Assessment of Heavy Metal Contamination and Human Health Risk in Parapenaeus longirostris from Coastal Tunisian Aquatic Ecosystems
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Areas
2.1.1. Bizerte Lagoon
2.1.2. Gulf of Gabes
2.1.3. Bay of Monastir
2.1.4. Kerkennah Site
2.2. Sample Collection and Preparation
2.3. Chemical Analysis
2.4. Pollution Assessment
2.5. Health Risk Assessment
2.5.1. Estimated Daily Intake
2.5.2. Estimated Weekly Intake
2.5.3. Percentage of Provisional Tolerable Weekly Intake
2.5.4. Maximum Daily Intake
2.5.5. Maximum Weekly Intake
2.5.6. Daily Intake Limit
2.5.7. Maximum Acceptable Daily Intake
2.5.8. Maximum Allowable Intake Rate
2.5.9. Target Hazard Quotient
2.5.10. Hazard Index
2.5.11. Cancer Risks
2.5.12. Relative Risk
2.6. Statistical Analysis
3. Results
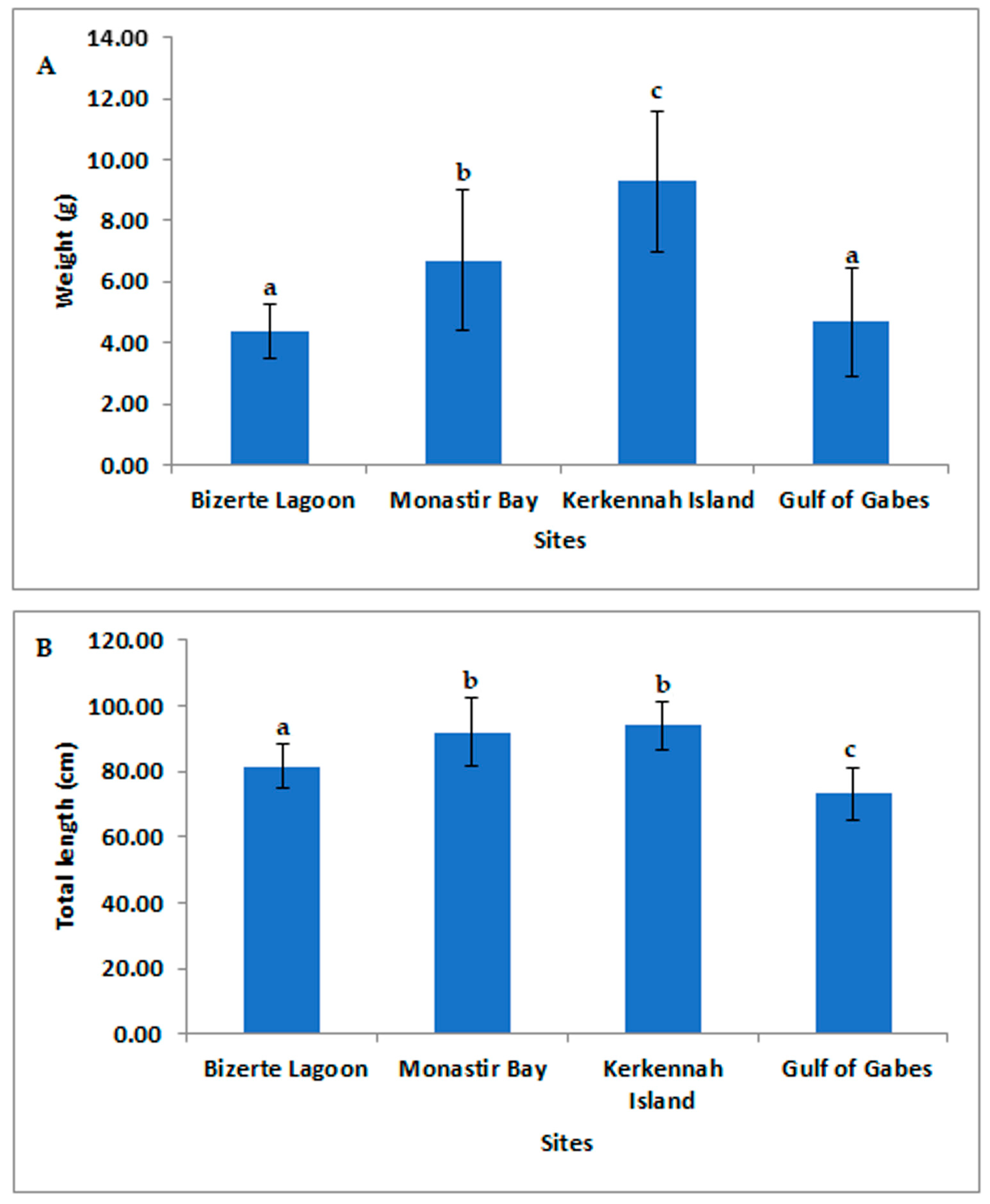
3.1. Study of Morphometric Parameters
3.2. Heavy Metal Concentrations
3.3. Comparative Analysis of Metal Concentrations Across Study Sites
3.4. Shrimp’s Level of Metal Pollution
3.5. Health Risk Assessment Related to the Consumption of Parapenaeus longirostris
4. Discussion
4.1. Heavy Metal Content and Level of Pollution in Shrimp
4.2. Human Health Risk Assessment
4.3. Comparison of Metal Concentrations Derived from Literature Data
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cd | Cadmium |
| Cu | Copper |
| Pb | Lead |
| Zn | Zinc |
| Hg | Mercury |
| NIST | National Institute of Standards and Technology |
| LOD | Limit of Detection |
| RSD | Relative Standard Deviation |
| PI | Pollution Index |
| EDI | Estimated Daily Intake |
| EWI | Estimated Weekly Intake |
| %PTWI | Percentage of Provisional Tolerable Weekly Intake |
| MDI | Maximum Daily Intake |
| MWI | Maximum Weekly Intake |
| CRlim | Daily Intake Limit |
| CRmm | Monthly Consumption Rate Limit |
| THQ | Target Hazard Quotient |
| HI | Hazard Index |
| CR | Cancer Risks |
| RR | Relative Risk |
| EF | Exposure Frequency |
| ED | Exposure Duration |
| FIR | Food Ingestion Rate |
| CF | Conversion Factor |
| WAB | Weight of Average Body |
| TA | Total exposure time |
| USEPA | United States Environmental Protection Agency |
| IR | Ingestion Rate |
| BW | Body Weight |
| ARL | Acceptable Risk Level |
| CSF | Cancer Slope Factor |
| RfD | Oral Reference Dose |
| Tap | Average Time Span |
| MS | Meal Size |
| THQi | Target Hazard Quotient |
| SD | Standard Deviation |
| ANOVA | Analysis of Variance |
| LSD | Least Significant Difference |
| p-value | Probability value |
| STIR | Société Tunisienne des Industries de Raffinage |
| SOTULUB | Société Tunisienne de Lubrifiants |
| SOCOMENA | Société de Construction Mécanique et Navale |
| MPI | Metal Pollution Index |
References
- Mahmudiono, T.; Esfandiari, Z.; Zare, A.; Sarkhoshkalat, M.; Mehri, F.; Fakhri, Y. Concentration of Potentially Toxic Elements in Fillet Shrimps of Mediterranean Sea: Systematic Review, Meta-Analysis and Health Risk Assessment. Food Chem. X 2024, 21, 101206. [Google Scholar] [CrossRef]
- Ben Ameur, W.; Annabi, A.; Ben Said, S.; Marini, M. First Assessment of Consumer Health Risks from Metal Accumulation in Fish from the Bizerte and Ghar El Melh Lagoons: The Case of Sarpa salpa (Linnaeus, 1758). Int. J. Environ. Anal. Chem. 2025, 1–36. [Google Scholar] [CrossRef]
- Oros, A.; Galatchi, M. Long-Term Heavy Metal Bioaccumulation in Sprat (Sprattus sprattus) from the Romanian Black Sea: Ecological and Human Health Risks in the Context of Sustainable Fisheries. Fishes 2025, 10, 178. [Google Scholar] [CrossRef]
- Gong, C.-H.; Wang, Z.-L.; Zhang, Y.-Q.; Qi, X.-H.; Cao, P.-T.; Li, Q.; Wang, W.-J.; Wang, P.; Yang, Y. Organ-Level Concentrations of Heavy Metals (Cr, Ni, Cu, Zn, As, Cd, Hg and Pb) in Five Aquatic Organisms from Lianyungang in China and Associated Heath Risk Assessment. J. Food Compos. Anal. 2025, 139, 107089. [Google Scholar] [CrossRef]
- Prabakaran, K.; Charoenpong, C.; Bureekul, S.; Wang, X.; Sompongchaiyakul, P. Heavy Metal Contamination in Marine Fish from the Andaman Sea: Influence of Habitat and Health Risk Assessment. Mar. Pollut. Bull. 2025, 210, 117299. [Google Scholar] [CrossRef] [PubMed]
- Ghosn, M.; Mahfouz, C.; Chekri, R.; Khalaf, G.; Guérin, T.; Jitaru, P.; Amara, R. Seasonal and Spatial Variability of Trace Elements in Livers and Muscles of Three Fish Species from the Eastern Mediterranean. Environ. Sci. Pollut. Res. 2020, 27, 12428–12438. [Google Scholar] [CrossRef]
- Okbah, M.A.A.; Dango, E.A.S.; Zokm, G.M.E. Heavy Metals in Fish Species from Mediterranean Coast, Tripoli Port (Libya): A Comprehensive Assessment of the Potential Adverse Effects on Human Health. Egypt. J. Aquat. Biol. Fish. 2018, 22, 149–164. [Google Scholar] [CrossRef]
- Ra, W.-J.; Yoo, H.J.; Kim, Y.-H.; Yun, T.; Soh, B.; Cho, S.Y.; Joo, Y.; Lee, K.-W. Heavy Metal Concentration According to Shrimp Species and Organ Specificity: Monitoring and Human Risk Assessment. Mar. Pollut. Bull. 2023, 197, 115761. [Google Scholar] [CrossRef]
- Ramos-Miras, J.J.; Sanchez-Muros, M.J.; Renteria, P.; De Carrasco, C.G.; Roca-Perez, L.; Boluda-Navarro, M.; Pro, J.; Martín, J.A.R. Potentially Toxic Element Bioaccumulation in Consumed Indoor Shrimp Farming Associated with Diet, Water and Sediment Levels. Environ. Sci. Pollut. Res. 2023, 30, 121794–121806. [Google Scholar] [CrossRef]
- Dghim, A.; Ben Ameur, W.; Annabi, A. Assessment of the Accumulation of Trace Metals and Oxidative Stress Response Biomarkers in the Portunid Portunus segnis. Appl. Sci. 2023, 13, 7197. [Google Scholar] [CrossRef]
- Lin, H.; Luo, X.; Yu, D.; He, C.; Cao, W.; He, L.; Liang, Z.; Zhou, J.; Fang, G. Risk Assessment of As, Cd, Cr, and Pb via the Consumption of Seafood in Haikou. Sci. Rep. 2024, 14, 19549. [Google Scholar] [CrossRef]
- Draz, A.; Qazi, M.A.; Hussain, T.; Ahmad, O.; Nazir, M.M.; Bhatti, M.B.; Hussain, N.; Sherzada, S. Heavy Metals Concentration and Human Health Risk Assessment in Selected Shrimp Species of Pakistan. Food Addit. Contam. Part B 2025, 18, 78–85. [Google Scholar] [CrossRef]
- Hossain, M.B.; Bhuiyan, N.Z.; Kasem, A.; Hossain, M.K.; Sultana, S.; Nur, A.-A.U.; Yu, J.; Albeshr, M.F.; Arai, T. Heavy Metals in Four Marine Fish and Shrimp Species from a Subtropical Coastal Area: Accumulation and Consumer Health Risk Assessment. Biology 2022, 11, 1780. [Google Scholar] [CrossRef]
- Sultana, S.; Hossain, M.B.; Choudhury, T.R.; Yu, J.; Rana, M.S.; Noman, M.A.; Hosen, M.M.; Paray, B.A.; Arai, T. Ecological and Human Health Risk Assessment of Heavy Metals in Cultured Shrimp and Aquaculture Sludge. Toxics 2022, 10, 175. [Google Scholar] [CrossRef]
- Hidayati, N.V.; Prudent, P.; Asia, L.; Vassalo, L.; Torre, F.; Widowati, I.; Sabdono, A.; Syakti, A.D.; Doumenq, P. Assessment of the Ecological and Human Health Risks from Metals in Shrimp Aquaculture Environments in Central Java, Indonesia. Environ. Sci. Pollut. Res. 2020, 27, 41668–41687. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Dong, K.F.; Xiao, G.; Ma, D. Heavy Metal Concentrations in Aquatic Organisms (Fishes, Shrimp and Crabs) and Health Risk Assessment in China. Mar. Pollut. Bull. 2020, 159, 111505. [Google Scholar] [CrossRef] [PubMed]
- Skordas, K.; Lolas, A.; Gounari, C.; Georgiou, K.; Neofitou, N.; Vafidis, D. Trace Element Content and Potential Human Health Risk from Consumption of the Deep-Water Rose Shrimp Parapenaeus longirostris (Crustacea: Decapoda) from Pagasitikos Gulf, Greece. J. Chem. Health Risks 2022, 12, 675–683. [Google Scholar] [CrossRef]
- Zaaboub, N.; Martins, M.V.A.; Terroso, D.L.; Helali, M.A.; Béjaoui, B.; El Bour, M.; Boukef-BenOmrane, I.; Pereira, A.L.; Dardon, U.; Ennouri, R.; et al. Geochemical and mineralogical fingerprints of the sediments supply and early diagenetic processes in the Bizerte Lagoon (Tunisia). J. Sediment. Environ. 2016, 1, 432–448. [Google Scholar] [CrossRef]
- Ben Ameur, W.; Hanen, G.; Ben Hassine, S.; Safouen, G.; El Megdiche, Y.; Mhadhbi, T.; Annabi, A.; Touil, S.; Driss, M.R. Bioaccumulation of Polycyclic Aromatic Hydrocarbons in Solea Solea from Bizerte and Ghar El Melh Lagoons (Tunisia) and Human Health Risk Assessment. J. Environ. Sci. Health Part A 2021, 56, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- MeHSIP-PPIF (2013) Études de Faisabilité—Dépollution Intégrée du lac de Bizerte. Mediterranean Hot Spot Investment Programme—Project Preparation and Implementation Facility, Technical Assistance Funded by the European Union—FEMIP Support Fund. Final Version Published September 2013. Available online: http://www.ecopact.tn/sites/default/files/2022-05/Etude-de-Faisabilite-Bizerte.pdf (accessed on 1 June 2025).
- Khammassi, M.; Jourde, J.; Zaabar, W.; Laabidi, S.; Sauriau, P.-G.; Achouri, M.S. Inventory and New Records of Benthic Amphipods from Macrophytes and Fine Sand Communities of the Bizerte Lagoon (Tunisia, SW Mediterranean Sea). Mar. Biodivers. Rec. 2019, 12, 24. [Google Scholar] [CrossRef]
- Ben Ameur, W.; El Megdiche, Y.; Ennaceur, S.; Mhadhbi, T.; Ben Hassine, S.; Annabi, A.; De Lapuente, J.; Driss, M.R.; Borràs, M.; Eljarrat, E. Biomarkers Responses and Polybrominated Diphenyl Ethers and Their Methoxylated Analogs Measured in Sparus aurata from the Lagoon of Bizerte, Tunisia. Environ. Sci. Pollut. Res. 2022, 29, 38618–38632. [Google Scholar] [CrossRef] [PubMed]
- Hamdaoui, B.; Ennouri, R.; Fatnassi, M.; Zarrouk, H.; Romdhane, N.; Chalghaf, M.; Mili, S. Review of the Situation of Tunisian Lagoon of Bizerta Using Marine Spatial Planning as a Key to Sustainable Blue Growth. J. Biomed. Res. Environ. Sci. 2022, 3, 149–162. [Google Scholar] [CrossRef]
- Karray, S. Etude Écotoxicologique et Phylogéographique de la Coque Cerastoderma glaucum Issue du Golfe de Gabès: Réponse Adaptative (In Situ et In Vivo) au Stress Métallique et Structure Génétique. Ph.D. Thesis, Université de Sfax, Sfax, Tunisie, 2015. [Google Scholar]
- Chouba, L.; Mzoughi-Aguir, N. Les métaux traces (Cd, Pb, Hg) et les hydrocarbures totaux dans les sédiments superficiels de la frange côtière du golfe de Gabès. INSTM Bull. Mar. Freshw. Sci. 2006, 33, 93–100. [Google Scholar] [CrossRef]
- Khiari, N.; Atoui, A.; Khalil, N.; Charef, A.; Aleya, L. Dynamics of Sediments along with Their Core Properties in the Monastir-Bekalta Coastline (Tunisia, Central Mediterranean). J. Afr. Earth Sci. 2017, 134, 320–331. [Google Scholar] [CrossRef]
- Brahim, M.; Atoui, A.; Ben Ali, M.R. Sedimentary Dynamics in Monastir Bay of the Tunisian Water. INSTM Bull. Mar. Freshw. Sci. 2017, 44, 175–184. [Google Scholar] [CrossRef]
- Chouchene, K.; Rocha-Santos, T.; Ksibi, M. Types, Occurrence, and Distribution of Microplastics and Metals Contamination in Sediments from South West of Kerkennah Archipelago, Tunisia. Environ. Sci. Pollut. Res. 2021, 28, 46477–46487. [Google Scholar] [CrossRef]
- Annabi, A.; El Mouadeb, R.; Herrel, A. Distinctive Accumulation Patterns of Heavy Metals in Sardinella aurita (Clupeidae) and Mugil Cephalus (Mugilidae) Tissues. Environ. Sci. Pollut. Res. 2018, 25, 2623–2629. [Google Scholar] [CrossRef]
- Adebiyi, F.M.; Ore, O.T.; Ogunjimi, I.O. Evaluation of Human Health Risk Assessment of Potential Toxic Metals in Commonly Consumed Crayfish (Palaemon hastatus) in Nigeria. Heliyon 2020, 6, e03092. [Google Scholar] [CrossRef]
- Miri, M.; Akbari, E.; Amrane, A.; Jafari, S.J.; Eslami, H.; Hoseinzadeh, E.; Zarrabi, M.; Salimi, J.; Sayyad-Arbabi, M.; Taghavi, M. Health Risk Assessment of Heavy Metal Intake Due to Fish Consumption in the Sistan Region, Iran. Environ. Monit. Assess. 2017, 189, 583. [Google Scholar] [CrossRef]
- Tkachenko, H.; Kurhaluk, N.; Kasiyan, O.; Kamiński, P. Dietary Nutrients and Health Risks from Exposure to Some Heavy Metals through the Consumption of the Farmed Common Carp (CYPRINUS CARPIO). J Environ. Health Sci. Eng. 2021, 19, 793–804. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Integrated Risk Information System. 2008. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=2776 (accessed on 15 December 2021).
- Mohiuddin, M.; Hossain, M.B.; Ali, M.M.; Kamal Hossain, M.; Habib, A.; Semme, S.A.; Rakib, M.R.J.; Rahman, M.A.; Yu, J.; Al-Sadoon, M.K.; et al. Human Health Risk Assessment for Exposure to Heavy Metals in Finfish and Shellfish from a Tropical Estuary. J. King Saud Univ. Sci. 2022, 34, 102035. [Google Scholar] [CrossRef]
- Chakma, S.; Rahman, M.A.; Jaman, M.N.; Al-Azim; Nag, S.K.; Ali, M.K.; Hoque, M.S.; Chakma, K. Assessing Trace Elements Bioaccumulation in Coastal River Fish and Shellfish: Implications for Human Health and Risk Evaluation. Biol. Trace Elem. Res. 2025, 203, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Guidance for Assessing Chemical Contamination Data for Use in Fish Advisories II Risk Assessment and Fish Consumption Limits. EPA/823–B94-004. United States Environmental Protection Agency Washington. 2000. Available online: https://www.epa.gov/sites/default/files/2015-06/documents/volume2.pdf (accessed on 15 May 2025).
- Abdel-Kader, H.H.; Mourad, M.H. Estimation of Cadmium in Muscles of Five Freshwater Fish Species from Manzalah Lake, and Possible Human Risk Assessment of Fish Consumption (Egypt). Biol. Trace. Elem. Res. 2023, 201, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Berhanu, G.; Lemma, H.; Mekonnen, S.; Dadi, D. Heavy Metals in Wastewater and Fish Collected from Waste Stabilization Pond and Human Health Risks in Southwestern Ethiopia. Front. Environ. Health 2024, 3, 1386827. [Google Scholar] [CrossRef]
- Cobbinah, R.T.; Boadi, N.O.; Saah, S.A.; Agorku, E.S.; Badu, M.; Kortei, N.K. Cancer Risk from Heavy Metal Contamination in Fish and Implications for Public Health. Sci. Rep. 2025, 15, 24162. [Google Scholar] [CrossRef]
- Giri, S.; Singh, A.K. Human Health Risk and Ecological Risk Assessment of Metals in Fishes, Shrimps and Sediment from a Tropical River. Int. J. Environ. Sci. Technol. 2015, 12, 2349–2362. [Google Scholar] [CrossRef]
- Demissie, S.; Mekonen, S.; Awoke, T.; Teshome, B.; Mengistie, B. Examining Carcinogenic and Noncarcinogenic Health Risks Related to Arsenic Exposure in Ethiopia: A Longitudinal Study. Toxicol. Rep. 2024, 12, 100–110. [Google Scholar] [CrossRef]
- Batvari, B.P.D.; Sivakumar, S.; Shanthi, K.; Lee, K.-J.; Oh, B.-T.; Krishnamoorthy, R.; Kamala-Kannan, S. Heavy Metals Accumulation in Crab and Shrimps from Pulicat Lake, North Chennai Coastal Region, Southeast Coast of India. Toxicol. Ind. Health 2016, 32, 1–6. [Google Scholar] [CrossRef]
- Tahity, T.; Islam, M.R.U.; Bhuiyan, N.Z.; Choudhury, T.R.; Yu, J.; Noman, M.A.; Hosen, M.M.; Quraishi, S.B.; Paray, B.A.; Arai, T.; et al. Heavy Metals Accumulation in Tissues of Wild and Farmed Barramundi from the Northern Bay of Bengal Coast, and Its Estimated Human Health Risks. Toxics 2022, 10, 410. [Google Scholar] [CrossRef]
- Miao, X.; Zhang, Q.; Hao, Y.; Zhang, H. The Size Screening Could Greatly Degrade the Health Risk of Fish Consuming Associated to Metals Pollution—An Investigation of Angling Fish in Guangzhou, China. Toxics 2023, 11, 54. [Google Scholar] [CrossRef]
- Aytekin, T.; Kargın, D.; Çoğun, H.Y.; Temiz, Ö.; Varkal, H.S.; Kargın, F. Accumulation and Health Risk Assessment of Heavy Metals in Tissues of the Shrimp and Fish Species from the Yumurtalik Coast of Iskenderun Gulf, Turkey. Heliyon 2019, 5, e02131. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF (accessed on 15 May 2025).
- Biswas, C.; Soma, S.S.; Rohani, M.F.; Rahman, M.H.; Bashar, A.; Hossain, M.S. Assessment of heavy metals in farmed shrimp, Penaeus monodon sampled from Khulna, Bangladesh: An inimical to food safety aspects. Heliyon 2021, 7, e06587. [Google Scholar] [CrossRef]
- Kwaansa-Ansah, E.E.; Nti, S.O.; Opoku, F. Heavy Metals Concentration and Human Health Risk Assessment in Seven Commercial Fish Species from Asafo Market, Ghana. Food Sci. Biotechnol. 2019, 28, 569–579. [Google Scholar] [CrossRef]
- FAO/WHO. Summary of Evaluations Performed by the Joint FAO/WHO Expert Committee on Food Additives (JECFA 1956-2003)(First through Sixty First Meetings); ILSI Press International Life Sciences Institute: Washington, DC, USA, 2004. [Google Scholar]
- JECFA. Summary and Conclusions of the 61st Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); JECFA/61/SC; JECFA: Rome, Italy, 2003. [Google Scholar]
- Soegianto, A. Bioaccumulation of Heavy Metals in Some Commercial Animals Caught from Selected Coastal Waters of East Java, Indonesia. Res. J. Agric. Biol. Sci. 2008, 4, 881–885. [Google Scholar]
- Opuene, K.; Agbozu, I. Relationships Between Heavy Metals in Shrimp (Macrobrachium felicinum) and Metal Levels in The Water Column and Sediments of Taylor Creek. Int. J. Environ. Res. 2008, 2, 343–348. [Google Scholar]
- Hossain, M.; Khan, Y. Trace metals in Penaeid shrimp and Spiny lobster from the Bay of Bengal. Sci. Asia 2001, 27, 165–168. [Google Scholar] [CrossRef]
- Miramand, P.; Guyot, T.; Rybarczyk, H.; Elkaim, B.; Mouny, P.; Dauvin, J.C.; Bessineton, C. Contamination of the Biological Compartment in the Seine Estuary by Cd, Cu, Pb, and Zn. Estuaries 2001, 24, 1056. [Google Scholar] [CrossRef]
- Ismail, A.; Jusoh, N.R.; Ghani, I.A. Trace Metal Concentrations in Marine Prawns off the Malaysian Coast. Mar. Pollut. Bull. 1995, 31, 108–110. [Google Scholar] [CrossRef]
- Ahdy, H.H.H.; Abdallah, A.M.; Tayel, F. Assessment of Heavy Metals and nonessential content of some edible and soft tissues. Egypt. J. Aquat. Res. 2007, 33, 85–97. [Google Scholar]
- Krishnamurti, J.R.V.N. Concentration of metals in shrimps and crabs from Thane- Bassein creek system, Maharastra. Indian J. Mar. Sci. 1999, 28, 92–95. [Google Scholar]
- Frías-Espericueta, M.G.; Izaguirre-Fierro, G.; Valenzuela-Quiñonez, F.; Osuna-López, J.I.; Voltolina, D.; López-López, G.; Muy-Rangel, M.D.; Rubio-Castro, W. Metal Content of the Gulf of California Blue Shrimp Litopenaeus stylirostris (Stimpson). Bull. Environ. Contam. Toxicol. 2007, 79, 214–217. [Google Scholar] [CrossRef]
- Baboli, M.J.; Velayatzadeh, M. Determination of heavy metals and trace elements in the muscles of marine shrimp, Fenneropenaeus merguiensis from Persian Gulf, Iran. J. Anim. Plant Sci. 2013, 23, 786–791. [Google Scholar]
- Everaarts, J.M.; Boon, J.P.; Kastoro, W.; Fischer, C.V.; Razak, H.; Sumanta, I. Copper, Zinc and Cadmium in Benthic Organisms from the Java Sea and Estuarine and Coastal Areas around East Java. Neth. J. Sea Res. 1989, 23, 415–426. [Google Scholar] [CrossRef]
- Abdennour, C.; Smith, B.D.; Boulakoud, M.S.; Samraoui, B.; Rainbow, P.S. Trace metals in marine, brackish and freshwater prawns (Crustacea, Decapoda) from northeast Algeria. Hydrobiologia 2000, 432, 217–227. [Google Scholar] [CrossRef]
- Guhathakurta, H.; Kaviraj, A. Heavy Metal Concentration in Water, Sediment, Shrimp (Penaeus monodon) and Mullet (Liza parsia) in Some Brackish Water Ponds of Sunderban, India. Mar. Pollut. Bull. 2000, 40, 914–920. [Google Scholar] [CrossRef]
- Çoğun, H.; Yüzereroğlu, T.A.; Kargin, F.; Firat, Ö. Seasonal Variation and Tissue Distribution of Heavy Metals in Shrimp and Fish Species from the Yumurtalik Coast of Iskenderun Gulf, Mediterranean. Bull. Environ. Contam. Toxicol. 2005, 75, 707–715. [Google Scholar] [CrossRef]
- Ip, C.C.M.; Li, X.D.; Zhang, G.; Wong, C.S.C.; Zhang, W.L. Heavy Metal and Pb Isotopic Compositions of Aquatic Organisms in the Pearl River Estuary, South China. Environ. Pollut. 2005, 138, 494–504. [Google Scholar] [CrossRef]
- Páez-Osuna, F.; Tron-Mayen, L. Distribution of Heavy Metals in Tissues of the Shrimp Penaeus californiensis from the Northwest Coast of Mexico. Bull. Environ. Contam. Toxicol. 1995, 55, 209–215. [Google Scholar] [CrossRef]
- Everaarts, J.M.; Nieuwenhuize, J. Heavy Metals in Surface Sediment and Epibenthic Macroinvertebrates from the Coastal Zone and Continental Slope of Kenya. Mar. Pollut. Bull. 1995, 31, 281–289. [Google Scholar] [CrossRef]
- Pourang, N.; Dennis, J.H. Distribution of Trace Elements in Tissues of Two Shrimp Species from the Persian Gulf and Roles of Metallothionein in Their Redistribution. Environ. Int. 2005, 31, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Everaarts, J.M.; Swennen, C. Heavy metals (Zn, Cu, Cd, Pb) in some benthic invertebrate species and in sediment from three coastal areas in Thailand and Malaysia. Sci. Asia 1987, 13, 189. [Google Scholar] [CrossRef]
- Vazquez, F.G.; Sharma, V.K.; Mendoza, Q.A.; Hernandez, R. Metals in Fish and Shrimp of the Campeche Sound, Gulf of Mexico. Bull. Environ. Contam. Toxicol. 2001, 67, 756–762. [Google Scholar] [CrossRef]
- Carbonell, G.; Ramos, C.; Tarazona, J.V. Heavy Metals in Shrimp Culture Areas from the Gulf of Fonseca, Central America. II. Cultured Shrimps. Bull. Environ. Contam. Toxicol. 1998, 60, 260–265. [Google Scholar] [CrossRef]
- Abdel-Salam, H.; Hamdi, S.A.H. Heavy Metals Monitoring Using Commercially Important Crustacean and Mollusks collected from Egyptian and Saudi Arabia Coasts. Anim. Vet. Sci. 2014, 2, 49. [Google Scholar] [CrossRef][Green Version]
- Abdennour, C. Copper, zinc and haemocyanin concentrations in four caridean decapods (Crustacea): Size relationships. Hydrobiologia 1997, 346, 1–9. [Google Scholar] [CrossRef]
- Harding, L.; Goyette, D. Metals in Northeast Pacific Coastal Sediments and Fish, Shrimp, and Prawn Tissues. Mar. Pollut. Bull. 1989, 20, 187–189. [Google Scholar] [CrossRef]

| Elements | EDI (mg/kgbw/day) | EWI (mg/kgbw/week) | %PTW | PI | MDI | MWI | CRlim (kg/day) | CRmm (meals/month) | THQ | CR | RR (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | |||
| Cd | 3.30 × 10−4 | 1.46 × 10−3 | 2.31 × 10−3 | 1.02 × 10−2 | 0.47 | 2.08 | 0.20 | 350.00 | 75.00 | 2450.00 | 525.00 | 0.35 | 0.08 | 46.93 | 20.03 | 3.30 × 10−4 | 1.46 × 10−3 | 2.08 × 10−5 | 9.17 × 10−5 | 0.20 |
| Cu | 1.02 × 10−3 | 4.51 × 10−3 | 7.16 × 10−3 | 3.16 × 10−2 | 2.92 × 10−3 | 1.29 × 10−2 | 0.02 | 112.90 | 24.19 | 790.32 | 169.35 | 4.52 | 0.97 | 605.60 | 258.40 | 2.56 × 10−5 | 1.13 × 10−4 | nd | nd | 1.56 × 10−2 |
| Zn | 1.63 × 10−3 | 7.21 × 10−3 | 1.14 × 10−2 | 5.05 × 10−2 | 2.33 × 10−3 | 1.03 × 10−2 | 0.01 | 70.71 | 15.15 | 494.95 | 106.06 | 21.21 | 4.55 | 2844.48 | 1213.72 | 5.44 × 10−6 | 2.40 × 10−5 | nd | nd | 3.30 × 10−3 |
| Pb | 2.95 × 10−3 | 1.30 × 10−2 | 2.07 × 10−2 | 9.12 × 10−2 | 1.18 | 5.21 | 0.90 | 39.11 | 8.38 | 273.74 | 58.66 | 0.14 | 0.03 | 18.35 | 7.83 | 8.43 × 10−4 | 3.72 × 10−3 | 2.51 × 10−7 | 1.11 × 10−6 | 0.51 |
| Elements | EDI (mg/kgbw/day) | EWI (mg/kgbw/week) | %PTW | PI | MDI | MWI | CRlim (kg/day) BL | CRmm (meals/month) | THQ | CR | RR (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | |||
| Cd | 3.63 × 10−4 | 1.60 × 10−3 | 2.54 × 10−3 | 1.12 × 10−2 | 0.52 | 2.29 | 0.22 | 318.18 | 68.18 | 2227.27 | 477.27 | 0.32 | 0.07 | 42.67 | 18.21 | 3.63 × 10−4 | 1.60 × 10−3 | 2.29 × 10−5 | 1.01× 10−4 | 0.22 |
| Cu | 1.17 × 10−3 | 5.17 × 10−3 | 8.20 × 10−3 | 3.62 × 10−2 | 3.35× 10−3 | 1.48 × 10−2 | 0.02 | 98.59 | 21.13 | 690.14 | 147.89 | 3.94 | 0.85 | 528.83 | 225.65 | 2.93 × 10−5 | 1.29 × 10−4 | nd | nd | 0.02 |
| Zn | 1.68 × 10−3 | 7.43 × 10−3 | 1.18 × 10−2 | 5.20 × 10−2 | 2.40× 10−3 | 1.06 × 10−2 | 0.01 | 68.63 | 14.71 | 480.39 | 102.94 | 20.59 | 4.41 | 2760.82 | 1178.02 | 5.61 × 10−6 | 2.48 × 10−5 | nd | nd | 0.34 × 10−2 |
| Pb | 2.69 × 10−3 | 1.19 × 10−2 | 1.88 × 10−2 | 8.31 × 10−2 | 1.08 | 4.75 | 0.82 | 42.94 | 9.20 | 300.61 | 64.42 | 0.15 | 0.03 | 20.16 | 8.60 | 7.68 × 10−5 | 3.39 × 10−3 | 2.28 × 10−6 | 1.01 × 10−6 | 0.47 |
| Elements | EDI (mg/kgbw/day) | EWI (mg/kgbw/week) | %PTW BL | PI | MDI | MWI | CRlim (kg/day) | CRmm (meals/month) | THQ | CR | RR (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | |||
| Cd | 3.79 × 10−4 | 1.67 × 10−3 | 2.54 × 10−3 | 1.12 × 10−2 | 0.54 | 2.39 | 0.23 | 304.35 | 65.22 | 2130.43 | 456.52 | 0.30 | 0.07 | 40.81 | 17.41 | 3.79 × 10−4 | 1.67 × 10−3 | 2.39 × 10−5 | 1.05 × 10−4 | 0.23 |
| Cu | 8.58 × 10−4 | 3.79 × 10−3 | 8.20 × 10−3 | 3.62 × 10−2 | 2.45 × 10−3 | 1.08 × 10−2 | 0.02 | 134.62 | 28.85 | 942.31 | 201.92 | 5.38 | 1.15 | 722.06 | 308.10 | 2.14 × 10−5 | 9.46 × 10−5 | nd | nd | 0.01 |
| Zn | 1.85 × 10−3 | 8.15 × 10−3 | 1.18 × 10−2 | 5.20 × 10−2 | 2.64 × 10−3 | 1.16 × 10−2 | 0.01 | 62.50 | 13.39 | 437.50 | 93.75 | 18.75 | 4.02 | 2514.32 | 1072.84 | 6.16 × 10−6 | 2.72 × 10−5 | nd | nd | 3.73 × 10−3 |
| Pb | 2.74 × 10−3 | 1.21 × 10−2 | 1.88 × 10−2 | 8.31 × 10−2 | 1.10 | 4.83 | 0.83 | 42.17 | 9.04 | 295.18 | 63.25 | 0.15 | 0.03 | 19.79 | 8.44 | 7.82 × 10−5 | 3.45 × 10−4 | 2.33 × 10−7 | 1.03 × 10−5 | 0.47 |
| Elements | EDI (mg/kgbw/day) | EWI (mg/kgbw/week) | %PTW BL | PI | MDI | MWI | CRlim (kg/day) | CRmm (meals/month) | THQ | CR | RR (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | |||
| Cd | 2.97 × 10−4 | 1.31 × 10−3 | 2.08 × 10−3 | 9.17 × 10−3 | 0.42 | 1.87 | 0.18 | 388.89 | 83.33 | 2722.22 | 583.33 | 0.39 | 0.08 | 52.15 | 22.25 | 2.97 × 10−4 | 1.31 × 10−3 | 1.87 × 10−5 | 8.26 × 10−5 | 0.18 |
| Cu | 8.25 × 10−4 | 3.64 × 10−3 | 5.77 × 10−3 | 2.55 × 10−2 | 2.36 × 10−3 | 1.04 × 10−2 | 0.02 | 140.00 | 30.00 | 980.00 | 210.00 | 5.60 | 1.20 | 750.94 | 320.42 | 2.06 × 10−5 | 0.91 × 10−4 | nd | nd | 1.25 × 10−2 |
| Zn | 1.68 × 10−3 | 7.43 × 10−3 | 1.18 × 10−2 | 5.20 × 10−2 | 2.40 × 10−3 | 1.06 × 10−2 | 0.01 | 68.63 | 14.71 | 480.39 | 102.94 | 20.59 | 4.41 | 2760.82 | 1178.02 | 5.61 × 10−6 | 2.48 × 10−5 | nd | nd | 0.34 × 10−2 |
| Pb | 1.91 × 10−3 | 8.44 × 10−3 | 1.34 × 10−2 | 5.91 × 10−2 | 0.77 | 3.38 | 0.58 | 60.34 | 12.93 | 422.41 | 90.52 | 0.21 | 0.05 | 28.32 | 12.08 | 5.47 × 10−4 | 2.41 × 10−3 | 1.63 × 10−6 | 7.18 × 10−6 | 0.33 |
| Region | Species | Zn | Cu | Pb | Cd | References |
|---|---|---|---|---|---|---|
| Indonesia | Penaeus merguensis | 2.13 | 1.166 | 0.022 | 0.0002 | [51] |
| Nigeria | Macrobrachium felicinum | 2.516 | 12.4–20.5 | 0.350 | 0.08 | [52] |
| Bangladesh | Panaeus monodon | 24.2–35.7 | 12.2–21.3 | 0.8–1.3 | 0.2–0.4 | [53] |
| Pakistan | Palaemon longirostris | 79–89 | 67.5–100.7 | 0.31–1.0 | 0.05–0.23 | [54] |
| Malaysia | Panaeus monodon | 13.030 | 3.567 | ND | 0.002 | [55] |
| Egypt | Penaeus indicus | 19–27 | 20–45 | 0.03–0.14 | 0.04–1.47 | [56] |
| India | Penaeus indicus | 41.9–72.0 | 16.9–37.4 | 0.02–0.09 | 0.04–0.1 | [57] |
| Gulf of California | Litopenaeus stylirostris | 57.8 | 25.4 | 5.3 | 0.66 | [58] |
| Persian Gulf | Fenneropenaeus merguiensis | 13.8 | 1.26 | 0.41 | 0.18 | [59] |
| Malay Peninsula Coast | Penaeus sp. | 60–85 | 60–130 | 0.7–3.4 | 0.2–0.9 | [60] |
| Algeria | Parapenaeus longirostris | 81–100 | 100–125 | 0.5–2.6 | 0.4–0.9 | [61] |
| Sundarbans, India | P. monodon | 1184 | -- | 32.1 | 0.74 | [62] |
| Gulf of Iskenderun, Turkey | Penaeus semisulcatus | 53.7 | 32.2 | 19.1 | 3.47 | [63] |
| China | Metapenaeus ensis | 15.8 | 28.0 | 0.135 | 0.001–0.003 | [64] |
| Mexico | Penaeus californiensis | 2–17 | 3.7–8.8 | -- | 0.01–0.3 | [65] |
| Kenya Coastal Area | Penaeus sp. | 49–102 | 45–90 | 0.1–0.6 | 1.1–8.5 | [66] |
| Persian Gulf | P. merguiensis | 47.3 | 20.3 | -- | 0.31 | [67] |
| Java Sea | P. merguiensis | 26–109 | 5–120 | -- | 0.6–13.9 | [68] |
| Gulf of Mexico | P. setiferus | 107 | 17.3 | 7.73 | 6.11 | [69] |
| Gulf of Fonseca | Panaeus monodon | 19–30 | 2.1–6.9 | 0.035–0.5 | 0.002–0.03 | [70] |
| Egypt and Saudi Arabia | Erugosquilla massavensis | 29–61 | 105–190 | 8.5–11.5 | 0.25–0.60 | [71] |
| England Coast | Palaemon elegans | 113–180 | 105–162 | -- | -- | [72] |
| NE Pacific Coast | Penaeus sp. | 48–53 | 14–20 | 0.7–1.3 | 0.1 | [73] |
| Tunisia | Parapenaeus longirostris | 1.04 (0.99–1.12) | 0.59 (0.50–0.71) | 1.56 (1.16–1.79) | 0.21 (0.18–0.23) | Present Study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Ameur, W.; Annabi, A.; Rania, K.; Marini, M. Assessment of Heavy Metal Contamination and Human Health Risk in Parapenaeus longirostris from Coastal Tunisian Aquatic Ecosystems. Pollutants 2025, 5, 23. https://doi.org/10.3390/pollutants5030023
Ben Ameur W, Annabi A, Rania K, Marini M. Assessment of Heavy Metal Contamination and Human Health Risk in Parapenaeus longirostris from Coastal Tunisian Aquatic Ecosystems. Pollutants. 2025; 5(3):23. https://doi.org/10.3390/pollutants5030023
Chicago/Turabian StyleBen Ameur, Walid, Ali Annabi, Kaddachi Rania, and Mauro Marini. 2025. "Assessment of Heavy Metal Contamination and Human Health Risk in Parapenaeus longirostris from Coastal Tunisian Aquatic Ecosystems" Pollutants 5, no. 3: 23. https://doi.org/10.3390/pollutants5030023
APA StyleBen Ameur, W., Annabi, A., Rania, K., & Marini, M. (2025). Assessment of Heavy Metal Contamination and Human Health Risk in Parapenaeus longirostris from Coastal Tunisian Aquatic Ecosystems. Pollutants, 5(3), 23. https://doi.org/10.3390/pollutants5030023







