primers (27F and 1492R) [32]. PCR reactions were performed in 25 µL volumes with initial denaturation at 95 °C for 3 min, followed by 30 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 1.5 min, and a final extension at 72 °C for 10 min. Sanger sequencing was performed by Macrogen (Seoul, Republic of Korea). Each sequence was read at an average depth of 500–800 bp with double-end reads to ensure accuracy. Sequences were manually validated using Chromas software (version 2.6.5). The acquired sequences were compared against the NCBI database using the Basic Local Alignment Search Tool (BLAST) to identify the closest matching bacterial species. Multiple sequence alignments were performed using ClustalW in MEGA 11 (version 11.0.10). A Neighbor-Joining tree was constructed with the Maximum Composite Likelihood model, and 1000 bootstrap replicates were applied to evaluate branch support [33]. Partial sequences of isolated glacier-associated bacterial strains were submitted to NCBI under accession numbers PQ805136–PQ805144.
2.6. Statistical Analyses
Statistical analyses were performed to compare multiple groups, to identify significant differences. For pairwise comparisons, independent t-tests were applied. Analysis of variance (ANOVA) was also performed for some parameters where needed. Data are presented as mean ± standard deviation (SD) from three biological replicates (n = 3). Statistical significance was considered at p < 0.05, and significant differences in figures are mentioned with statistical groups shown with different alphabets.
3. Results
3.1. Elemental Analyses of Glacial Samples
For the elemental analysis of glacial samples (ice and associated sediments), nine key elements were evaluated. These included essential nutrients, metal co-factors, and potentially toxic heavy metals. The samples analyzed were glacial ice-water (Ggi, Sgi, Sgif, and Hgi) and glacial sediments (Ggs, Sgs, and Hgs).
3.1.1. Analysis of Nutritional Elements Present in Glacial Samples
Potassium (K) and iron (Fe) are essential elements found in Earth’s crust and are vital for biological processes. In this study, we observed that these elements were present in relatively small amounts within the glacial ice samples (Ggi, Sgi, Sgif), with Fe up to 1.25 ppm and K up to 3.42 ppm (Figure 2). However, the Shispar glacier ice (Hgi) exhibited significantly higher concentrations of Fe (18.4 ppm, p < 0.01) and K (6.8 ppm, p < 0.05) compared to other ice-water samples.
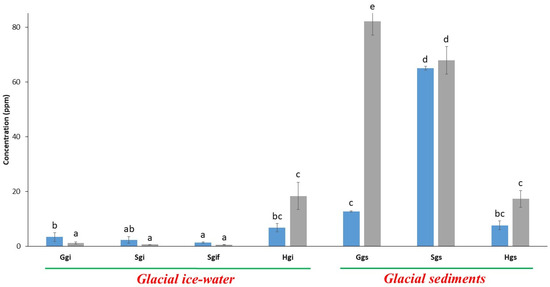
Figure 2.
Analysis of nutritional elements present in glacial samples collected from HKH region. Blue and grey bars represent potassium (K) and iron (Fe) concentrations, respectively. The error bars in the graph represent standard deviations, where n = 3. Letters, i.e., abcde, represent different statistical classes.
Furthermore, Fe and K concentrations were significantly higher in glacial sediments than in their corresponding ice-water samples (p < 0.001). The highest Fe concentration (82.14 ppm) was recorded in Gilgit glacier sediments (Ggs), while the maximum K concentration (64.9 ppm) was found in Swat glacier sediments (Sgs). Notably, all three glacial sediment samples exhibited significant variation in Fe levels, with the lowest concentration observed in Shispar glacier sediments (Hgs, 17.3 ppm, p < 0.05) (Figure 2).
3.1.2. Analysis of Elements as Co-Factors, Present in Glacial Samples
Copper (Cu), nickel (Ni), and zinc (Zn) are essential metal co-factors involved in enzymatic processes, particularly in bacteria and archaea. In all glacial samples, these three elements were present at concentrations below 2.5 ppm (Figure 3).
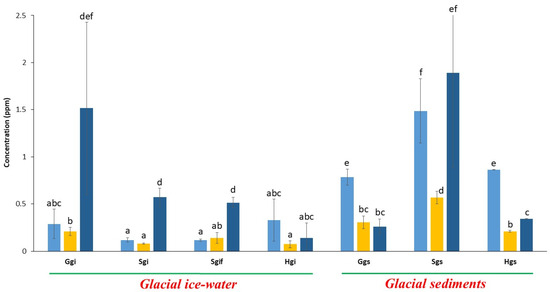
Figure 3.
Analysis of co-factor elements present in glacial samples collected from HKH region. Sky blue, yellow and dark blue bars represent copper (Cu), nickel (Ni) and zinc (Zn) concentrations, respectively. The error bars in the graph represent standard deviations, where n = 3. Letters, i.e., abcdef, represent different statistical classes.
When comparing these elements, Zn was found in significantly higher concentrations than Cu and Ni in four out of seven sample categories (Ggi, Sgi, Sgif, and Sgs, p < 0.05). In contrast, Cu was significantly elevated in Gilgit (Ggs) and Shispar (Hgs) glacial sediments compared to Zn and Ni (p < 0.01).
Across all sample types, Cu and Ni were consistently more abundant in sediment samples than in their corresponding ice-water samples (p < 0.001). Notably, the Swat glacier sediments (Sgs) exhibited the highest concentrations of all three elements, i.e., Zn (1.9 ppm), Cu (1.5 ppm), and Ni (0.6 ppm) (Figure 3). This suggests a pattern of metal enrichment in sediment layers, possibly due to the natural weathering processes and particle retention.
3.1.3. Analysis of Potentially Toxic Heavy Metals Present in Glacial Samples
Lead (Pb), cadmium (Cd), chromium (Cr), and arsenic (As) are toxic heavy metals with known environmental and health risks. Our analysis revealed that arsenic was the most enriched heavy metal across glacial samples, with concentrations ranging from 3.13 ppm in Hunza glacier ice (Hgi) to 11.02 ppm in Swat glacier sediments (Sgs) (Figure 4a). Arsenic levels were significantly higher in glacial sediments compared to their respective ice-water samples in Swat and Hunza glaciers (p < 0.01). Among ice samples, Ghamurbam glacier ice (Ggi) showed the highest arsenic concentration (6.71 ppm, p < 0.05), while Shispar glacier ice (Hgi) had the lowest (3.13 ppm). Notably, arsenic concentrations were comparable between ice and sediment samples in Swat and Hunza glaciers, suggesting localized accumulation of this element (Figure 4a).
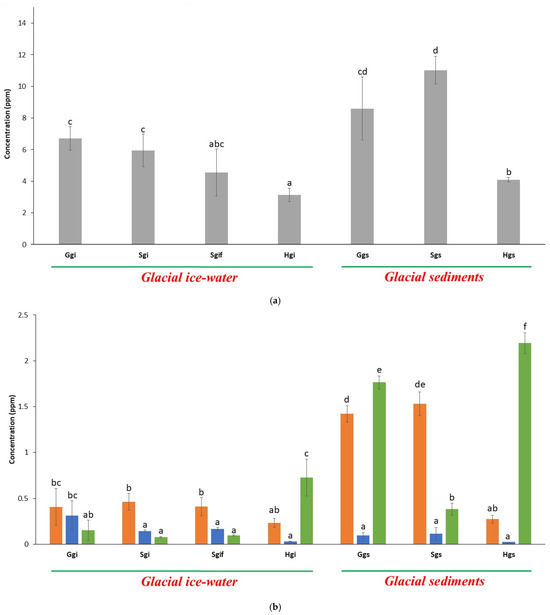
Figure 4.
(a) Evaluation of arsenic contamination present in glacial samples collected from HKH region. The error bars in the graph represent standard deviations, where n = 3. Letters, i.e., abcd, represent different statistical classes. (b) Evaluation of heavy metals present in glacial samples collected from HKH region. Orange, blue and green bars represent lead (Pb), cadmium (Cd) and chromium (Cr) concentrations, respectively. The error bars in the graph represent standard deviations where n = 3. Letters, i.e., abcdef, represent different statistical classes.
For the other three toxic heavy metals, Pb was significantly elevated in Gilgit and Swat glacier sediments compared to their corresponding ice-water samples (p < 0.001). The highest Pb concentration was observed in Swat glacier sediments (Sgs) at 1.53 ppm, while comparable Pb levels between ice and sediments were noted in Shispar glacier, indicating an even distribution of lead across this glacier (Figure 4b).
Chromium (Cr) concentrations were consistently higher in glacier sediment samples than in ice-water samples across all three sites (p < 0.01). Among the three glaciers, Shispar (Hgs) showed the highest Cr concentration at 2.2 ppm, reflecting localized geological input.
In contrast, cadmium (Cd) showed a reversed pattern, with significantly higher concentrations in Ghamurbam glacier ice (Ggi, 0.31 ppm) than in its corresponding sediment sample (0.09 ppm, p < 0.05). This anomalous distribution may reflect differences in Cd mobility or solubility in glacial environments (Figure 4b).
3.1.4. WHO and USEPA Comparison of Elemental Concentrations
Copper and Zinc concentrations remain well below threshold values set by WHO and USEPA drinking water standards (Supplementary Table S1), while other elements were detected as exceeding these standards. This may point towards the potential ecological and health concerns, including bioaccumulation in aquatic food chains. Particularly for our case, meltwater from glaciers will certainly dilute its constituents (including heavy mental contaminants) while reaching its downstream use for any biological purpose. An important factor to keep in mind is that the available threshold values are for drinking water [34,35] and not for other forms of water like surface water and environmental water. It is therefore important to compare these observations with some environmental tolerance thresholds for elements. Moreover, water monitoring is recommended to evaluate its impact on downstream ecosystems and human water use.
3.2. Microbial Analyses of the Studied Glacial Samples
Microbial growth analysis focused on ice-water samples, excluding sediments, to assess the abundance and diversity of culturable bacteria. Bacterial growth was evaluated as abundance (numbers) via optical density (OD) of the growth medium incubated under two temperature conditions, i.e., 4–8 °C (psychrophiles) and 12–18 °C (psychrotolerants). OD measurements revealed that bacterial growth was significantly higher at 12–18 °C across all samples compared to 4–8 °C (p < 0.001) (Figure 5). The highest OD value (1.45) was recorded in Sgif ice-water, while the 4–8 °C incubation consistently produced lower OD values ranging from 0.7 to 0.86 across all samples (p < 0.05). This indicates that most bacteria are potentially favored with moderate psychrotolerant conditions.
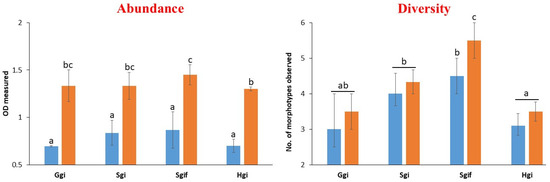
Figure 5.
Evaluation of cultivable bacterial growth (abundance and diversity) in two temperature conditions (4–8 °C, blue bars and 12–18 °C, orange bars) for the ice-water samples originating from HKH glaciers. Error bars in the graph represent standard deviations, where n = 3, while letters, i.e., abc, indicate different statistical classes.
Morphological analysis of isolated bacterial colonies on nutrient agar revealed significant differences in community diversity between sampling sites (p < 0.01). The Sgif sample exhibited the highest morphotype diversity with six distinct colony types, while the Hgi sample displayed the lowest diversity with only three morphotypes (Figure 5). Apart from Sgif, there were no statistically significant differences in bacterial diversity between the two incubation temperatures.
When comparing glacier sources, Swat glacier ice (Sgi) showed significantly higher microbial diversity than Gilgit (Ggi) and Hunza (Hgi) glacier ice samples (p < 0.05). These findings suggest that site-specific environmental conditions play a role in shaping microbial diversity within the glacial ecosystem. Further microbiological and genomic studies are underway to characterize the ecological functions of these glacial bacteria.
3.3. Identification and Phylogenetic Analysis of Bacteria Associated with Glacial Samples
Phylogenetic analysis of 16S rRNA gene sequences confirmed that the bacterial isolates from glacial samples share 99–100% homology with previously identified species in the NCBI database (Figure 6). This strong genetic similarity suggests that glacial environments harbor bacterial communities capable of adapting to extreme conditions. We performed multiple sequence alignments using the ClustalW algorithm in MEGA 11 and constructed a Neighbor-Joining tree based on the Maximum Composite Likelihood model. To ensure the robustness of our analysis, we applied 1000 bootstrap replicates, and the bacterial sequences were submitted to NCBI GenBank (accession numbers: PQ805136–PQ805144).
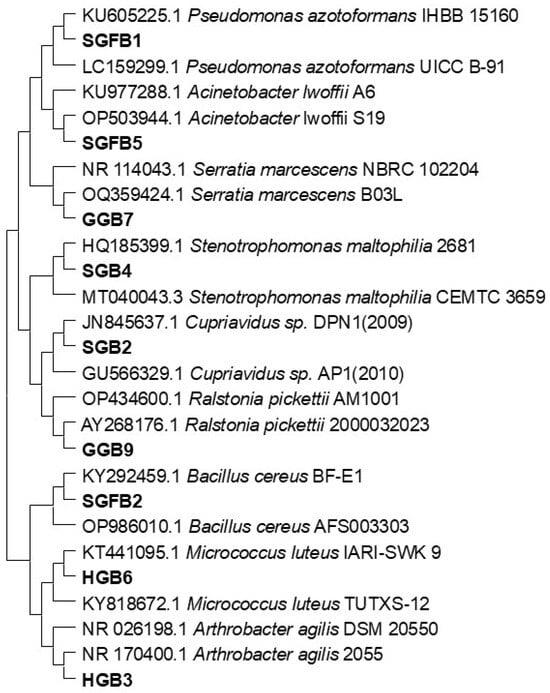
Figure 6.
Phylogenetic tree, based on 16s rRNA gene sequences of bacterial isolates purified from ice-water samples collected from HKH glaciers. Reference strains, taken from NCBI database, are included in analysis along with their respective sequences’ accession numbers. Bacterial strains reported in this study are mentioned in bold text.
The phylogenetic tree revealed that the bacterial isolates clustered into seven distinct groups, each associated with species known for their environmental adaptability and heavy metal resistance. Among these, SGFB1 showed close evolutionary ties to Pseudomonas azotofirmans (bootstrap = 98%), a species recognized for its ability to withstand environmental stress and detoxify heavy metals. Similarly, SGFB5 was identified as a strain related to Serratia marcescens (bootstrap = 96%), a bacterium frequently found in aquatic environments and known for its potential in bioremediation. Two isolates, GGB7 and SGB4, exhibited strong genetic similarity to Stenotrophomonas maltophilia (bootstrap = 97%), a bacterium notable for its dual resistance to heavy metals and antibiotics, highlighting its role in both metal detoxification and antimicrobial defense.
Another important group included SGB2, which was related to Cupriavidus sp. (bootstrap = 94%), a genus known for its capacity to survive in polluted environments by detoxifying heavy metals. Additionally, GGB9 and SGFB2 were closely aligned with Bacillus cereus (bootstrap = 99%), a widely studied bacterium that thrives in extreme environments and is involved in nutrient cycling and metal resistance. The isolate HGB6 showed genetic alignment with Micrococcus luteus (bootstrap = 95%), an extremophile bacterium known for its resilience in harsh environments. Lastly, HGB3 clustered with Arthrobacter agilis (bootstrap = 93%), a species typically found in heavy metal-contaminated soils, suggesting its potential role in biogeochemical cycling.
These findings provide compelling evidence that bacterial communities within glacial environments are not only diverse but also adapted to survive in the presence of elevated metal concentrations. The close evolutionary relationships between these isolates and known metal-resistant bacteria suggest that glacial microbes may play a critical role in metal detoxification and elemental cycling, contributing to the ecological balance in these extreme environments. Given their metal resistance and metabolic versatility, these bacteria may serve as potential candidates for biotechnological applications in bioremediation and as bioindicators for monitoring glacial pollution.
4. Discussion
This study provides an analysis of the elemental composition and bacterial community present in glacial ice-water and associated sediments from three glaciers in northern Pakistan. Our findings reveal significant differences in elemental concentrations between ice and sediment samples. The elevated concentrations of arsenic, cadmium, and lead in some samples may be attributed to a combination of geogenic sources (e.g., mineralized bedrock) [36] and anthropogenic factors [37], such as long-range atmospheric deposition. Swat glacier’s proximity to agricultural areas and settlements could explain localized contamination [38]. Furthermore, the phylogenetic analysis of bacterial isolates underscores the ecological adaptability of these microbial communities to heavy metal-rich environments.
4.1. Elemental Composition and Its Environmental Significance
The mountain glacier surfaces represent a place of varied biogenic organo-mineral and mineral compound accumulation [30]. The analysis of our study revealed that elemental concentrations, particularly for potassium (K) and iron (Fe), were significantly higher in sediments compared to ice-water samples (p < 0.001), consistent with previous findings [20,39,40]. Similarly, different heavy metals were reported, i.e., Fe as 1697–2215 and 10.2–22 ppm, Zn as 43–60 and 8–13 ppm, Ni as 4–9 and 0.3–1.5 ppm, Cu as 8–11 and 0.7–2 ppm, Cr as 7.6–11 and 0.8–1.9 ppm in glacial lake sediments and meltwater, respectively, while K was 312–678 and 16–40 μM in sediments and meltwater [41]. All these elements were significantly higher in sediments compared to the meltwater samples. This difference is likely due to the lower mobility of these elements and their tendency to bind to sedimentary particles [42]. Glaciated sediments are an important source for determining glacial environmental processes. Meltwater streams draining from Himalayan glaciers carry different types of sediments [43]. Earlier data [44] suggested the active geochemical weathering regime in a low-temperature environment. In another study, the mean concentrations of lead (0.51 ppm), cadmium (0.04 ppm), nickel (0.06 ppm), chromium (0.08 ppm), and iron (0.06 ppm) were found in lake and river water of Swat, Lower Dir and Upper Dir [45].
Among our samples, Gilgit glacier sediments (Ggs) exhibited the highest Fe concentration (82.14 ppm), a finding consistent with the region’s mineral-rich bedrock and weathering processes [43]. Pyrite (45.4–46.4% Fe and 0.02%Ni), chalcopyrite (29.89%Fe and 34.63%Cu), and iron-arsenide (Lollingite: 29.86%Fe, 65.7%As) are the polymetallic deposits of the Kargah Gilgit-Baltistan region [20] that corresponds to the study area of this work too. Moreover, Fe was reported as 0.6–14 ppm in Mingyong glacier, China [46], 0–1.34 ppm in groundwater of Gilgit-Baltistan [37] and in Hunza River water as 55.2 ppm (at Wakhan), 66.9 ppm (at Karakoram Block) and 87.2 ppm at Kohistan Block (Pakistan) [47]. Karakoram and Kohistan blocks of that work [48] corroborate our Hunza and Gilgit regions, thus reinforcing our findings. The high Co/Ni ratio (1.3–16.4) and low Mo/Ni ratio (0.43–0.94) in Kargah (Gilgit-Baltistan) polymetallic deposits suggested a mafic (including sulfide deposits) source for the mineralization. Earlier data also showed the narrow variations in Pb isotope values, suggesting a single Pb source in this area [20].
Recently, the chemical composition showed the seasonal variation of ionic concentration within river water of the Tibetan Plateau with a higher proportion of K/Na [49]. Just like our observations, several other studies in the region also reported K as 1.8 ppm in Batura (Himalaya-Pakistan) glacier meltwater [50], 144–440 ppm in glacier samples from Kamri, Burzil, Siachin, Baltoro, Shigar Basin, Biafo and Panmah [51], 79–363 ppm in samples from Ghulmet, Ghulkin and Hopar glaciers of the Hunza valley in Karakoram Range, Pakistan [52], 0.2–20 ppm in groundwater of Gilgit-Baltistan [37] and 22–120 ppm in Mingyong Glacier, China [46].
In contrast, arsenic (As) concentrations were notably elevated in Swat glacier sediments (Sgs) (11.02 ppm), suggesting the potential anthropogenic input from agricultural or industrial activities in the vicinity [38]. Arsenic-rich groundwaters are also found in geothermal areas and in areas of mining activity where oxidation of sulfide minerals has occurred. Arsenic is mainly a byproduct in the smelting of copper and lead ores, reported from Chitral and Gilgit-Baltistan regions [36]. Moreover, the sulfide oxidation rate was found to be 5 times higher in glacial catchments compared to nonglacial catchments [53], which may explain the higher As concentration in our glaciated samples.
Considering the metal concentrations, a recent study evaluated the contamination of the surface of Arctic, Antarctic, and Caucasian glaciers. Particularly, Cu, Zn, Ni, Cd, and Pb were found as 1.8–17.4, 3.1–85.7, 1.3–19, <0.005–0.112, and 0.2–30 pp within cryoconite sediments of Caucasian glaciers [39], which are at almost the same altitude as our samples from Gilgit and Hunza. Another study reported the heavy metals in the water of Hunza River and its tributaries, located in Gilgit–Baltistan, Pakistan. Their average concentrations were 4.44, 5.48, and 15.6 ppm Cr; 5.78, 6.10, and 21.7 ppm Ni; and 5.56, 5.29, and 6.56 ppm Cu in Wakhan, Karakoram, and Kohistan Blocks, respectively [47]. A potential explanation for heavy metals in our glacial samples may relate to the mineral resources of the bedrock, for instance, Cr sourced from Chilas chromite (for Gilgit), Dargai chromite (for Swat-Malakand), Ni sourced from ultramafic rocks of Teru, Yasin and Pakora areas of Shyok Suture. Nickel also occurs in Malam Jabba (Swat), Shangla-Alpurai areas, Gilgit, Nagar and Hunza rivers [48]. In Gilgit-Baltistan, mineral enriched with metallic components are reported as Zincian Tetrahedrite (8.56% As, 39.28% Cu and 5.97% Zn), Galena (85.95% Pb), Native Silver (0.7% Cd), Bornite (62.83% Cu, 0.03% Cr), and Covellite (66.13% Cu) [20], thus explaining the potential sources of elements observed in our work.
Very recently, Khan and colleagues [37] aimed to gain insight into hydrochemical genesis and quality of groundwater from mountainous Gilgit-Baltistan (western high Himalayas, Pakistan). Their analyses explained 75.7% of the total variance, where the combination of natural and anthropogenic activities was found to influence the groundwater hydrochemistry [37]. The rocks in Gilgit-Baltistan are rich in minerals such as copper, gold, silver, lead, zinc, iron, and precious gemstones [48]. This may provide clues for the observations of elemental concentrations in our glacial samples.
A particularly striking observation was the anomalous distribution of cadmium (Cd), which was significantly higher in Ghamurbam glacier ice (0.31 ppm) compared to its corresponding sediments (0.09 ppm, p < 0.05). This pattern may be attributed to the high solubility of cadmium [42] in glacial meltwater [45], facilitating its mobilization from upstream sources. This suggests a need for longitudinal studies to evaluate seasonal shifts in metal transport under changing climate conditions [50].
Our findings are consistent with earlier research highlighting the dual influence of geological and anthropogenic sources on elemental composition in glacial environments [54]. Similar patterns of heavy metal enrichment, particularly for arsenic and lead, have been reported in studies of alpine glaciers [45]. These results highlight the complex interactions between geological and potentially anthropogenic inputs in shaping the elemental profiles within glacial environments.
4.2. Microbial Diversity and Adaptation to Glacial Environments
The potential threat of resurrection of age-old microbiome in glaciers and its incorporation into surface waters has raised a major concern for epidemics and exposure of life to paleomicrobiota [51]. This has led scientists to observe the microbial flora embedded within glaciers. In our work, microbial growth analysis demonstrated that bacterial abundance was significantly higher at 12–18 °C compared to 4–8 °C (p < 0.001). Similarly—from ice, sediments and meltwaters from Ghulmet, Ghulkin and Hopar glaciers of Hunza valley in Karakoram Range, Pakistan—CFU per gm/mL of the sample was reported to be 1.34 × 102–3.55 × 107 and 6.50 × 104–1.65 × 109 at 5 °C and 25 °C, respectively [52]. Moreover, bacterial communities in ice, water, and sediments of Batura glacier were investigated and 27 cold-adapted bacteria were isolated at 4 °C and 15 °C; they exhibited growth at a wide range of temperatures (4–35 °C) [32], just like our observations at two different temperatures, i.e., 4–8 °C and 12–18 °C. Another study from the region, i.e., Mingyong glacier of southwestern China, determined the microbial diversity showing high bacterial abundance with 2.24 × 103 to 5.56 × 103 OTUs in their samples [46]. Additionally, the microbial diversity observed in our study mirrors the findings from Polar and Himalayan regions, where psychrotolerant bacteria play pivotal roles in element cycling and contaminant degradation [20]. This aligns with Garcia-Lopez et al. [7], who found similar anthropogenic enrichment in polar glaciers. These observations support the dominance of cold-adapted taxa in alpine systems, suggesting a predominance of psychrotolerant species adapted to fluctuating glacial conditions [55].
The Swat glacier ice (Sgi) exhibited the greatest diversity of bacterial morphotypes, while the Hunza glacier ice (Hgi) showed the lowest (p < 0.05). These findings align with other studies indicating that microbial diversity in glacial environments is shaped by microclimatic variability [56] and nutrient availability [57]. The observed bacterial diversity also reflects functional adaptations to heavy metal stress. To this extent, a very recent study evaluated bacteria isolated from supraglacial lake debris and meltwater in Dook Pal glacier (Chitral–Pakistan) for antibiotic and metal resistance. A total of 92.5% of their bacterial isolates from lake debris and 83.3% of isolates from meltwater harbored metal resistance [58]. In our case, the presence of Stenotrophomonas maltophilia SGB4—relative bacteria known for antibiotic and metal resistance—suggests active microbial strategies for coping with toxic environments, as reported earlier [59]. Similarly, the presence of Cupriavidus sp. SGB2 would emphasize the bacterial capability for bioremediation through metal detoxification [60].
4.3. Phylogenetic Insights and Functional Implications
The phylogenetic analysis grouped the bacterial isolates into seven distinct clusters, each linked to species with established roles in metal resistance and environmental resilience [61]. Notably, SGFB1 aligned closely with Pseudomonas azotofirmans (bootstrap = 98%), a bacterium recognized for its ability to tolerate and detoxify heavy metals [62]. Similarly, SGB2 was linked to Cupriavidus sp. (bootstrap = 94%), reinforcing the role of glacial microbial communities in mitigating metal contamination [63]. Interestingly, GGB9 and SGFB2 grouped with Bacillus cereus (bootstrap = 99%), a species renowned for its metabolic versatility and ability to survive under extreme environmental conditions. Their role in nutrient cycling under harsh conditions is well-documented. Recent studies investigated the bacterial diversity within various glaciers of Pakistan and found Proteobacteria as the dominant phylum, accompanied by Actinobacteria, Firmicutes, and Bacteroidetes [32,51]. Moreover, bacterial structure and function were examined in the lake debris and meltwater of Dook Pal Glacier (Hindu Kush region), where Proteobacteria dominated in lake debris (33.1–94.5%), while Proteobacteria (36.7–50.5%) and Firmicutes (44–62%) dominated in meltwater [41]. Interestingly, the predominant phyla in Mingyong glacier (China) were Proteobacteria, Deinococcus-Thermus, Firmicutes, Actinobacteria, and Nitrospirae [46].
4.4. Study Limitations and Future Directions
Our findings underscore the critical role of glaciers not only as reservoirs of fresh water but also as dynamic ecosystems influencing elemental fluxes and microbial processes. While this study provides insights into the elemental and microbial composition of glacial samples, several limitations must be acknowledged. Future studies should expand sampling to additional glaciers across northern Pakistan and the Himalayan-Karakoram region to provide a more comprehensive understanding of spatial variation in elemental distribution and microbial adaptation. The detection of arsenic, lead, and cadmium may pose ecological risks, including potential bioaccumulation in aquatic ecosystems and contamination of downstream drinking water supplies. Understanding these risks is essential to assess the broader environmental impacts of glacial melt in regions reliant on glacial runoff. Based on our findings, future studies are suggested to specify the consequences of bioaccumulation, such as affecting aquatic life and human health. It is furthermore recommended to use the specific approaches to understanding these risks, such as through long-term monitoring and risk assessment models. Moreover, future work should employ isotopic tracing or geochemical modeling to quantify the contribution of varied sources [64]. Multi-element fingerprinting techniques [65] may further help differentiate natural from anthropogenic sources. For microbiological context, the focus on culturable bacteria may underestimate the full microbial diversity, particularly from metagenomic perspectives. Future research should employ shotgun metagenomics to capture the genetic potential [66] of these ecosystems and identify key genes involved in metal detoxification and stress adaptation. Although we identified the bacterial species from metal-contaminated glacier environments, the underlying genetic mechanisms for metal tolerance (e.g., metal efflux pumps, resistance genes) remain unexamined.
Additionally, long-term monitoring across multiple seasons is essential to evaluate how glaciers impact metal mobility and microbial functioning retreat. Also, for glacier conservation, cumulative efforts focused on sustainable development should be adopted to control and mitigate the ever-challenging climate change. Further interdisciplinary research is needed to bridge the gap between glacial microbiology, hydrology, and environmental chemistry, ensuring a comprehensive understanding of these fragile ecosystems in a rapidly changing world.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/earth6030071/s1, Table S1. Comparison of heavy metal concentrations in our ice-water compared with WHO and USEPA limits in drinking water.
Author Contributions
Conceptualization, R.N. and K.J.; methodology, K.J. and N.u.H.S.; software, K.J. and S.A.; validation, R.N., K.J. and Ö.D.; formal analysis, K.J. and S.F.K.; investigation, K.J. and S.F.K.; resources, R.N. and A.J.A.; data curation, R.N. and A.A.T.; writing—original draft preparation, K.J. and S.F.K.; writing—review and editing, S.F.K. and R.N.; visualization, A.A.T. and R.N.; supervision, R.N.; project administration, R.N.; funding acquisition, R.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was partially supported by the funds under TWAS-IsDB research project (No. 2021-Joint Research Grant RG-507175), awarded to RN.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Sequencing data is available in the publicly accessible repository of NCBI. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors acknowledge the Department of Environmental Sciences at CUI-Abbottabad for logistic support and for assistance in the Instrumental Lab of the institution. Moreover, Muhammad Ibrahim is acknowledged for his assistance in glacier sampling.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Lehmann-Konera, S.; Ruman, M.; Kozioł, K.; Gajek, G.; Polkowska, Ż. Glaciers as an Important Element of the World Glacier Monitoring Implemented in Svalbard. In Glacier Evolution in a Changing World; Godone, D., Ed.; Intech Open Limited: London, UK, 2017. [Google Scholar] [CrossRef]
- Jones, D.B.; Harrison, S.; Anderson, K.; Betts, R.A. Mountain rock glaciers contain globally significant water stores. Sci. Rep. 2018, 8, 2834. [Google Scholar] [CrossRef] [PubMed]
- Bolch, T.; Shea, J.M.; Liu, S.; Azam, F.M.; Gao, Y.; Gruber, S.; Zhang, Y. Status and change of the cryosphere in the extended Hindu Kush Himalaya region. In The Hindu Kush Himalaya Assessment: Mountains, Climate Change, Sustainability and People; Springer: Cham, Switzerland, 2019; pp. 209–255. [Google Scholar]
- Rasul, G.; Molden, D. The global social and economic consequences of mountain cryospheric change. Front. Environ. Sci. 2019, 7, 91. [Google Scholar] [CrossRef]
- Milner, A.M.; Khamis, K.; Battin, T.J.; Brittain, J.E.; Barrand, N.E.; Füreder, L.; Brown, L.E. Glacier shrinkage driving global changes in downstream systems. Proc. Natl. Acad. Sci. USA 2017, 114, 9770–9778. [Google Scholar] [CrossRef]
- Pandit, A.; Ramsankaran, R.A.A.J. Identification of potential sites for future lake formation and expansion of existing lakes in glaciers of Chandra Basin, Western Himalayas, India. Front. Earth Sci. 2020, 8, 500116. [Google Scholar] [CrossRef]
- Garcia-Lopez, E.; Moreno, A.M.; Cid, C. Microbial community structure and metabolic networks in Polar Glaciers. In Metagenomics: Basics, Methods and Applications; Intech Open Limited: London, UK, 2019; pp. 501–505. [Google Scholar]
- Hotaling, S.; Hood, E.; Hamilton, T.L. Microbial ecology of mountain glacier ecosystems: Biodiversity, ecological connections and implications of a warming climate. Environ. Microbiol. 2017, 19, 2935–2948. [Google Scholar] [CrossRef]
- Cameron, K.A.; Müller, O.; Stibal, M.; Edwards, A.; Jacobsen, C.S. Glacial microbiota are hydrologically connected and temporally variable. Environ. Microbiol. 2020, 22, 3172–3187. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, K.; Liu, Y.; Vick-Majors, T.J.; Wang, F.; Ji, M. Temporal variation of bacterial community and nutrients in Tibetan glacier snowpack. Cryosphere 2022, 16, 1265–1280. [Google Scholar] [CrossRef]
- Ren, Z.; Gao, H.; Luo, W.; Elser, J.J. Bacterial communities in surface and basal ice of a glacier terminus in the headwaters of Yangtze River on the Qinghai–Tibet Plateau. Environ. Microbiome 2022, 17, 12. [Google Scholar] [CrossRef]
- Shen, L.; Liu, Y.; Wang, N.; Adhikari, N.P. Genomic insights of Dyadobacter tibetensis Y620-1 isolated from ice core reveal genomic features for succession in glacier environment. Microorganisms 2019, 7, 211. [Google Scholar] [CrossRef]
- Kobayashi, K.; Takeuchi, N.; Kagami, M. High prevalence of parasitic chytrids infection of glacier algae in cryoconite holes in Alaska. Sci. Rep. 2023, 13, 3973. [Google Scholar] [CrossRef]
- Fiołka, M.J.; Takeuchi, N.; Sofińska-Chmiel, W.; Mieszawska, S.; Treska, I. Morphological and physicochemical diversity of snow algae from Alaska. Sci. Rep. 2020, 10, 19167. [Google Scholar] [CrossRef]
- Escudero, C.; Amils, R. Dark biosphere: Just at the very tip of the iceberg. Environ. Microbiol. 2022, 25, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Reeksting, B.J.; Hoffmann, T.D.; Tan, L.; Paine, K.; Gebhard, S. In-depth profiling of calcite precipitation by environmental bacteria reveals fundamental mechanistic differences with relevance to application. Appl. Environ. Microbiol. 2020, 86, e02739-19. [Google Scholar] [CrossRef] [PubMed]
- Varliero, G.; Lebre, P.H.; Frey, B.; Fountain, A.G.; Anesio, A.M.; Cowan, D.A. Glacial water: A dynamic microbial medium. Microorganisms 2023, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Mania, I.; Gorra, R.; Colombo, N.; Freppaz, M.; Martin, M.; Anesio, A.M. Prokaryotic diversity and distribution in different habitats of an alpine rock glacier-pond system. Microb. Ecol. 2019, 78, 70–84. [Google Scholar] [CrossRef]
- Thaysen, E.M.; McMahon, S.; Strobel, G.J.; Butler, I.B.; Ngwenya, B.T.; Heinemann, N.; Edlmann, K. Estimating microbial growth and hydrogen consumption in hydrogen storage in porous media. Renew. Sustain. Energy Rev. 2021, 151, 111481. [Google Scholar] [CrossRef]
- Sanyal, A.; Antony, R.; Samui, G.; Thamban, M. Microbial communities and their potential for degradation of dissolved organic carbon in cryoconite hole environments of Himalaya and Antarctica. Microbiol. Res. 2018, 208, 32–42. [Google Scholar] [CrossRef]
- Gong, F.; Wang, Y.; Ueda, T.; Zhang, D. Modeling and mesoscale simulation of ice-strengthened mechanical properties of concrete at low temperatures. J. Eng. Mech. 2017, 143, 04017022. [Google Scholar] [CrossRef]
- Pugnaire, F.I.; Morillo, J.A.; Peñuelas, J.; Reich, P.B.; Bardgett, R.D.; Gaxiola, A.; Van Der Putten, W.H. Climate change effects on plant-soil feedbacks and consequences for biodiversity and functioning of terrestrial ecosystems. Sci. Adv. 2019, 5, eaaz1834. [Google Scholar] [CrossRef]
- IPCC. Sixth Assessment Report—Climate Change 2022: Impacts, Adaptation and Vulnerability; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2022. [Google Scholar]
- Kannojia, P.; Sharma, P.K.; Sharma, K. Climate change and soil dynamics: Effects on soil microbes and fertility of soil. In Climate Change and Agricultural Ecosystems; Woodhead Publishing: Cambridge, UK, 2019; pp. 43–64. [Google Scholar]
- Bradley, J.A.; Singarayer, J.S.; Anesio, A.M. Microbial community dynamics in the forefield of glaciers. Proc. R. Soc. B 2014, 281, 20140882. [Google Scholar] [CrossRef]
- Wietrzyk-Pełka, P.; Rola, K.; Szymański, W.; Węgrzyn, M.H. Organic carbon accumulation in the glacier forelands with regard to variability of environmental conditions in different ecogenesis stages of High Arctic ecosystems. Sci. Total Environ. 2020, 717, 135151. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.; Muraleedharan, P.M.; Babu, C.P. Mid-troposphere transport of Middle-East dust over the Arabian Sea and its effect on rainwater composition and sensitive ecosystems over India. Sci. Rep. 2017, 7, 13676. [Google Scholar] [CrossRef]
- Neelavannan, K.; Sen, I.S.; Lone, A.M.; Gopinath, K. Microplastics in the high-altitude Himalayas: Assessment of microplastic contamination in freshwater lake sediments, Northwest Himalaya (India). Chemosphere 2022, 290, 133354. [Google Scholar] [CrossRef] [PubMed]
- Zeb, B.; Alam, K.; Sorooshian, A.; Chishtie, F.; Ahmad, I.; Bibi, H. Temporal characteristics of aerosol optical properties over the glacier region of northern Pakistan. J. Atmos. Sol.-Terr. Phys. 2019, 186, 35–46. [Google Scholar] [CrossRef]
- Brighenti, S.; Tolotti, M.; Bruno, M.C.; Wharton, G.; Pusch, M.T.; Bertoldi, W. Ecosystem shifts in Alpine streams under glacier retreat and rock glacier thaw: A review. Sci. Total Environ. 2019, 675, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sharma, M. Cultural and morphological characterization of antagonistic Trichoderma isolates. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1041–1048. [Google Scholar] [CrossRef]
- Ali, P.; Chen, F.; Hassan, F.; Sosa, A.; Khan, S.; Badshah, M.; Shah, A.A. Bacterial community characterization of Batura Glacier in the Karakoram Range of Pakistan. Int. Microbiol. 2021, 24, 183–196. [Google Scholar] [CrossRef]
- Rehman, R.; Kazmi, S.F.; Irshad, M.; Bilal, M.; Hafeez, F.; Ahmed, J.; Nazir, R. Microalgae-Assisted Treatment of Wastewater Originating from Varied Sources, Particularly in the Context of Heavy Metals and Antibiotic-Resistant Bacteria. Water 2024, 16, 3305. [Google Scholar] [CrossRef]
- USEPA. National Primary Drinking Water Regulations—Arsenic and Clarifications to Compliance and New Source Monitoring Rule; EPA 816-F-01-004; United States Environmental Protection Agency: Washington, DC, USA, 2001. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 13 June 2025).
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; WHO Library Cataloguing-in-Publication Data; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0. [Google Scholar]
- Kazmi, A.H. Preliminary report on Geol. and Mineral occurrences in lower Hunza valley. Geol. Surv. Pak. 1951, 534, 13. [Google Scholar]
- Khan, M.H.; Xiao, Y.; Yang, H.; Wang, L.; Zhang, Y.; Hu, W.; Wang, J.; Liu, G.; Liu, W. Identification of hydrochemical fingerprints, quality and formation dynamics of groundwater in western high Himalayas. Environ. Monit. Assess. 2024, 196, 305. [Google Scholar] [CrossRef]
- Kam, E.; Yümün, Z. Geographical distribution of toxic elements in Northeast Marmara Sea sediments and analysis of toxic element pollution by various pollution index methods (Istanbul/Turkey). Appl. Ecol. Environ. Res. 2021, 19, 1869–1893. [Google Scholar] [CrossRef]
- Abakumov, E.; Tembotov, R.; Polyakov, V.; Ivanov, M.; Mavlyudov, B.; Kushnov, I.; Nizamutdinov, T.; Yaneva, R.; Zhiyanski, M. Concentration of Trace Elements in Cryoconites of Mountain and Polar Regions of the World. Geosciences 2023, 13, 188. [Google Scholar] [CrossRef]
- Hussain, Z.; Tao, C.; Li, C.F.; Liao, S.; Alam, M.; Farhan, M.; Zhang, H.; Hussain, A. Mineralogy, Fluid Inclusions, and Isotopic Study of the Kargah Cu-Pb Polymetallic Vein-Type Deposit, Kohistan Island Arc, Northern Pakistan: Implication for Ore Genesis. Minerals 2021, 11, 1266. [Google Scholar] [CrossRef]
- Ilahi, N.; Bahadur, A.; Wang, W.; Degen, A.A.; Kang, S.; Sajjad, W.; Shang, Z. Diversity, distribution, and function of bacteria in the supraglacial region hit by glacial lake outburst flood in northern Pakistan. Environ. Sci. Eur. 2022, 34, 73. [Google Scholar] [CrossRef]
- Suska-Malawska, M.; Vyrakhamanova, A.; Ibraeva, M.; Poshanov, M.; Sulwiński, M.; Toderich, K.; Mętrak, M. Spatial and In-Depth Distribution of Soil Salinity and Heavy Metals (Pb, Zn, Cd, Ni, Cu) in Arable Irrigated Soils in Southern Kazakhstan. Agronomy 2022, 12, 1207. [Google Scholar] [CrossRef]
- Kowalska, J.B.; Nicia, P.; Gąsiorek, M.; Zadrożny, P.; Węgrzyn, M.H.; Waroszewski, J. Are natural or anthropogenic factors influencing potentially toxic elements’ enrichment in soils in proglacial zones? An example from Kaffiøyra (Oscar II Land, Spitsbergen). Int. J. Environ. Res. Public Health 2022, 19, 13703. [Google Scholar] [CrossRef]
- Ahmad, S.; Ansari, Z.; Mulhim, M. Sedimentological and mineralogical characteristics of active glacial sediments in the Indian Himalaya regions. Geol. Ecol. Landsc. 2022, 6, 265–276. [Google Scholar] [CrossRef]
- Khan, M.; Ellahi, A.; Niaz, R.; Ghoneim, M.E.; Tag-eldin, E.; Rashid, A. Water quality assessment of alpine glacial blue water lakes and glacial-fed rivers. Geomat. Nat. Hazards Risk 2022, 13, 2597–2617. [Google Scholar] [CrossRef]
- Li, H.; Taj, M.K.; Ji, X.; Zhang, Q.; Lin, L.; Zhou, Z.; Wei, Y. Bacterial communities in soil samples from the Mingyong Glacier of southwestern China. Pak. J. Pharm. Sci. 2017, 30, 689–696. [Google Scholar]
- Muhammad, S.; Ahmad, K. Heavy metal contamination in water and fish of the Hunza River and its tributaries in Gilgit–Baltistan: Evaluation of potential risks and provenance. Environ. Technol. Innov. 2020, 20, 101159. [Google Scholar] [CrossRef]
- Malkani, M.S.; Mahmood, Z. Mineral Resources of Pakistan: A Review. Dir. Gen. Geol. Surv. Pak. 2016, 128, 2–25. [Google Scholar]
- Yu, Z.; Wu, G.; Li, F.; Chen, M.; Tran, T.V.; Liu, X.; Gao, S. Glaciation enhanced chemical weathering in a cold glacial catchment, western Nyaingêntanglha Mountains, central Tibetan Plateau. J. Hydrol. 2021, 597, 126197. [Google Scholar] [CrossRef]
- Hodson, A.; Porter, P.; Lowe, A.; Mumford, P. Chemical denudation and silicate weathering in Himalayan glacier basins: Batura Glacier, Pakistan. J. Hydrol. 2002, 262, 193–208. [Google Scholar] [CrossRef]
- Amin, A.; Khan, I.U.; Amin, M.; Fatima, M.; Sajjad, W.; Shah, T.A.; Dawoud, T.M.; Wondmie, G.F. Resurrected microorganisms: A plethora of resting bacteria underway for human interaction. AMB Express 2024, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Rafiq, M.; Haleem, A.; Iqbal, N.; Khan, M.; Shah, A.A.; Hasan, F. Glaciochemistry and Pigment Producing Ability of Bacteria from the Roof of the World, the Glaciers of Karakoram, Pakistan. Geomicrobiol. J. 2023, 40, 143–151. [Google Scholar] [CrossRef]
- Liu, W.; Xu, Z.; Jiang, H.; Zhou, X.; Zhao, T.; Li, Y. Lithological and glacial controls on sulfide weathering and the associated CO2 budgets in the Tibetan Plateau: New constraints from small catchments. Geochim. Cosmochim. Acta 2023, 343, 341–352. [Google Scholar] [CrossRef]
- Paudyal, R.; Kang, S.; Huang, J.; Tripathee, L.; Zhang, Q.; Li, X.; Sillanpää, M. Insights into mercury deposition and spatiotemporal variation in the glacier and melt water from the central Tibetan Plateau. Sci. Total Environ. 2017, 599, 2046–2053. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, W.; Zhao, Y.; Zhang, H.; Shan, B. Basin-scale comprehensive assessment of cadmium pollution, risk, and toxicity in riverine sediments of the Haihe Basin in north China. Ecol. Indic. 2017, 81, 295–301. [Google Scholar] [CrossRef]
- Rafiq, M.; Hayat, M.; Zada, S.; Sajjad, W.; Hassan, N.; Hasan, F. Geochemistry and bacterial recovery from Hindu Kush Range glacier and their potential for metal resistance and antibiotic production. Geomicrobiol. J. 2019, 36, 326–338. [Google Scholar] [CrossRef]
- Sajjad, W.; Ilahi, N.; Haq, A.; Shang, Z.; Nabi, G.; Rafiq, M.; Bahadur, A.; Banerjee, A.; Kang, S. Bacteria populating freshly appeared supraglacial lake possess metals and antibiotic-resistant genes. Environ. Res. 2024, 247, 118288. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.N.; Huber, C.; Asimakopoulos, A.G.; Steinnes, E.; Mikkelsen, Ø. Trace elements and polychlorinated biphenyls (PCBs) in terrestrial compartments of Svalbard, Norwegian Arctic. Sci. Total Environ. 2019, 685, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Spolaor, A.; Moroni, B.; Luks, B.; Nawrot, A.; Roman, M.; Larose, C.; Cappelletti, D. Investigation on the sources and impact of trace elements in the annual snowpack and the Firn in the Hansbreen (Southwest Spitsbergen). Front. Earth Sci. 2021, 8, 536036. [Google Scholar] [CrossRef]
- Dhakar, K.; Pandey, A. Microbial Ecology from the Himalayan Cryosphere Perspective. Microorganisms 2020, 8, 257. [Google Scholar] [CrossRef]
- Margesin, R.; Collins, T. Microbial ecology of the cryosphere (glacial and permafrost habitats): Current knowledge. Appl. Microbiol. Biotechnol. 2019, 103, 2537–2549. [Google Scholar] [CrossRef] [PubMed]
- Hotaling, S.; Lutz, S.; Dial, R.J.; Anesio, A.M.; Benning, L.G.; Fountain, A.G.; Hamilton, T.L. Biological albedo reduction on ice sheets, glaciers, and snowfields. Earth-Sci. Rev. 2021, 220, 103728. [Google Scholar] [CrossRef]
- Giraldo, J.P.S. Application of the Geochemical Fractionation of Metals in Sediments for Environmental Analysis of a Water Reservoir. Case Riogrande Ii (Antioquia-Colombia). In Fractionation; IntechOpen: London, UK, 2018. [Google Scholar]
- Pawlak, F.; Koziol, K.; Polkowska, Z. Chemical hazard in glacial melt? The glacial system as a secondary source of POPs (in the Northern Hemisphere). A systematic review. Sci. Total Environ. 2021, 778, 145244. [Google Scholar] [CrossRef]
- Gul, J.; Muhammad, S.; Liu, S.Y.; Ullah, S.; Ahmad, S.; Hayat, H.; Tahir, A.A. Spatio-temporal changes in the six major glaciers of the Chitral River basin (Hindukush Region of Pakistan) between 2001 and 2018. J. Mt. Sci. 2020, 17, 572–587. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).