Liquid crystal polymers, through covalent bonding, combine the spontaneous long range orientational order of the liquid crystal state with the entropically driven random coil configurations of the skeletal polymer chains in a single material [
1]. The competition between these two rather different processes leads to some unusual properties [
2]. For a side-chain liquid crystal polymer, the long range orientational ordering of the mesogenic side-chains leads to a distortion of the isotropic random coil configuration of the polymer chains to form either an oblate (−ve) or prolate (+ve) ellipsoid, depending on the nature of the coupling between the polymer backbone and the mesogenic side groups. In this work we have focused on an exemplary side-chain liquid crystal polymer formed by the copolymerization of 4-cyanophenyl-4′-(6-propenoyloxyhexanoxyl) benzoate
Scheme 1a [
3] and 2-hydroxyethyl acrylate
Scheme 1b.
These above monomers are copolymerized together to yield a random atactic copolymer (CBZ6) containing 6 mol% of II, which is used, together with a diisocyanate cross-linker, to lightly cross-link the polymer chains to form elastomeric networks. The formed polymer exhibits a glass transition of 33 °C and a nematic-isotropic transition at 129 °C [
4]. Mitchell et al. (1991) used small-angle neutron scattering coupled with isotopic labelling of the polymer chains to measure the shape of the polymer chains in a monodomain of this material [
5]. The chains were extended by about 10% in the direction of the director of the monodomain. Later work studied the consequences of copolymerizing monomers with opposite signs of coupling, which at a particular composition yield a nematic polymer with zero coupling between the polymer chain and the long orientational ordering of the mesogenic side-groups [
6].
Now, if we consider a cross-linked system, the change in the shape of the polymer chain trajectories will be transmitted to the external shape of the sample, and by switching the long range orientational order on and off, we have a molecular mechanism for yielding a macroscopic shape change. These materials exhibit a rich variety of behaviors, which has been reviewed by Davis [
7]. In this work, we combine these fascinating materials with ferro particles. The idea of mixing ferroparticles with liquid crystals was first discussed by de Gennes and Brochard in 1970 [
8]. The novel properties of liquid crystal elastomers are best illustrated by creating a mono-domain texture and then performing the cross-linking. The development of a monodomain texture can be achieved by using a number of techniques, one of which is to use a magnetic field to generate a uniform alignment.
In this work, we have utilized a ferrofluid as a source of sub-micron-sized single domain ferri or ferro-magnetic materials, in this case magnetite, suspended in a carrier fluid. The magnetic particles are coated with a surfactant to prevent particle agglomeration. In this work, we have used a sample of dry ferrofluid obtained from Ferrofluidics Ltd. Transmission electron microscopy revealed that the dry ferrofuid consisted of spherical particles 10 nm in diameter. Trial experiments revealed that single phase mixtures of these nanoparticles were formed with the low molecular weight liquid crystal BLO88, in which 1% by weight was dispersed using dichloromethane as a co-host. In the same manner, 1% by weight or 0.28% by volume of the nanoparticles were dispersed in the side-chain liquid crystal polymer CBZ6. No phase separation was observed, but when the nanoparticle fraction was increased to 1.42% by volume or above, a certain level of phase separation could be observed in a light microscope.
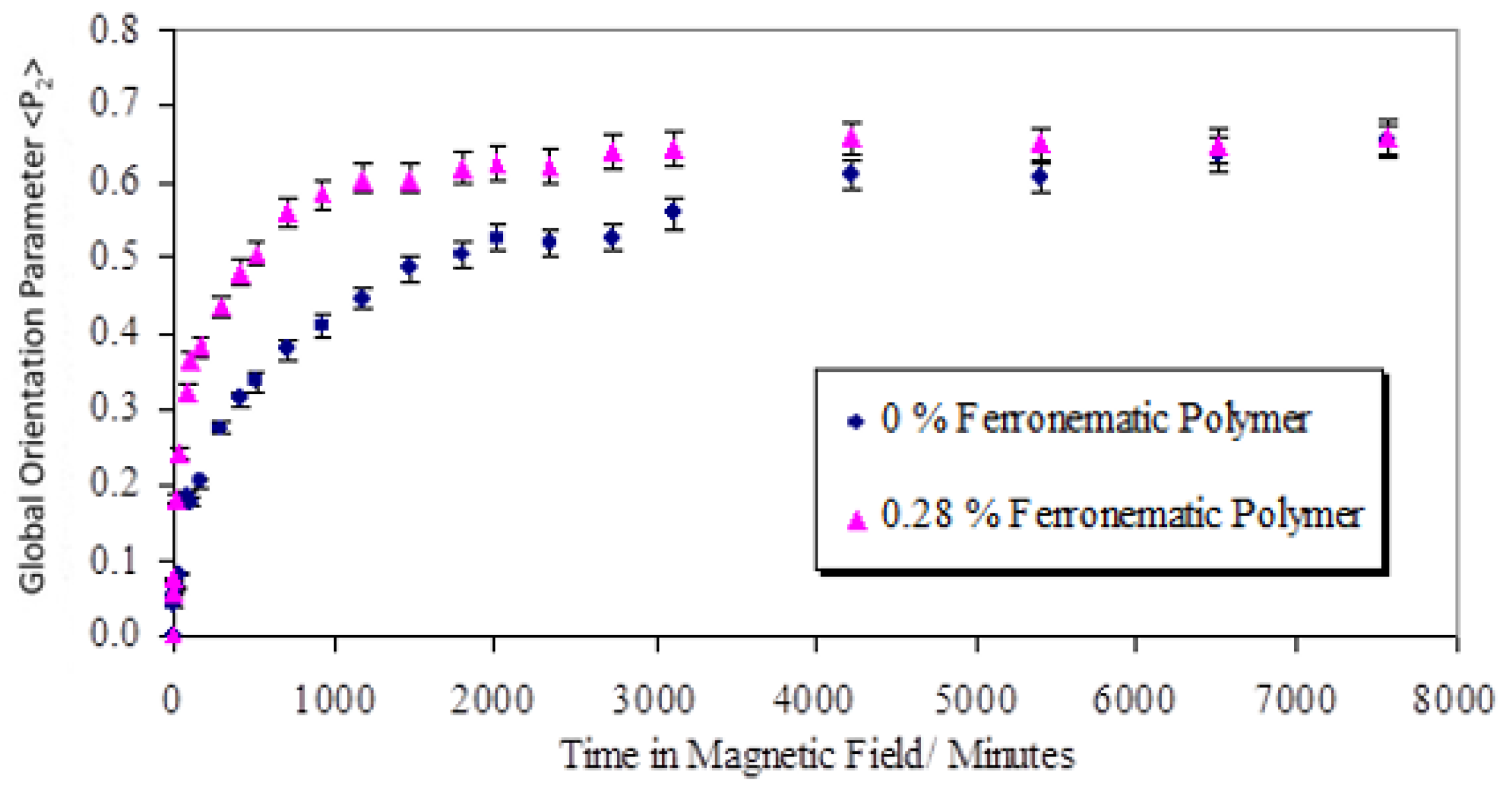
Figure 1 shows a plot of the global level of preferred orientation P
2, evaluated using wide-angle X-ray scattering [
9], for a sample of CBZ6 held in a 2.1 T magnetic field at a temperature of 90 °C as function of time compared with the behavior of the same polymer containing 0.28% by volume of the ferro nanoparticles. These results show a marked reduction in the time (by a factor of 4) required to form a monodomain when the nanoparticles are present.
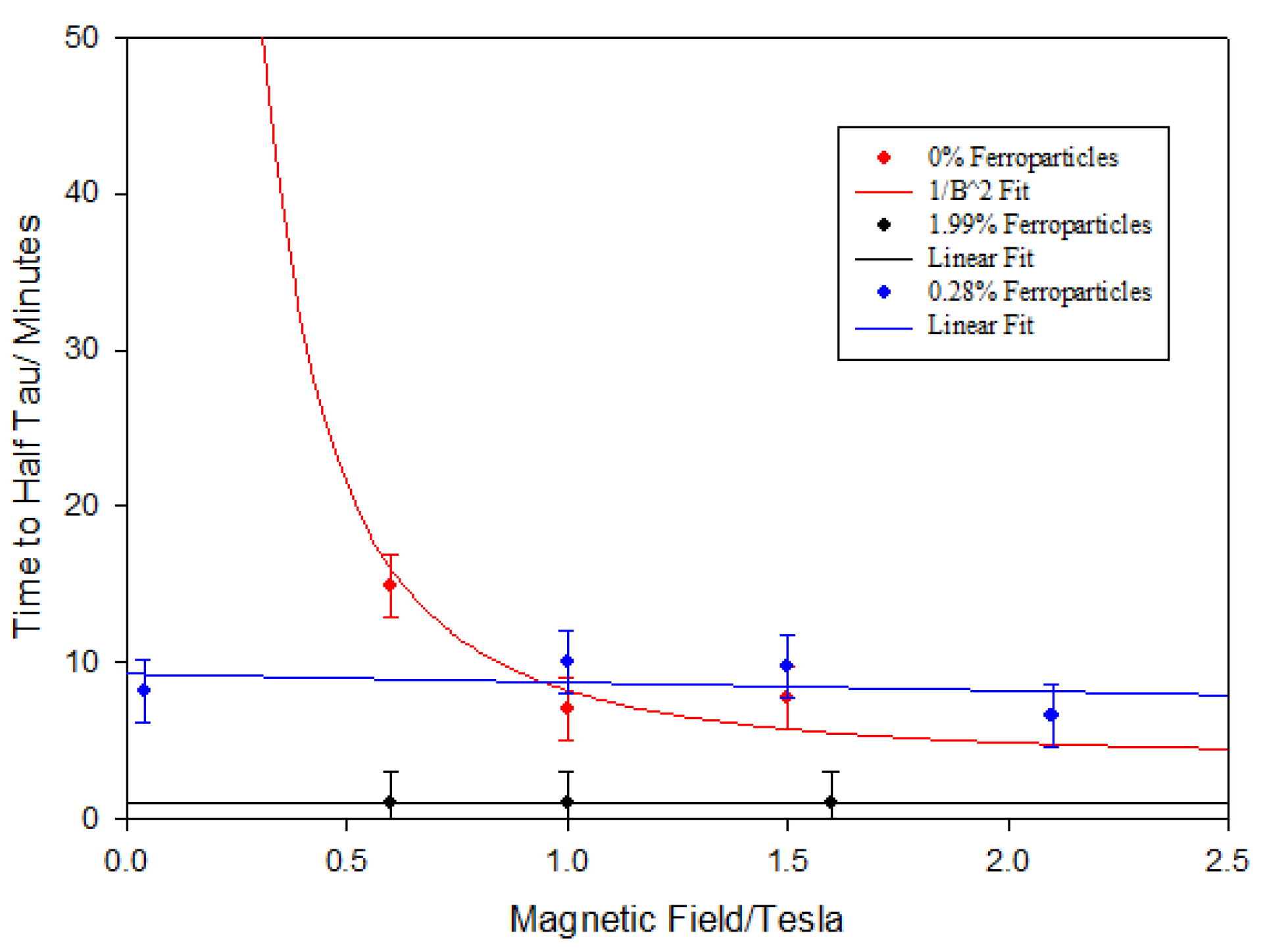
A range of ferronematic polymers was prepared with ferroparticle volume fractions from 0.07% to 28.4%. We repeated the measurements made in
Figure 1, and we evaluated the time taken to reach half the value of the monodomain orientation as a function of the applied magnetic field. In
Figure 2, we plot the results for three mixtures.
Figure 2 shows that the liquid crystal polymer behavior follows the expected B
−2 behavior for the alignment of liquid crystal directors where the molecules exhibit paramagnetic behavior [
10]. The two systems containing relatively small volume fractions of the ferroparticles show contrasting behavior, in that the alignment time is independent of the external magnetic field strength. This is a striking observation and is due to change in the mechanism of formation of a monodomain. For the pure polymer, the only coupling between the material and the magnetic field is via the anisotropy of the paramagnetic susceptibility, and this gives rise to a strong dependence on the strength of the applied magnetic field. It is well known that the individual nanoparticles can easily be magnetized in any direction with a low magnetic field. As a consequence, we can rapidly obtain the formation of a set of dipole magnets that then form chains, and we attribute the field independence of the alignment time to the fact that the alignment mechanism is one that is largely driven by the influence of these chains on the alignment, in a similar manner to that observed in polymer-stabilized liquid crystals [
11].
The formation of a liquid crystal elastomer involves a cross-linking reaction, which uses the hydroxyl sites built into the copolymers to react with an diisocynanate molecule. Initial tests were performed using hexamehylene diisocyanate (HDI). Unfortunately, it was found, in contrast to our previous studies [
5], that the cross-linking reaction was too rapid and that cross-linking occurred before a monodomain formed; it seems likely that the iron present catalyzed carbamate formation [
12]. We switched the cross-linking agent to a less reactive system in the form of hydrogenated 4,4 methylene bis (cyclohexyl isocyanate) (HDMI), and monodomain formation prior to cross-linking was realized.
Figure 3 shows the dimensions of monodomains formed from the ferronematic polymer and the side-chain liquid crystal polymer alone as a function of the temperature. The monodomains shrink in the direction parallel to the monodomain director and expand in the normal direction. The ferronematic elastomer exhibits a large level of shrinkage compared to the monodomain prepared without ferroparticles.
Combining the long range order of the liquid crystal state with the entropic nature of the polymer chains leads to fascinating new behavior. We have shown here that it is possible to prepare homogenous mixtures of a side-chain liquid crystal polymer and nanoparticles of iron oxide coated with a surfactant. Small volume fractions lead to a dramatic change in the behavior of the material in an external magnetic field. The formation of a monodomain is independent of the applied magnetic field strength. Cross-linked monodomains show anisotropic shape changes with temperature, which are larger than those observed for elastomers prepared without nanoparticles. The addition of nanoparticles to these materials leads to new behavior. This work was supported in the UK by the Engineering and Physical Science Research council and in Portugal by the Portuguese Foundation for Science and Technology (FCT) through the project reference UID/Multi/04044/2013. We thank Peter Harris for the transmission electron microscopy measurements.