Potentially Toxic Elements in Local Cigarettes and Marijuana Leaves of Bauchi State, Nigeria: Public Health and Environmental Implications
Abstract
1. Introduction
1.1. Global Burden of PTEs
1.2. Purpose of the Study
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Preparation
2.3. Sample Digestion and Analysis
2.4. Statistical Analysis
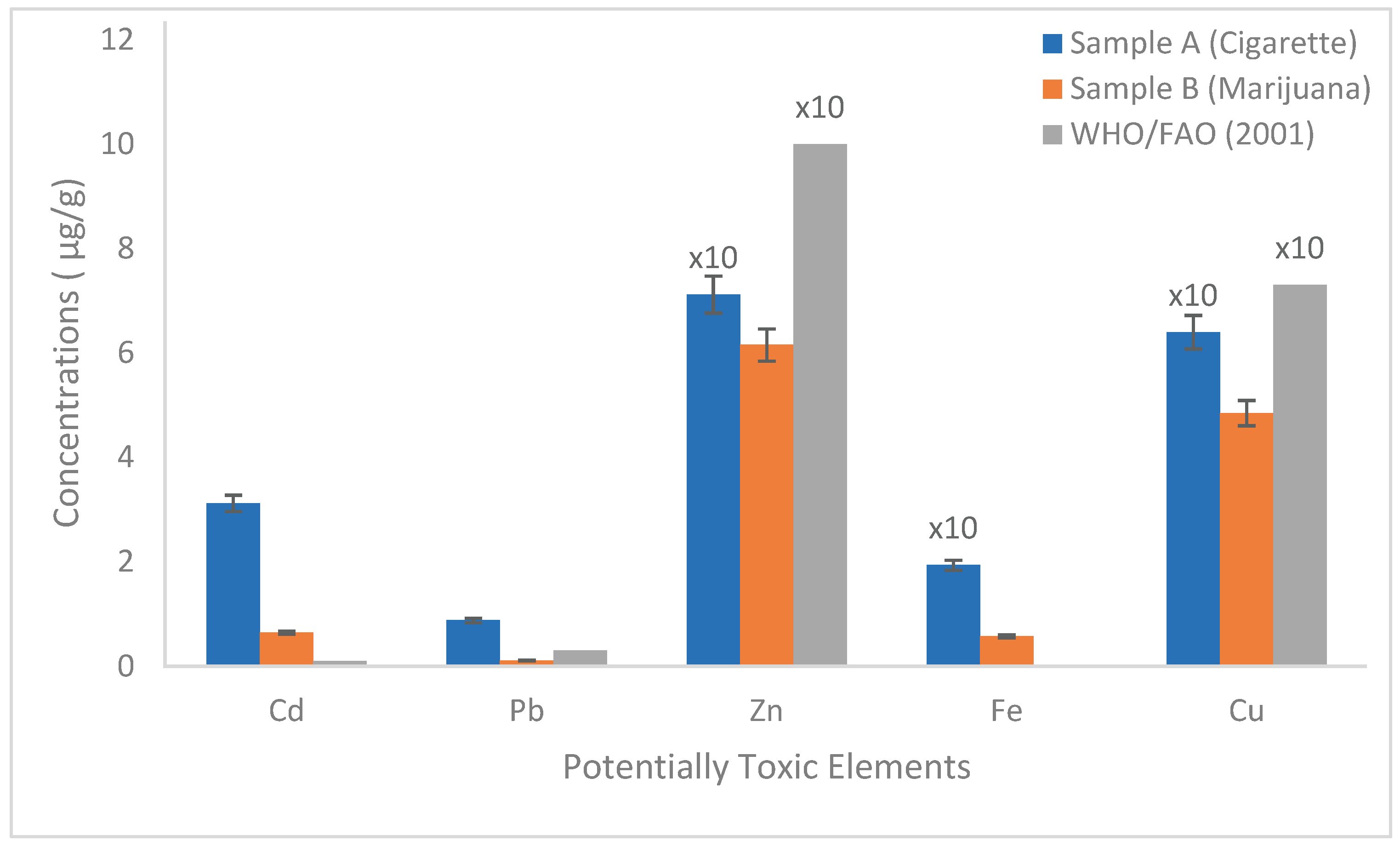
3. Results and Discussion
Study Limitations
4. Conclusions
- Strengthen and enforce quality control measures for tobacco and cannabis products to ensure PTE concentrations meet safe limits established by the WHO.
- Launch targeted campaigns to educate the public about the health risks of PTEs exposure from smoking, emphasizing Cd and Pb toxicity.
- Mitigate soil contamination by promoting clean irrigation, safe fertilizers, and regular monitoring of agricultural lands used for tobacco and cannabis cultivation.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAS | Atomic Absorption Spectroscopy |
| Cd | Cadmium |
| COPD | Chronic Obstructive Pulmonary Disease |
| Cu | Copper |
| Fe | Iron |
| FAO | Food and Agriculture Association |
| Hg | Mercury |
| IARC | International Agency for Research on Cancer |
| IQ | Intelligence Quotient |
| LMIC | Low- and Middle-Income Countries |
| Pb | Lead |
| PTE. | Potentially Toxic Elements |
| UK | United Kingdom |
| US | United States |
| WHO | World Health Organization |
| Zn | Zinc |
References
- Aliyu, A.S.; Umar, N.Y.; Sani, F.A.; Abubakar, U.U.; Umar, M.A. Epidemiological Study on the Prevalence of Cigarette Smoking and Factors Associated with It among Nursing Students at Aliko Dangote College of Nursing Sciences Bauchi State, Nigeria. ARC J. Public Health Community Med. 2020, 5, 8–17. [Google Scholar] [CrossRef]
- Masindi, V.; Muedi, K.L. Environmental Contamination by Heavy Metals. In Heavy Metals; Hosam El-Din, M., Saleh Refaat Aglan, F., Eds.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Springer: Basel, Swizerland, 2012; pp. 133–164. [Google Scholar] [CrossRef]
- Siame, T.; Muzandu, K.; Kataba, A.; M’kandawire, E. Comparative determination of human health risks associated with consumption of groundwater contaminated with lead in selected areas surrounding the former lead mine in Kabwe and non-mining areas in Lusaka, Zambia. Int. J. Community Med. Public Health 2023, 10, 4089. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Das, S.; Sultana, K.W.; Ndhlala, A.R.; Mondal, M.; Chandra, I. Heavy Metal Pollution in the Environment and Its Impact on Health: Exploring Green Technology for Remediation. Environ. Health Insights 2023, 17, 11786302231201259. [Google Scholar] [CrossRef] [PubMed]
- Siame, T.; Aminu, M.B.; Ogunsanya, T.I.; Isiaka, A.B.; Aduwa, S.; Akagbue, B.O.; Durodola, O.; Bolaji, O. A comparison of studies examining the effects of arsenic on global human health and specific regions in Nigeria. GSC Adv. Res. Rev. 2024, 18, 22–31. [Google Scholar] [CrossRef]
- Adnan, M.; Xiao, B.; Xiao, P.; Zhao, P.; Li, R.; Bibi, S. Research Progress on Heavy Metals Pollution in the Soil of Smelting Sites in China. Toxics 2022, 10, 231. [Google Scholar] [CrossRef]
- Ahmadi, M.; Akhbarizadeh, R.; Haghighifard, N.J.; Barzegar, G.; Jorfi, S. Geochemical determination and pollution assessment of heavy metals in agricultural soils of southwestern of Iran 05 Environmental Sciences 0503 Soil Sciences. J. Environ. Health Sci. Eng. 2019, 17, 657–669. [Google Scholar] [CrossRef]
- Barakat, A.; Ennaji, W.; Krimissa, S.; Bouzaid, M. Heavy metal contamination and ecological-health risk evaluation in peri-urban wastewater-irrigated soils of Beni-Mellal city (Morocco). Int. J. Environ. Health Res. 2020, 30, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Lou, Z.; Xiao, R.; Ren, Z.; Lv, X. Source analysis and source-oriented risk assessment of heavy metal pollution in agricultural soils of different cultivated land qualities. J. Clean. Prod. 2022, 341, 130942. [Google Scholar] [CrossRef]
- Xing, W.; Yang, H.; Ippolito, J.A.; Zhao, Q.; Zhang, Y.; Scheckel, K.G.; Li, L. Atmospheric deposition of arsenic, cadmium, copper, lead, and zinc near an operating and an abandoned lead smelter. J. Environ. Qual. 2020, 49, 1667–1678. [Google Scholar] [CrossRef]
- Caruso, R.V.; O’Connor, R.J.; Stephens, W.E.; Cummings, K.M.; Fong, G.T. Toxic Metal Concentrations in Cigarettes Obtained from U.S. Smokers in 2009: Results from the International Tobacco Control (ITC) United States Survey Cohort. Int. J. Environ. Res. Public Health 2013, 11, 202–217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ren, T.; Chen, X.; Ge, Y.; Zhao, L.; Zhong, R. Determination of heavy metals in cigarettes using high-resolution continuum source graphite furnace atomic absorption spectrometry. Anal. Methods 2017, 9, 4033–4043. [Google Scholar] [CrossRef]
- Musah, B.I. Effects of heavy metals and metalloids on plant-animal interaction and biodiversity of terrestrial ecosystems-an overview. Environ. Monit. Assess. 2024, 197, 12. [Google Scholar] [CrossRef]
- Okereafor, U.; Makhatha, M.; Mekuto, L.; Uche-Okereafor, N.; Sebola, T.; Mavumengwana, V. Toxic Metal Implications on Agricultural Soils, Plants, Animals, Aquatic life and Human Health. Int. J. Environ. Res. Public Health. 2020, 17, 2204. [Google Scholar] [CrossRef]
- Shahjahan, M.; Taslima, K.; Rahman, M.S.; Al-Emran, M.; Alam, S.I.; Faggio, C. Effects of heavy metals on fish physiology—A review. Chemosphere 2022, 300, 134519. [Google Scholar] [CrossRef] [PubMed]
- Angon, P.B.; Islam, S.; Kc, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Cadmium and Cadmium Compounds (Group 1) 1993: 119. Available online: https://www.inchem.org/documents/iarc/vol58/mono58-2.html (accessed on 28 July 2025).
- Tellez-Plaza, M.; Jones, M.R.; Dominguez-Lucas, A.; Guallar, E.; Navas-Acien, A. Cadmium Exposure and Clinical Cardiovascular Disease: A Systematic Review. Curr. Atheroscler. Rep. 2013, 15, 356. [Google Scholar] [CrossRef] [PubMed]
- Lanphear, B.; Navas-Acien, A.; Bellinger, D.C. Lead Poisoning. N. Engl. J. Med. 2024, 391, 1621–1631. [Google Scholar] [CrossRef]
- León, O.L.L.; Pacheco, J.M.S. Effects of Lead on Reproductive Health. In Lead Chemistry; Chooto, P., Ed.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Collins, J.F.; Klevay, L.M. Copper. Adv. Nutr. 2011, 2, 520–522. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The Essential Toxin: Impact of Zinc on Human Health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed]
- Rafati Rahimzadeh, M.; Rafati Rahimzadeh, M.; Kazemi, S.; Moghadamnia, A.R.; Ghaemi Amiri, M.; Moghadamnia, A.A. Iron; Benefits or threatens (with emphasis on mechanism and treatment of its poisoning). Hum. Exp. Toxicol. 2023, 42, 9603271231192361. [Google Scholar] [CrossRef] [PubMed]
- Mamtani, R.; Stern, P.; Dawood, I.; Cheema, S. Metals and Disease: A Global Primary Health Care Perspective. J. Toxicol. 2011, 193, 307. [Google Scholar] [CrossRef]
- Pappas, R.S.; Fresquez, M.R.; Martone, N.; Watson, C.H. Toxic Metal Concentrations in Mainstream Smoke from Cigarettes Available in the USA. J. Anal. Toxicol. 2014, 38, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Atayese, M.; Eigbadon, A.; Oluwa, K.; Adesodun, J. Heavy metal contamination of amaranthus grown along major highways in Lagos, Nigeria. Afr. Crop Sci. J. 2010, 16, 225–235. [Google Scholar] [CrossRef][Green Version]
- Anyanwu, B.O.; Ezejiofor, A.N.; Igweze, Z.N.; Orisakwe, O.E. Heavy Metal Mixture Exposure and Effects in Developing Nations: An Update. Toxics 2018, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Fasinu, P.S.; Orisakwe, O.E. Heavy Metal Pollution in Sub-Saharan Africa and Possible Implications in Cancer Epidemiology. Asian Pac. J. Cancer Prev. 2013, 14, 3393–3402. [Google Scholar] [CrossRef]
- Dryburgh, L.M.; Bolan, N.S.; Grof, C.P.; Galettis, P.; Schneider, J.; Lucas, C.J.; Martin, J.H. Cannabis contaminants: Sources, distribution, human toxicity and pharmacologic effects. Br. J. Clin. Pharmacol. 2018, 84, 2468–2476. [Google Scholar] [CrossRef]
- Nate, S. Untested, Unsafe? Cannabis Users Show Higher Lead and Cadmium Levels. Environ. Health Perspect. 2023, 131, 094001. [Google Scholar]
- Oomen, P.; Andree, R.; Rigter, S.; Van Laar, M. An Experiment with a Closed Cannabis Chain: Baseline Report on Contaminant Analysis. Ministry of Justice and Security, The Hague: South Holland, The Netherlands, 2018. Available online: https://www.government.nl/binaries/government/documenten/reports/2018/06/20/an-experiment-with-a-closed-cannabis-chain/Rapportage_Knottnerus_Closed_Cannabis_chain.pdf (accessed on 28 July 2025).
- Bengyella, L.; Kuddus, M.; Mukherjee, P.; Fonmboh, D.J.; Kaminski, J.E. Global impact of trace non-essential heavy metal contaminants in industrial cannabis bioeconomy. Toxin Rev. 2022, 41, 1215–1225. [Google Scholar] [CrossRef]
- Gatzke-Kopp, L.M.; Riis, J.L.; Ahmadi, H.; Piccerillo, H.L.; Granger, D.A.; Blair, C.B.; Thomas, E.A. Environmental tobacco smoke exposure is associated with increased levels of metals in children’s saliva. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.P.; Novirsa, R.; Jeong, H.; Nugraha, W.C.; Addai-Arhin, S.; Viet, P.H.; Tominaga, N.; Ishibashi, Y.; Arizono, K. Mercury, cadmium, and lead in cigarettes from international markets: Concentrations, distributions and absorption ability of filters. J. Toxicol. Sci. 2021, 46, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.W. Levels of Heavy Metals in Popular Cigarette Brands and Exposure to These Metals via Smoking. Sci. World J. 2012, 2012, 729430. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, R.J.; Schneller, L.M.; Caruso, R.V.; Stephens, W.E.; Li, Q.; Yuan, J.; Fong, G.T. Toxic metal and nicotine content of cigarettes sold in China, 2009 and 2012. Tob. Control. 2015, 24, iv55–iv59. [Google Scholar] [CrossRef] [PubMed]
- Raju, N.J.; Kofod, M.; Isenbeck-Schröter, M.; Müller, G. Heavy metal content of Indian cigarettes. Toxicol. Environ. Chem. 1999, 72, 215–219. [Google Scholar] [CrossRef]
- WorldWeather. Bauchi, Nigeria, Minimum and Maximum Forecast Temperature Annually. 2024. Available online: https://www.worldweatheronline.com/bauchi-weather/bauchi/ng.aspx (accessed on 28 July 2025).
- Altmaier, S. The Big Four Heavy Metals in Cannabis: Sample Preparation and Analysis via ICP-MS. Cannabis Sci. Technol. 2021, 4, 24–29. Available online: https://www.cannabissciencetech.com/view/the-big-four-heavy-metals-in-cannabis-sample-preparation-and-analysis-via-icp-ms (accessed on 28 July 2025).
- Sharma, A.; Sharma, V. Forensic analysis of cigarette ash using ATR-FTIR spectroscopy and chemometric methods. Microchem. J. 2022, 178, 107406. [Google Scholar] [CrossRef]
- Siame, T.; Muzandu, K.; Kataba, A.; Weisiyu, Q.; M’kAndawire, E. A comparative assessment of Lead (Pb) concentration and physicochemical parameters in groundwater from the Kabwe mine and Lusaka non-mine sites, Zambia. Discov. Environ. 2024, 2, 101. [Google Scholar] [CrossRef]
- Hina, B.; Rizwani, G.; Naseeb, U.; Huma, A.; Hyder, Z. Application of Atomic Absorption Spectroscopy to determine Mineral and Heavy Metal distribution level of Medicinal Plants. J. Anal. Tech. Res. 2023, 5, 26–32. [Google Scholar] [CrossRef]
- Anal, J.M.H.; Chase, P. Trace elements analysis in some medicinal plants using graphite furnace-atomic absorption spectroscopy. Environ. Eng. Res. 2016, 21, 247–255. [Google Scholar] [CrossRef]
- Meier, E.; Vandrey, R.; Rubin, N.; Pacek, L.R.; Jensen, J.A.; Donny, E.C.; Hecht, S.S.; Carmella, S.G.; Murphy, S.E.; Luo, X.; et al. Cigarette Smokers Versus Cousers of Cannabis and Cigarettes: Exposure to Toxicants. Nicotine Tob. Res. 2020, 22, 1383–1389. [Google Scholar] [CrossRef]
- WHO Food Additives and Contaminants. Joint Codex Alimentarius Commission, FAO/WHO Food Standards Programme; ALINORM 01/12A; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Aftab, K.; Iqbal, S.; Khan, M.R.; Busquets, R.; Noreen, R.; Ahmad, N.; Kazimi, S.G.T.; Karami, A.M.; Al Suliman, N.M.S.; Ouladsmane, M. Wastewater-Irrigated Vegetables Are a Significant Source of Heavy Metal Contaminants: Toxicity and Health Risks. Molecules 2023, 28, 1371. [Google Scholar] [CrossRef]
- Weggler, K.; McLaughlin, M.J.; Graham, R.D. Effect of Chloride in Soil Solution on the Plant Availability of Biosolid-Borne Cadmium. J. Environ. Qual. 2004, 33, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Silbergeld, E. Facilitative mechanisms of lead as a carcinogen. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2003, 533, 121–133. [Google Scholar] [CrossRef]
- Yabe, J.; Nakayama, S.M.; Ikenaka, Y.; Yohannes, Y.B.; Bortey-Sam, N.; Oroszlany, B.; Muzandu, K.; Choongo, K.; Kabalo, A.N.; Ntapisha, J. Lead poisoning in children from townships in the vicinity of a lead–zinc mine in Kabwe, Zambia. Chemosphere 2015, 119, 941–947. [Google Scholar] [CrossRef]
- Atta, M.I.; Zehra, S.S.; Dai, D.-Q.; Ali, H.; Naveed, K.; Ali, I.; Sarwar, M.; Ali, B.; Iqbal, R.; Bawazeer, S.; et al. Amassing of heavy metals in soils, vegetables and crop plants irrigated with wastewater: Health risk assessment of heavy metals in Dera Ghazi Khan, Punjab, Pakistan. Front. Plant Sci. 2023, 13, 1080635. [Google Scholar] [CrossRef]
- Srivastava, V.; Sarkar, A.; Singh, S.; Singh, P.; de Araujo, A.S.F.; Singh, R.P. Agroecological Responses of Heavy Metal Pollution with Special Emphasis on Soil Health and Plant Performances. Front. Environ. Sci. 2017, 5, 64. [Google Scholar] [CrossRef]
- Needleman, H. Lead poisoning. Annu. Rev. Med. 2004, 55, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J. Zinc in Human Nutrition. Nutr. Res. Rev. 1988, 1, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, M.J.; Zubillaga, M.; Lysionek, A.; Sarabia, M.I.; Caro, R.; De Paoli, T.; Hager, A.; Weill, R.; Boccio, J. Zinc as an essential micronutrient: A review. Nutr. Res. 2000, 20, 737–755. [Google Scholar] [CrossRef]
- Pourkhabbaz, A.; Pourkhabbaz, H. Investigation of toxic metals in the tobacco of different Iranian cigarette brands and related health issues. Iran. J. Basic Med. Sci. 2012, 15, 636–644. [Google Scholar]
- Aboubakar, A.; Douaik, A.; Mewouo, Y.C.M.; Madong, R.C.B.A.; Dahchour, A.; El Hajjaji, S. Determination of background values and assessment of pollution and ecological risk of heavy metals in urban agricultural soils of Yaoundé, Cameroon. J. Soils Sediments 2021, 21, 1437–1454. [Google Scholar] [CrossRef]
- Alloway, B.J. Zinc in Soils and Crop Nutrition, 2nd ed.; International Zinc Association; International Fertilizer Association (IFA): Brussels, Belgium; Paris, France, 2008. [Google Scholar]
- Liehr, J.; Jones, J. Role of Iron in Estrogen-Induced Cancer. Curr. Med. Chem. 2001, 8, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Sisodia, R. Heavy Metal Contamination of Medicinal Plants in India—A Perspective. Asian J. Water Environ. Pollut. 2023, 20, 9–17. [Google Scholar] [CrossRef]
- USEPA. Aquatic Life Criteria—Iron. Environmental Protection Agency. 2023. Available online: https://www.epa.gov/wqc/aquatic-life-criteria-and-methods-toxics (accessed on 28 July 2025).
- Yang, Y.; Wu, J.; Wang, L.; Ji, G.; Dang, Y. Copper homeostasis and cuproptosis in health and disease. MedComm 2024, 5, e724. [Google Scholar] [CrossRef] [PubMed]
- Giller, K.E.; Witter, E.; McGrath, S.P. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol. Biochem. 1998, 30, 1389–1414. [Google Scholar] [CrossRef]
- Zerihun, A.; Chandravanshi, B.S.; Debebe, A.; Mehari, B. Levels of selected metals in leaves of Cannabis sativa L. cultivated in Ethiopia. SpringerPlus 2015, 4, 359. [Google Scholar] [CrossRef]
- Proshad, R.; Zhang, D.; Uddin, M.; Wu, Y. Presence of cadmium and lead in tobacco and soil with ecological and human health risks in Sichuan province, China. Environ. Sci. Pollut. Res. Int. 2020, 27, 18355–18370. [Google Scholar] [CrossRef] [PubMed]
- Järup, L.; Akesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Fine, J.M.; Nadas, A. Metallic particles in inhaled air and their effects on health. Environ. Health Perspect. 2012, 120, 1669–1674. [Google Scholar]

| Equipment | Model | Manufacturer |
|---|---|---|
| Muffle furnance | Lenton ECF 12/10 | Gallenkamp, Chicago, IL, USA |
| Mortar and pestle | Wood | Locally made |
| Hot plate | Oven BS (Welland series) | Gallenkamp, Chicago, IL, USA |
| Beaker | Glass 1000 series | Pyrex, Sunderland, UK |
| Volumetric flask | Glass Class A | Pyrex, Sunderland, UK |
| Spatula | Metal 2082 | BJB Enterprises, Tustin, CA, USA |
| Weighing balance | Mettle PC 400 | Gallenkamp, Chicago, IL, USA |
| Substance | Heavy Metal Concentration (µg/g) | Source of Contamination | Health Effects | Citation |
|---|---|---|---|---|
| Marijuana | Pb: 7.9–10.2; Cd: 3.2–4.7 | Absorbed from soil during plant growth | Cancer, cognitive impairment, cardiovascular disease | [65] |
| Cigarettes | Pb: 0.44–2.64; Cd: 0.86–1.81; As: 0.17–0.86 | Soil contamination, fertilizers used in tobacco cultivation | Lung cancer, cardiovascular diseases, neurological disorders | [5,66] |
| Cigarettes | Pb: 0.88; Cd: 3.12; Cu: 64, Zn: 71.2, Fe: 19.2 | Application of additives during tobacco processing. Phosphate fertilizer and pesticides use during tobacco cultivation | Causes serious long-term health effects, especially via inhalation | Current study |
| Marijuana | Pb: 0.11; Cd: 0.645; Cu: 4.85, Zn: 6.155, Fe: 0.575 | Low contamination | Causes serious long-term health effects, especially via inhalation | Current study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siame, T.; Abolade, Y.A.; Omotayo, F.; Nyarko, A.J.; Aminu, M.B.; Ogwurumba, U.A.; Akagbue, B.O.; Abdulmalik, F.; Zabidi, H. Potentially Toxic Elements in Local Cigarettes and Marijuana Leaves of Bauchi State, Nigeria: Public Health and Environmental Implications. Pollutants 2025, 5, 26. https://doi.org/10.3390/pollutants5030026
Siame T, Abolade YA, Omotayo F, Nyarko AJ, Aminu MB, Ogwurumba UA, Akagbue BO, Abdulmalik F, Zabidi H. Potentially Toxic Elements in Local Cigarettes and Marijuana Leaves of Bauchi State, Nigeria: Public Health and Environmental Implications. Pollutants. 2025; 5(3):26. https://doi.org/10.3390/pollutants5030026
Chicago/Turabian StyleSiame, Tasha, Yisa Adeniyi Abolade, Famodu Omotayo, Albert Junior Nyarko, Mu’awiya Baba Aminu, Uchechukwu Anthony Ogwurumba, Bertha Onyenachi Akagbue, Fatima Abdulmalik, and Hareyani Zabidi. 2025. "Potentially Toxic Elements in Local Cigarettes and Marijuana Leaves of Bauchi State, Nigeria: Public Health and Environmental Implications" Pollutants 5, no. 3: 26. https://doi.org/10.3390/pollutants5030026
APA StyleSiame, T., Abolade, Y. A., Omotayo, F., Nyarko, A. J., Aminu, M. B., Ogwurumba, U. A., Akagbue, B. O., Abdulmalik, F., & Zabidi, H. (2025). Potentially Toxic Elements in Local Cigarettes and Marijuana Leaves of Bauchi State, Nigeria: Public Health and Environmental Implications. Pollutants, 5(3), 26. https://doi.org/10.3390/pollutants5030026









