1. Introduction
Dioxins, a class of persistent organic pollutants (POPs), are hazardous environmental pollutants characterized by high toxicity, strong lipophilicity, and a pronounced tendency to bioaccumulate in human and animal tissues [
1]. Dioxins and related compounds have been widely detected in various environmental matrices including ambient air, soil, water, and biota, with concentrations varying according to local pollution sources and environmental conditions. Background levels in unpolluted areas typically range from sub-picogram to low picogram levels per cubic meter in air and per gram in soil and sediment, whereas industrial zones, waste incinerators, and contaminated sites often exhibit concentrations orders of magnitude higher (e.g., 1–100 pg TEQ/m
3 in air, >1000 pg TEQ/g in soils) [
2,
3]. These compounds bioaccumulate in the food chain, especially in fatty tissues of animals, leading to elevated levels in animal-derived foods such as fish, meat, and dairy products from polluted regions [
4]. Understanding this spatial variability is crucial for assessing human exposure and guiding environmental regulations. The term ‘dioxins’ encompasses a group of chemically related compounds, including polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs), which are by-products of various industrial and combustion processes, including waste incineration, metal smelting, chlorine bleaching of paper pulp, and the production of certain chlorinated chemicals such as herbicides and disinfectants [
5]. Although these compounds are grouped under the umbrella of dioxin-like compounds due to their shared mechanism of toxicity via the aryl hydrocarbon receptor (AhR), PCDDs, PCDFs, and dioxin-like PCBs differ significantly in their congener-specific toxicity, persistence, and sources. PCDDs and PCDFs are primarily by-products of combustion, while dioxin-like PCBs, such as PCB-126, were intentionally manufactured and often persist in electrical equipment and building materials. Importantly, the toxic equivalency factors (TEFs) assigned to these groups reflect differences in their potency and health risks, necessitating separate consideration in exposure assessment and risk characterization [
6,
7]. Clarifying these distinctions enhances the understanding of exposure sources and informs targeted public health interventions [
6,
7]. Additionally, dioxin-like polychlorinated biphenyls (dl-PCBs) share similar toxicological properties—primarily through AhR activation—but differ in chemical structure, sources, and congener profiles. While PCDDs and PCDFs predominantly originate from combustion-related activities, dl-PCBs were widely used as industrial chemicals before their ban and persist in the environment due to their stability. Polychlorinated biphenyls (PCBs) are a group of 209 synthetic chlorinated compounds once extensively used as dielectric fluids in transformers, capacitors, and other electrical equipment. Only a subset of 12 congeners, known as dioxin-like PCBs (dl-PCBs), exhibit toxicological behavior similar to dioxins through AhR-mediated pathways, while the majority of PCBs do not possess this dioxin-like activity [
8]. These compounds are unintentionally generated as by-products of industrial activities and waste incineration, resulting in widespread environmental dissemination. Human exposure to dioxins occurs predominantly through dietary intake—especially via the consumption of animal fat-rich foods—although inhalation and dermal absorption also represent relevant exposure pathways. Concentrations of dioxins in food items vary significantly depending on the pollution level of the environment. In unpolluted regions, levels in animal fat generally remain below 1 pg TEQ/g lipid, whereas in contaminated areas such as industrial zones and hotspots, concentrations can exceed 20 pg TEQ/g lipid, especially in fish and dairy products [
3,
4]. Human biomonitoring reflects this variability, with breast milk concentrations ranging globally from 5 to 20 pg TEQ/g lipid in general populations, and higher values in populations residing near pollution sources [
4]. Due to their lipophilic nature, dioxins accumulate in maternal adipose tissue and can be transferred to the fetus via the placenta and to the infant through breast milk. Beyond their toxicological and bioaccumulative profiles, dioxins are also classified as endocrine-disrupting chemicals (EDCs), exogenous compounds that interfere with endogenous hormonal signaling mechanisms [
6,
7]. Although dioxins have been described as EDCs, it is important to clarify that their primary mode of action is mediated via the aryl hydrocarbon receptor (AhR) pathway, rather than classical estrogen receptor signaling. Binding to AhR triggers transcriptional changes that modulate xenobiotic metabolism and disrupt multiple physiological processes, including development and immune function [
6,
7]. Interactions between the aryl hydrocarbon receptor (AhR) and estrogen receptor signaling pathways may contribute to estrogen-like effects observed in vitro, such as the stimulation of uterine epithelial proliferation in certain biological contexts [
9]. This nuanced understanding helps reconcile diverse toxicological profiles of dioxins and informs risk assessment frameworks. In vitro studies have demonstrated estrogenic properties, such as stimulating uterine epithelial cell proliferation [
10]. EDCs, including dioxins, are increasingly acknowledged for their ability to interfere with developmental and reproductive processes, even at low, environmentally relevant exposure levels [
11]. Among the various exposure pathways, lactational transfer via breast milk represents a particularly sensitive and direct route for infant exposure to dioxins during critical developmental windows. Given the well-documented vulnerability of the neonatal period, the presence of lipophilic toxicants in breast milk warrants focused investigation. Similarly, a study by Yang et al. [
12] in Korea compared dioxin and PCB concentrations in breast milk from mothers in two regions with different industrial exposure. The findings revealed significantly higher levels of these compounds in mothers residing in more industrialized areas, raising concerns regarding infant intake exceeding WHO tolerable thresholds.
A number of reviews have compiled international data on dioxin and organochlorine pollution in human breast milk across diverse geographical regions. For instance, Ulaszewska et al. [
13] reviewed data from over 20 countries and revealed marked geographical variation in the levels of PCDD/Fs and dl-PCBs, with higher concentrations commonly observed in industrialized areas. Similarly, Helou et al. [
14] and Klincic et al. [
15] reported significant levels of organochlorine pesticides and PCBs in breast milk from Lebanon and Croatia, respectively, highlighting regional exposure differences and the influence of environmental and regulatory factors. In their systematic review, Meghdad et al. [
16] confirmed the global presence of these compounds in maternal milk and emphasized the potential health implications for infants. More recently, Lehmann et al. [
17] and Brambilla et al. [
18] provided updated evidence on the presence of multiple environmental pollutants in breast milk, including EDCs, and assessed the implications for neurodevelopment and early-life exposure risk. Finally, the 2024 review by Serreau et al. [
1] offered a comprehensive and updated synthesis of current data, reinforcing the persistence of these pollutants in maternal milk despite regulatory efforts.
Numerous studies have documented the presence of dioxins in human breast milk, raising concerns about potential health risks to nursing infants. Concentrations of dioxins in human breast milk exhibit substantial geographic variation, reflecting local environmental pollution and regulatory history. In minimally polluted regions, average levels typically remain below 10 pg TEQ/g lipid, while in industrialized or contaminated areas, values exceeding 20 pg TEQ/g lipid have been reported, raising concerns about infant exposure surpassing WHO tolerable intake limits [
1]. Continuous biomonitoring and harmonization of analytical protocols remain essential to track exposure trends and inform public health interventions. For example, research conducted in industrialized and urban areas of the UK and Europe has found detectable levels of dioxins and polychlorinated biphenyls (PCBs) in breast milk, with environmental exposure from waste incinerators and industrial activities contributing significantly to maternal body burden [
19], many of which, like dioxins, act as EDCs [
11]. Similarly, research in Italy’s Campania region, an area affected by illegal waste disposal and burning, reported significant correlations between environmental dioxin risk factors and dioxin levels in breast milk [
19]. Supporting these findings, Giovannini et al. [
20] assessed dioxin levels in breast milk samples from women in Caserta and Naples and identified key environmental risk factors, including illegal waste burning and regional pollution sources. Building on this evidence, Rivezzi et al. [
21] developed a general model of dioxin pollution based on breast milk samples from 94 women in the same areas, demonstrating that elevated dioxin and dl-PCB levels were associated with proximity to waste combustion sites and maternal age.
Dietary habits have also been implicated in maternal dioxin exposure. The Norwegian Mother and Child Cohort Study (MoBa) demonstrated that higher maternal intake of dioxins and PCBs during pregnancy was associated with reduced birth weight and length in newborns [
22]. Additionally, the European NewGeneris study found that maternal diets rich in red and white meat, low-fat dairy, and fast food were linked to higher dioxin levels in maternal and cord blood, as well as reduced gestational age and birth weight [
23]. Consistent with these findings, a study by Schecter et al. [
24] in Bien Hoa City, Vietnam, a known dioxin hotspot, confirmed that food intake, particularly of animal-origin products, was the predominant source of human exposure to dioxins, underscoring the critical role of diet in dioxin body burden.
Conversely, some studies have not found a significant association between maternal dioxin exposure and adverse infant outcomes. For example, a large-scale longitudinal study in Japan by Ae et al. [
25] reported a consistent and significant decline in dioxin concentrations in human breast milk from 1998 to 2015, suggesting that national regulatory efforts and industrial reforms effectively reduced maternal exposure over time. Furthermore, research in Vietnam indicated that while dioxin levels in breast milk were higher in areas near pollution hotspots, the concentrations were within ranges found in other regions, and the health implications for infants remained unclear [
26]. Complementing these findings, Nguyet Anh et al. [
27] identified specific maternal risk factors—including longer residence in affected areas, higher maternal age, and elevated BMI—as being significantly associated with increased dioxin concentrations in breast milk collected from mothers living in a well-documented dioxin hotspot in Vietnam. This term refers to highly contaminated areas such as Bien Hoa, where extensive use of Agent Orange during the Vietnam War has led to long-standing environmental dioxin pollution, confirmed by multiple environmental and biomonitoring studies.
Given the heterogeneity of findings in the literature, there is a pressing need for an integrative narrative review that explores maternal dioxin exposure—via dietary and environmental routes—and its implications for early-life transfer through lactation. Although numerous studies have addressed aspects of dioxin toxicity, bioaccumulation, and infant exposure, the evidence remains fragmented across populations, geographies, and methodologies. This review aims to critically examine current knowledge, highlight major conceptual and empirical gaps, and offer a contextualized understanding of exposure pathways and health implications. By synthesizing diverse findings, we seek to inform future research priorities and public health strategies, particularly in light of growing concern over endocrine-disrupting compounds during sensitive developmental windows [
28,
29,
30].
To ensure comparability across studies and strengthen risk assessment frameworks, standardized analytical protocols for detecting dioxins in human breast milk are essential. Commonly used techniques include high-resolution gas chromatography coupled with high-resolution mass spectrometry (HRGC-HRMS), which remains the gold standard due to its sensitivity and congener specificity. Emerging methods, such as bioassay-based screening tools (e.g., the CALUX assay), offer cost-effective alternatives for preliminary evaluation, particularly in resource-limited settings. However, significant inter-laboratory variation in sample preparation, lipid adjustment, and expression of results (e.g., pg TEQ/g lipid) continues to hinder data harmonization and cross-regional comparison. A clearer focus on methodological transparency and harmonized reporting metrics is needed to improve the interpretability and global comparability of biomonitoring data.
Novel Contributions of the Review
This narrative review offers both conceptual and methodological contributions to the existing literature on lactational exposure to dioxins and dl-PCBs.
Conceptually, it expands the traditional geographic scope of reviews on this topic by integrating findings from underrepresented regions such as Southeast Asia (Vietnam), sub-Saharan Africa (Uganda), and Southern Europe (Italy). These regions are often excluded or underexplored in systematic reviews that focus predominantly on high-income countries with robust environmental monitoring systems. This inclusion enables a more globally balanced perspective on exposure risks and emphasizes the influence of socioeconomic and regulatory disparities.
Methodologically, while adhering to PRISMA 2020 guidelines for transparency and rigor, this review adopts a flexible narrative approach that enables synthesis across diverse study designs and exposure pathways, including the integration of the following:
- ▪
Dietary exposure assessments;
- ▪
Spatial modeling of environmental pollution;
- ▪
Longitudinal biomonitoring data;
- ▪
Mixed exposure–outcome frameworks (e.g., combining neurodevelopmental, growth, and endocrine outcomes).
This broader approach allows for the identification of multi-factorial exposure determinants (e.g., maternal age, BMI, residence duration, dietary patterns) that might be overlooked in narrowly focused systematic reviews. Thus, the review not only summarizes concentrations of dioxins in breast milk but also contextualizes them within social, environmental, and regulatory settings, offering practical insights for public health surveillance and risk communication, especially in regions with limited biomonitoring infrastructure.
2. Materials and Methods
This narrative review employed a structured literature search and selection process aimed at identifying relevant studies examining concentrations of dioxins and dioxin-like PCBs in human breast milk, as well as maternal and environmental determinants influencing exposure.
This structured narrative review incorporates selected elements of systematic methodology (e.g., database searching, inclusion criteria, independent screening) to enhance transparency and reproducibility, without fully adhering to the PRISMA framework.
Study selection was conducted in accordance with the PRISMA 2020 guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) to enhance transparency and reproducibility.
2.1. Search Strategy and Data Sources
A structured search was conducted across four major databases: PubMed, Scopus, Web of Science, and Embase. In addition, Google Scholar was consulted in a supplementary capacity to identify potentially relevant grey or uncaptured literature referenced in the included studies. The search covered publications from January 2000 to March 2024. Only English-language publications were considered due to limitations in translation resources and to ensure consistency in data extraction and evaluation. The start date was selected to ensure the inclusion of recent and methodologically robust studies reflecting current environmental conditions, analytical capabilities, and regulatory frameworks relevant to dioxin exposure and human biomonitoring.
The search strategy used combinations of the following keywords: “dioxins,” “PCDD/Fs,” “PCBs,” “breast milk,” “human milk,” “maternal exposure,” “infant exposure,” “maternal diet,” “infant health,” “infant development,” “dietary exposure,” “bioaccumulation,” and “environmental pollution.” In addition to electronic database searches, the reference lists of all included studies were manually screened to identify further relevant literature not captured through initial queries.
No grey literature or non-peer-reviewed sources (e.g., journalistic investigations, institutional reports) were included in the final synthesis to reduce potential bias and enhance scientific rigor.
TOXLINE, now discontinued, was replaced by toxicology records indexed in PubMed and Embase.
While maintaining the descriptive scope of a narrative review, the inclusion of reproducible selection criteria and multiple reviewers enhanced methodological transparency. The goal was to capture a broad spectrum of findings and perspectives that contribute to understanding the presence of dioxins in human breast milk and associated maternal and environmental risk factors.
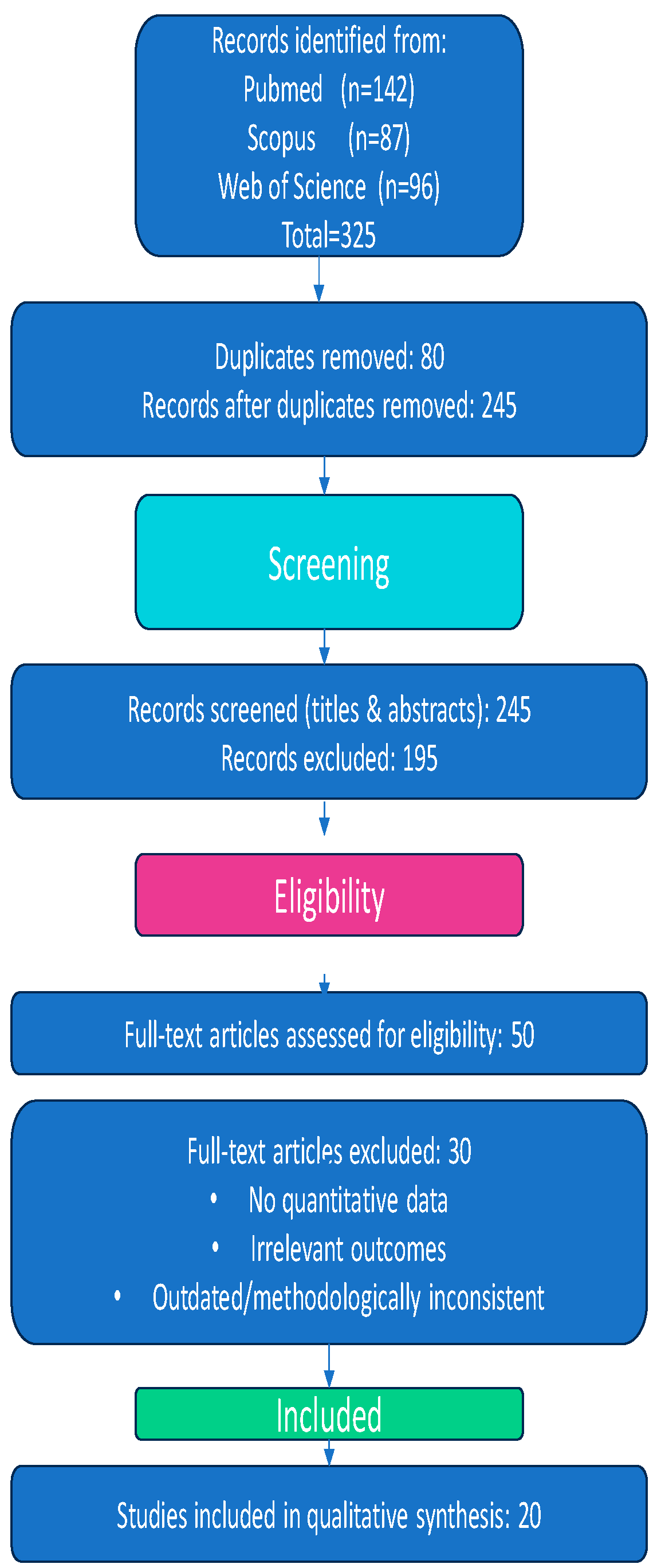
The initial search yielded 325 records:
- ▪
PubMed (n = 142);
- ▪
Scopus (n = 87);
- ▪
Web of Science (n = 96).
2.2. Screening and Eligibility
After removing 80 duplicate records, 245 unique records remained and were screened based on titles and abstracts.
Exclusion criteria were as follows:
- ▪
Non-human or in vitro studies;
- ▪
Reviews, editorials, or conference abstracts;
- ▪
Articles not published in English, due to lack of access to certified translation resources and to ensure methodological consistency in data interpretation.
Following abstract screening, 195 articles were excluded for not meeting eligibility criteria. The remaining 50 full-text articles were assessed in detail. At this stage, 30 additional studies were excluded for the following reasons:
- ▪
Lack of quantitative data on dioxins or dl-PCBs in breast milk;
- ▪
Lack of data linking exposure to maternal, dietary, or environmental determinants;
- ▪
Use of outdated or methodologically inconsistent approaches.
2.3. Review Process and Quality Control
To enhance rigor and minimize potential bias, the screening and eligibility assessment of titles, abstracts, and full texts were independently performed by at least two researchers. Discrepancies or disagreements during the review process were resolved through discussion, and when necessary, by consultation with a third reviewer to reach consensus. This approach ensured methodological consistency and reliability in study selection and data extraction.
2.4. Risk of Bias (Quality) Assessment
Due to the narrative nature of this review, formal risk-of-bias assessments utilizing standardized tools such as ROBINS-I or the Newcastle–Ottawa Scale were not performed. However, to ensure the reliability and rigor of the review process, study selection and data extraction were conducted independently by at least two reviewers, with any discrepancies resolved through discussion or, when necessary, by adjudication of a third reviewer. The methodological quality of included studies was qualitatively appraised during data synthesis, taking into consideration key factors including study design, sample size, exposure assessment methodologies, and the clarity and comprehensiveness of reporting. Records were systematically managed using spreadsheets instead of dedicated reference management software. Of the 325 records initially identified, 80 duplicates were removed, and 245 records underwent title and abstract screening, which resulted in the exclusion of 195 studies. Subsequently, 50 full-text articles were evaluated for eligibility, and 30 studies excluded based on predefined criteria. Ultimately, 20 studies were included in the final qualitative synthesis.
2.5. PRISMA Statement and Flowchart
Figure 1 summarizes the flow of study selection across all screening stages and provides reasons for exclusion.
2.5.1. Final Inclusion
A total of 20 studies met the predefined inclusion criteria and were incorporated into the final qualitative synthesis. These studies represent a diverse geographic and methodological spectrum, providing data on dioxin concentrations in human breast milk and related maternal or environmental factors from countries including Italy, Vietnam, Norway, the United Kingdom, Japan, and South Korea.
The exclusion of non-English publications, particularly those in Spanish, is acknowledged as a potential limitation that may have resulted in the omission of relevant evidence, especially from Latin American regions.
2.5.2. Inclusion Criteria
- ▪
“Peer-reviewed original research”
- ▪
“Quantitative measurement of polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), or dioxin-like polychlorinated biphenyls (dl-PCBs) in human breast milk”
- ▪
“Assessment of maternal characteristics, environmental exposure, or dietary sources”
- ▪
“Published in English between 2000 and 2024”
Furthermore, unlike traditional systematic reviews that focus exclusively on high-income countries, this review intentionally included studies from regions with limited environmental monitoring (e.g., sub-Saharan Africa, Southeast Asia), in order to better capture global exposure disparities and maternal vulnerability factors.
3. Results
This review synthesizes findings from twenty peer-reviewed studies published between 2002 and 2024 that investigated concentrations of polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), and dl-PCBs in human breast milk, along with associated maternal, dietary, and environmental determinants.
In a Korean study, Yang et al. [
12] measured PCDD/F and PCB levels in breast milk samples collected from mothers residing in two regions with distinct environmental profiles. Higher concentrations were observed in the urban–industrialized area compared to those in the rural reference area. These findings underscore the influence of urban pollution and maternal dietary habits, particularly fish and meat consumption, on maternal body burden and estimated infant intake [
12].
A global assessment by Fiedler et al. [
19] analyzed persistent organic pollutant (POP) concentrations in breast milk samples collected from primiparous women across multiple regions, including areas with varying levels of industrialization and regulatory controls. The study identified marked regional disparities, with higher levels of dioxins and dl-PCBs in countries undergoing rapid industrial development or with limited environmental monitoring infrastructure. Correlation analyses revealed strong associations between contaminant levels and factors such as dietary patterns, environmental regulations, and national emission profiles. These findings not only reinforce the importance of spatial variability in exposure but also highlight the need for continued international biomonitoring efforts targeting lactational transfer.
Giovannini et al. [
20] investigated dioxin pollution in Italy’s Campania region, with a focus on areas historically impacted by illegal waste disposal. Analysis of breast milk samples from 94 women revealed elevated dioxin concentrations, particularly among those living near known waste-burning sites. Significant associations were identified between contaminant levels and maternal age as well as duration of residence in high-risk areas [
20].
A complementary study by Rivezzi et al. [
21], conducted in the same region, developed a spatial model of dioxin dispersion using breast milk data and environmental risk indicators. The findings supported the hypothesis of localized pollution, with elevated dioxin and dl-PCB concentrations observed in samples from women residing near combustion sources. The study highlighted cumulative exposure patterns driven by both environmental conditions and demographic characteristics [
21].
In the Norwegian Mother and Child Cohort Study, Papadopoulou et al. [
22] evaluated maternal dietary intake of dioxins and PCBs during pregnancy and its association with neonatal outcomes. The study found that higher intake of these compounds—particularly through fatty fish and meat—was significantly associated with reduced birth weight and length, indirectly suggesting that breast milk may contribute to continued postnatal exposure [
22].
Building upon this dietary perspective, Papadopoulou et al. [
23] examined data from the European NewGeneris cohort. The study found that maternal diets high in processed meat, low-fat dairy, and fast food were associated with elevated dioxin biomarker concentrations in both maternal and cord blood. Although the study did not focus exclusively on breast milk, the findings emphasized dietary intake as a primary route of maternal exposure, with potential implications for postnatal transfer [
23].
Costopoulou et al. [
31] conducted a detailed dietary exposure assessment in Greece, focusing on infant intake of dioxins and dl-PCBs through breast milk consumption. Their study combined national food pollution data with breastfeeding patterns to estimate that exclusively breastfed infants, particularly in regions with high fish consumption, may receive dioxin doses exceeding the tolerable daily intake levels. This highlighted regional dietary habits as a critical factor in infant exposure and underscored the need for ongoing biomonitoring and targeted risk communication strategies among breastfeeding mothers in areas with elevated environmental pollution.
Additionally, a large meta-analysis conducted by Govarts et al. [
32] within twelve European birth cohorts examined prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE), focusing on birth weight outcomes. The study reported a consistent inverse association between prenatal exposure to these persistent organic pollutants and infant birth weight, suggesting that even relatively low levels of exposure during pregnancy could negatively affect fetal growth and development.
In Japan, Ae et al. [
25] conducted a large-scale longitudinal survey monitoring dioxin concentrations in breast milk from 1998 to 2015. The study documented a consistent and statistically significant decline in contaminant levels over time. These reductions were attributed to strengthened national regulations and shifts in industrial activity, illustrating the positive impact of environmental policy on reducing maternal dioxin burden [
25].
In Vietnam, Tai et al. [
26] examined neurodevelopmental outcomes among infants exposed to dioxins via breastfeeding in a known dioxin hotspot. Although breast milk samples exhibited elevated dioxin concentrations, the study found inconclusive associations with early neurodevelopmental indicators. Nonetheless, it highlighted the necessity for further longitudinal research to evaluate potential long-term effects in exposed populations [
26]. Similarly, Patandin et al. [
33] reported that environmental exposure to PCBs and dioxins was associated with reduced cognitive abilities in children at 42 months, highlighting potential neurodevelopmental risks related to early-life exposure.
Similarly, Nishijo et al. [
34] conducted both cross-sectional and longitudinal studies in the same region, revealing that perinatal exposure to elevated TCDD levels was associated with significantly lower infant body weight and length, particularly among boys with higher TEQ-PCDD/Fs concentrations in breast milk. These findings reinforce the evidence for early postnatal growth retardation associated with dioxin exposure in contaminated areas.
In a more recent multi-country cohort study, Yim et al. [
35] assessed the effects of prenatal exposure to dioxins and PCBs on neurodevelopmental outcomes at six months of age, applying multi-pollutant modeling approaches. They reported that even low-level prenatal exposure to these compounds may be linked to subtle neurodevelopmental delays, underscoring early-life vulnerability to persistent organic pollutants and the need to consider cumulative and combined exposures in risk assessment models.
Moreover, Ames et al. [
36] conducted a comprehensive review of early-life exposure to per- and polyfluoroalkyl substances (PFAS) and its implications for child neurodevelopment. Although PFAS are chemically distinct from dioxins and PCBs, the authors emphasized shared features of developmental neurotoxicity and the importance of adopting a multi-pollutant perspective. Their findings suggest that early-life exposure to complex mixtures of environmental pollutants—including those transmitted via breast milk—may exert cumulative and potentially synergistic effects on neurodevelopmental trajectories.
In Spain, Ribas-Fitó et al. [
37] investigated prenatal exposure to organochlorine compounds and their impact on infant neurodevelopment. The study identified subtle neurodevelopmental delays associated with higher exposure levels, supporting evidence from other cohorts regarding early-life vulnerability to persistent organic pollutants.
Balalian et al. [
38] conducted a comprehensive systematic review of perinatal PCB exposure and child neurodevelopment. Synthesizing evidence from over 100 studies, their work highlighted consistent associations between prenatal PCB exposure and deficits across cognitive, motor, and behavioral domains. The review emphasized the considerable methodological variability across studies in exposure assessment and neurodevelopmental outcome measurement. Importantly, it underscored the significance of both prenatal and lactational transfer of PCBs, advocating for standardized protocols in future research and the inclusion of breastfeeding-related exposure metrics to better characterize infant risk profiles.
Schecter et al. [
24] investigated dietary exposure pathways in Bien Hoa City, Vietnam—a region with well-documented historical dioxin pollution. Analysis of locally sourced food and breast milk samples confirmed that consumption of animal-derived products constituted the primary contributor to maternal dioxin burden. These findings further supported the evidence for the interconnection between environmental pollution, dietary habits, and maternal-to-infant transfer [
24].
Finally, Nguyet Anh et al. [
27] examined maternal characteristics associated with elevated dioxin concentrations in breast milk samples collected from women residing near a confirmed dioxin-contaminated site in Vietnam. Furthermore, evidence from underrepresented regions—such as sub-Saharan Africa, Latin America and Southeast Asia—reinforces the global relevance of these concerns. Mochungong and Zhu [
39] reviewed environmental monitoring data across these regions and identified widespread pollution with dioxins, PCBs, and PBDEs in both human and environmental matrices. Their findings highlighted bioaccumulation through the food chain, inadequate regulatory oversight, and potential infant exposure via breastfeeding in contexts with limited environmental health infrastructure.
In a more targeted biomonitoring effort, Matovou et al. [
40] investigated the presence of multiple persistent organic pollutants in breast milk samples collected from mothers in Uganda. The study not only confirmed risks of infant dietary exposure but also identified associations between POP concentrations and altered maternal thyroid hormone levels, suggesting possible endocrine-disrupting effects of lactational exposure.
Key factors such as advanced maternal age, higher body mass index (BMI), and longer duration of residence were significantly correlated with elevated dioxin levels, underscoring the influence of chronic environmental exposure and individual vulnerability [
27].
Collectively, the reviewed studies provide robust and consistent evidence of the presence of dioxins and dioxin-like PCBs in human breast milk across diverse geographic settings and environmental scenarios. Primary contributing factors include residential proximity to pollution sources, dietary intake—particularly the consumption of animal fats and fish—and maternal characteristics such as age, body mass index (BMI), and length of residence in contaminated areas. While the health implications for infants were inconsistently addressed across the included studies, the findings underscore the utility of breast milk as a critical biomonitoring matrix for assessing maternal and environmental exposure to persistent organic pollutants (POPs).
The synthesis underscores the importance of considering context-specific exposure pathways and maternal determinants, especially in under-monitored settings. This broader perspective allows for a more comprehensive understanding of global disparities in infant exposure risk.
A summary of the main characteristics and findings of the ten included studies is provided in
Table 1.
4. Discussion
This review aimed to examine the maternal and environmental determinants of dioxin and dl-PCB concentrations in human breast milk, with the objective of clarifying the factors that influence infant exposure to these persistent organic pollutants. The ten studies included present a multifactorial depiction of pollution sources, exposure pathways, and both temporal and geographic trends, offering valuable insights into the complexities of human biomonitoring during early-life exposure. This suggests that infant exposure to persistent pollutants is not randomly distributed but rather reflects specific environmental patterns, often linked to socioeconomic and geographic inequalities. These spatial disparities warrant targeted environmental health interventions.
A central theme across the reviewed studies is the critical role of environmental proximity to pollution sources in influencing contaminant levels in breast milk. Evidence from industrialized or contaminated regions, such as southern Italy [
20,
21], Bien Hoa City in Vietnam [
26], and urban Korea [
12], consistently showed elevated concentrations of PCDDs, PCDFs, and dl-PCBs in maternal milk.
However, in addition to local sources, long-range atmospheric transport of persistent organic pollutants also significantly shapes regional exposure patterns. Studies have documented the presence of dioxins and dl-PCBs in remote regions such as the Arctic [
41], despite the absence of direct industrial activity. This phenomenon, driven by global volatilization and condensation cycles, underscores the transboundary nature of dioxin pollution and highlights the importance of coordinated international regulatory frameworks.
In terms of quantitative exposure, several studies reported actual dioxin concentrations in breast milk, expressed in WHO-TEQ units. For instance, Ae et al. [
25] documented a decline from approximately 20 pg WHO-TEQ/g fat in 1998 to 6 pg/g in 2015, demonstrating the effect of long-term regulatory measures. Similarly, Schecter et al. [
24] found mean concentrations exceeding 100 pg WHO-TEQ/g lipid in breast milk samples from Bien Hoa, Vietnam—a known dioxin hotspot. In Greece, Costopoulou et al. [
31] estimated infant dioxin intake via breastfeeding to be around 2.3 pg WHO-TEQ/kg/day, surpassing the WHO’s tolerable daily intake of 2 pg/kg/day. These data highlight the importance of contextualizing maternal and infant exposure within established toxicological thresholds and reinforce the public health relevance of biomonitoring efforts.
These studies highlight the enduring legacy of environmental pollution—stemming from sources such as illegal waste incineration, past military activity, or industrial emissions. Notably, Fiedler et al. [
19] provided comprehensive evidence that residential proximity to municipal and hazardous waste incinerators is a significant contributor to dioxin exposure, even in countries with advanced emission control technologies. Their review emphasized that, despite regulatory improvements, localized environmental burdens may persist and continue to affect vulnerable populations, reinforcing the importance of continuous environmental monitoring and spatially targeted interventions.
Dietary exposure also emerges as a consistent determinant. Studies from Norway [
22] and the pan-European NewGeneris cohort [
23] identify a high intake of animal-derived foods—particularly fatty fish, meat, and dairy products—as major contributors to maternal dioxin burden. Complementing these findings, Costopoulou et al. [
31] conducted a focused dietary exposure assessment within the Greek population, evaluating infant intake of dioxins and dl-PCBs through breastfeeding. Their analysis combined national food pollution data with breastfeeding patterns, revealing that exclusively breastfed infants, particularly in regions with high fish consumption, could receive dioxin doses exceeding the recommended tolerable daily intake levels. This study highlighted that regional dietary habits, particularly in communities with high fish consumption, can significantly influence infant exposure, even in the absence of direct environmental pollution. Therefore, public health strategies should extend beyond pollution control to incorporate culturally sensitive nutritional guidance and ongoing biomonitoring of breastfeeding mothers in affected areas.
Similar findings were reported by Schecter et al. [
24], who showed that contaminated food remains the primary exposure pathway among highly exposed populations in Vietnam. Taken together, these results emphasize the need for regionally tailored dietary recommendations, especially for lactating women residing in or near polluted areas.
Maternal characteristics—including age, parity, BMI, and duration of residence—also influence dioxin concentrations in breast milk. Older mothers and those with greater adiposity are more likely to accumulate and excrete lipophilic compounds such as dioxins and PCBs [
20,
27]. Additionally, evidence suggests that first-born infants may receive a higher toxic burden than their siblings, as early lactation serves as a primary excretion route for stored lipophilic pollutants in mothers. This finding underscores the role of parity as an independent determinant of infant exposure [
42]. Furthermore, studies from Vietnam [
26,
27] emphasize the role of cumulative lifetime exposure, showing that prolonged residence in contaminated areas increases body burden, even after environmental conditions have improved. These findings underscore the cumulative nature of dioxin exposure and the importance of adopting life-course approaches to exposure assessment and policy development. Interventions that focus solely on current environmental conditions may underestimate the actual risk.
In contrast, the longitudinal study from Japan by Ae et al. [
25] offers encouraging evidence of a sustained decline in dioxin concentrations in breast milk over time. This trend reflects the effectiveness of both national and international initiatives—such as the Stockholm Convention—in eliminating major sources of persistent organic pollutants. However, comparative data from other regions are less often longitudinal in design, limiting the ability to make direct comparisons. Nevertheless, several European studies (e.g., Patandin et al. [
33], Costopoulou et al. [
31]) report lower recent dioxin levels relative to earlier decades, indirectly supporting similar downward trends. These trends likely reflect the cumulative impact of EU-wide bans on incineration emissions and stricter food safety regulations. The Japanese findings thus align with broader global reductions but provide a rare long-term national perspective, underscoring the need for more harmonized, longitudinal biomonitoring across countries to assess the effectiveness of regulatory policies. These results illustrate that well-implemented regulatory frameworks can lead to tangible health-protective outcomes over time. Nonetheless, the absence of longitudinal data from low-income and heavily industrialized countries limits the completeness of global assessments regarding policy effectiveness.
Notably, several studies have gone beyond mere exposure assessment to evaluate potential health outcomes. For instance, Tai et al. [
26] investigated infant neurodevelopment in connection with postnatal dioxin exposure via breastfeeding; however, their findings were inconclusive. Similarly, Patandin et al. [
33] reported that environmental exposure to PCBs and dioxins adversely impacted cognitive abilities in Dutch children at 42 months of age. Papadopoulou et al. [
22] identified associations between prenatal dietary dioxin intake and reduced birth size, suggesting potential fetal vulnerability. This is further supported by a large meta-analysis conducted by Govarts et al. [
32], which pooled data from twelve European birth cohorts to investigate the impact of prenatal exposure to PCBs and DDE on fetal growth. The study reported a consistent inverse association between maternal exposure to these persistent pollutants and infant birth weight, reinforcing concerns that even low-dose prenatal exposure might adversely affect fetal development. Consistent with these findings, Yim et al. [
35] utilized a multi-pollutant modeling approach to investigate associations between prenatal exposure to dioxins and PCBs and neurodevelopment at six months of age. Their results suggested that even low-level prenatal exposure may negatively impact early cognitive and motor development, underscoring the need for more integrated risk assessment models that consider the combined effects of multiple pollutants. Similarly, Ribas-Fitó et al. [
37] in Spain reported subtle neurodevelopmental delays linked to prenatal exposure to organochlorine compounds, reinforcing evidence from diverse cohorts regarding the vulnerability of early neurodevelopment to persistent organic pollutants. A broader toxicological perspective is provided by Ames et al. [
36], who conducted a comprehensive review of early-life exposure to per- and polyfluoroalkyl substances (PFAS) and their neurodevelopmental implications. Although PFAS are chemically distinct from dioxins and PCBs, the review highlights overlapping mechanisms of developmental neurotoxicity and emphasizes the importance of assessing cumulative exposure to chemical mixtures. These findings highlight the need for integrative environmental health frameworks that consider co-exposure to diverse persistent pollutants, including those transferred via breast milk, when evaluating risks to child neurodevelopment. These associations, even if subtle or borderline significant, suggest that current “safe” exposure thresholds may not fully protect the developing brain. As such, precautionary principles should guide both risk communication and public health responses.
Supporting emerging evidence, Balalian et al. [
38] conducted a comprehensive systematic review focused on perinatal PCB exposure and child neurodevelopmental outcomes. Their synthesis of over 100 studies identified consistent evidence linking prenatal PCB exposure to deficits in cognitive, motor, and behavioral development. The review also highlighted substantial methodological heterogeneity across studies, particularly regarding exposure assessment and outcome measurement, and underscored the critical role of both prenatal and lactational PCB transfer. They called for standardized research protocols and the inclusion of breastfeeding-related exposure metrics to improve risk characterization and support targeted interventions. These findings indicate that although breast milk serves as a critical matrix for exposure biomonitoring, further interdisciplinary research is essential to clarify the health significance of early-life dioxin exposure.
Additional evidence from Vietnam supports these observations. Nishijo et al. [
34] conducted both cross-sectional and longitudinal studies in dioxin-contaminated areas, demonstrating that perinatal exposure to elevated levels of TCDD was associated with significantly lower weight and length in infants, particularly among boys with higher TEQ-PCDD/Fs in breast milk. These findings underscore the potential for early postnatal growth impairment due to maternal dioxin burden, reinforcing concerns regarding sex-specific vulnerability and the need for long-term developmental monitoring in highly exposed populations.
The inclusion of studies from diverse geographical regions and methodological approaches enabled a more holistic understanding of the factors influencing dioxin concentrations in breast milk. Nevertheless, inconsistencies in analytical methods, reporting formats, and exposure indicators may hinder direct comparisons across studies. Moreover, methodological inconsistencies across studies—such as variations in milk sampling time (e.g., colostrum versus mature milk), lipid normalization techniques, and detection sensitivity—introduce challenges in comparing exposure levels directly. These discrepancies may lead to under- or overestimation of true concentrations and complicate efforts to synthesize findings across different cohorts. Additionally, the restriction to English-language publications may have resulted in the exclusion of relevant data, particularly from non-English-speaking regions such as Latin America, where dioxin exposure is a documented concern. This language limitation represents a potential source of selection bias and underscores the need for future reviews to include multilingual sources when feasible. Despite this, consistent patterns emerged, particularly regarding the influence of diet, environmental context, and maternal physiology.
Further supporting the geographic scope of this review, Mochungong and Zhu [
39] compiled evidence of widespread pollution by dioxins, PCBs, and PBDEs across underrepresented regions such as sub-Saharan Africa, Latin America, and Southeast Asia. Their analysis emphasized the interplay among weak regulatory frameworks, contaminated food sources, and potential infant exposure through breastfeeding in low- and middle-income settings. Additionally, Matovou et al. [
40] conducted a biomonitoring study in Uganda, identifying multiple persistent organic pollutants in breast milk and revealing associations with altered maternal thyroid hormone homeostasis. These findings reinforce the need for inclusive global research efforts that consider regional disparities in environmental health infrastructure and exposure patterns.
Out of an initial pool of 325 articles, twenty studies ultimately met the predefined inclusion criteria and were incorporated into the final synthesis. Although this number may not capture the full extent of global variability in dioxin and dl-PCB pollution in breast milk, it represents a substantial and diverse evidence base compared to previous reviews. The increase in eligible studies reflects both expanding research interest and enhanced data availability across regions. Nevertheless, some geographic and methodological gaps remain. Future reviews could benefit from broader inclusion criteria, multilingual search strategies, and harmonized exposure metrics to further strengthen the evidence base. Despite these limitations, the included studies provide valuable insights into exposure determinants and highlight key directions for targeted public health interventions.
An additional limitation of this review is the absence of a formal risk of bias assessment using standardized tools such as ROBINS-I or the Newcastle–Ottawa Scale. Although study quality was qualitatively considered during data synthesis, the lack of a structured and quantitative evaluation limits the ability to comprehensively assess the internal validity and reliability of the included studies. Incorporating formal bias assessment tools in future reviews would enhance the rigor and interpretability of the findings.
We acknowledge that the generalizations regarding maternal exposure to dioxins and the influence of behavioral and environmental factors are based on a limited number of studies. This limitation may affect the accuracy and generalizability of the conclusions. Therefore, caution should be exercised when extrapolating these findings to broader populations. Further research with larger and more diverse cohorts is necessary to strengthen and validate these associations.
Returning to the aim of this review—to synthesize the current literature on maternal and environmental determinants of dioxin and dl-PCB concentrations in breast milk—the findings reveal that such exposures are neither random nor unavoidable. Instead, they are influenced by identifiable factors that can guide public health policies, environmental control measures, and maternal nutrition recommendations.
In summary, this body of evidence highlights the value of breast milk as a sentinel biofluid for evaluating human exposure to persistent organic pollutants. The reviewed studies collectively enhance our understanding of exposure determinants and potential intervention strategies aimed at minimizing mother-to-child contaminant transfer. Importantly, maternal exposure to dioxins and dioxin-like PCBs is shaped by modifiable environmental and behavioral factors—including dietary habits and residential proximity to pollution sources—which can be addressed through targeted actions. Breast milk remains both a crucial nutritional resource and a sensitive indicator of early-life exposure to toxicants. Nonetheless, notable research gaps persist, such as the underrepresentation of low- and middle-income countries, the absence of harmonized exposure protocols, and the scarcity of longitudinal data linking exposure to child health trajectories. Bridging these gaps is essential to avoid a two-tiered global health landscape in which vulnerable populations remain unmonitored and unprotected. Equity-driven biomonitoring frameworks are necessary to ensure that all infants—regardless of geography—receive the same level of health protection.
It is important to emphasize that breastfeeding offers well-established health benefits for both infants and mothers, as recognized by the World Health Organization, which continues to recommend breastfeeding even in regions with high environmental contaminant exposure (WHO, 2024) [
43]. These benefits include enhanced immune protection, optimal nutrition, and improved neurodevelopmental outcomes. Therefore, while concerns about exposure to dioxins and dl-PCBs through breast milk are valid, they should be carefully balanced against the substantial advantages of breastfeeding. This underscores the need for nuanced, interdisciplinary approaches that safeguard infant health without discouraging breastfeeding, focusing on minimizing environmental pollution and supporting maternal well-being. Breastfeeding should remain universally promoted due to its numerous proven benefits; however, the risk of chronic low-dose exposure in vulnerable infants necessitates carefully tailored strategies that protect both maternal–infant health and environmental integrity. Such strategies should include region-specific dietary guidance for lactating women, low-cost food preparation methods (e.g., trimming fish skin or visible fat), and targeted monitoring of local food sources—particularly in low-resource, fish-dependent communities.
Recommendations, Reflections, and Future Research Directions
This review identified several critical areas requiring attention to enhance understanding and management of dioxin and dl-PCB exposure via breast milk.
Standardization and Harmonization: Future research must prioritize the standardization of analytical methodologies, including harmonized TEQ-based protocols across laboratories, and address methodological variabilities such as sampling timing and lipid normalization techniques. Such efforts will improve comparability across studies and support more robust meta-analyses.
Longitudinal Studies and Surveillance: Extending longitudinal evaluations of health outcomes related to early-life exposure is vital for elucidating long-term effects on child development. Additionally, integrating human biomonitoring data into national and international exposure surveillance systems can facilitate more effective public health responses. Moreover, fostering cross-national cohort collaborations can enhance statistical power, reduce regional bias, and enable the tracking of transboundary exposure trends.
Policy and Intervention Strategies: Policymakers should leverage scientific evidence to implement context-specific interventions, including stricter zoning around pollution sources like incinerators, public education on dietary exposure reduction, and investment in environmental remediation, especially in historically contaminated and resource-limited areas. Importantly, these interventions must be culturally and economically tailored to affected communities. Community engagement should be embedded in policy design to ensure interventions are contextually relevant, socially accepted, and sustainably implemented.
Global Exposure Considerations: Addressing long-range pollutant transport and less visible exposure pathways is essential for achieving a globally coherent assessment of maternal and infant exposure. Coordinated international regulatory frameworks and guidance on tolerable intake levels are necessary to safeguard health worldwide.
Equity and Inclusion: Special focus should be placed on underrepresented low- and middle-income regions to avoid global health disparities. Equity-driven biomonitoring frameworks are essential to ensure vulnerable populations receive adequate protection.
These directions emphasize the importance of integrating scientific findings with public health policy and environmental management to minimize early-life exposure to harmful persistent organic pollutants while preserving the benefits of breastfeeding.