Adsorption of Methylene Blue from an Aqueous Solution by Carbon Materials: A Kinetic Study †
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Osman, A.I.; Ayati, A.; Farghali, M.; Krivoshapkin, P.; Tanhaei, B.; Karimi-Maleh, H.; Krivoshapkina, E.; Taheri, P.; Tracey, C.; Al-Fatesh, A.; et al. Advanced Adsorbents for Ibuprofen Removal from Aquatic Environments: A Review. Environ. Chem. Lett. 2024, 22, 373–418. [Google Scholar] [CrossRef]
- De Lima, A.F.; Fagnani, H.M.C.; Santos, W.L.F.; De Barros, M.A.S.D. Methylene Blue Adsorption in Hydrocarbons Textile Waste. Rev. Mater. 2020, 25, e-12885. [Google Scholar] [CrossRef]
- Joshi, S.; Shrestha, R.G.; Pradhananga, R.R.; Ariga, K.; Shrestha, L.K. High Surface Area Nanoporous Activated Carbons Materials from Areca Catechu Nut with Excellent Iodine and Methylene Blue Adsorption. C 2022, 8, 2. [Google Scholar] [CrossRef]
- Krstić, A.; Lolić, A.; Mirković, M.; Kovač, J.; Arsić, T.M.; Babić, B.; Kalijadis, A. Synthesis of Nitrogen Doped and Nitrogen and Sulfur Co-Doped Carbon Cryogels and Their Application for Pharmaceuticals Removal from Water. J. Environ. Chem. Eng. 2022, 10, 108998. [Google Scholar] [CrossRef]
- Babić, B.; Kaluderović, B.; Vračar, L.; Krstajić, N. Characterization of Carbon Cryogel Synthesized by Sol-Gel Polycondensation and Freeze-Drying. Carbon N. Y. 2004, 42, 2617–2624. [Google Scholar] [CrossRef]
- Tarantino, S.; Occhipinti, R.; Maraschi, F.; Zema, M.; Riccardi Pia, M.; Profumo, A.; Sturini, M. Porous metakaolin-based geopolymers for adsorption of Contaminants of Emerging Concern from wastewaters. Appl. Clay Sci. 2024, 259, 107502. [Google Scholar] [CrossRef]
- Alves de Castro, L.; Vilar-Yanez, S.; Gomez-Gonzales, M.; Acevedo-Garcia, P.; Prieto-Arnosa, A.; Redondo-Pineiro, Y.; Rivas, J. Understanding adsorption mechanisms and metal ion selectivity of superparamagnetic beads with mesoporous CMK-3 carbon and commercial activated carbon. Microporous Mesoporous Mater. 2024, 374, 113159. [Google Scholar] [CrossRef]
- Mihajlović, S.; Vukčević, M.; Pejić, B.; Grujić, A.P.; Ristić, M. Application of Waste Cotton Yarn as Adsorbent of Heavy Metal Ions from Single and Mixed Solutions. Environ. Sci. Pollut. Res. 2020, 27, 35769–35781. [Google Scholar] [CrossRef] [PubMed]
- Pathania, D.; Sharma, S.; Singh, P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab. J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef]
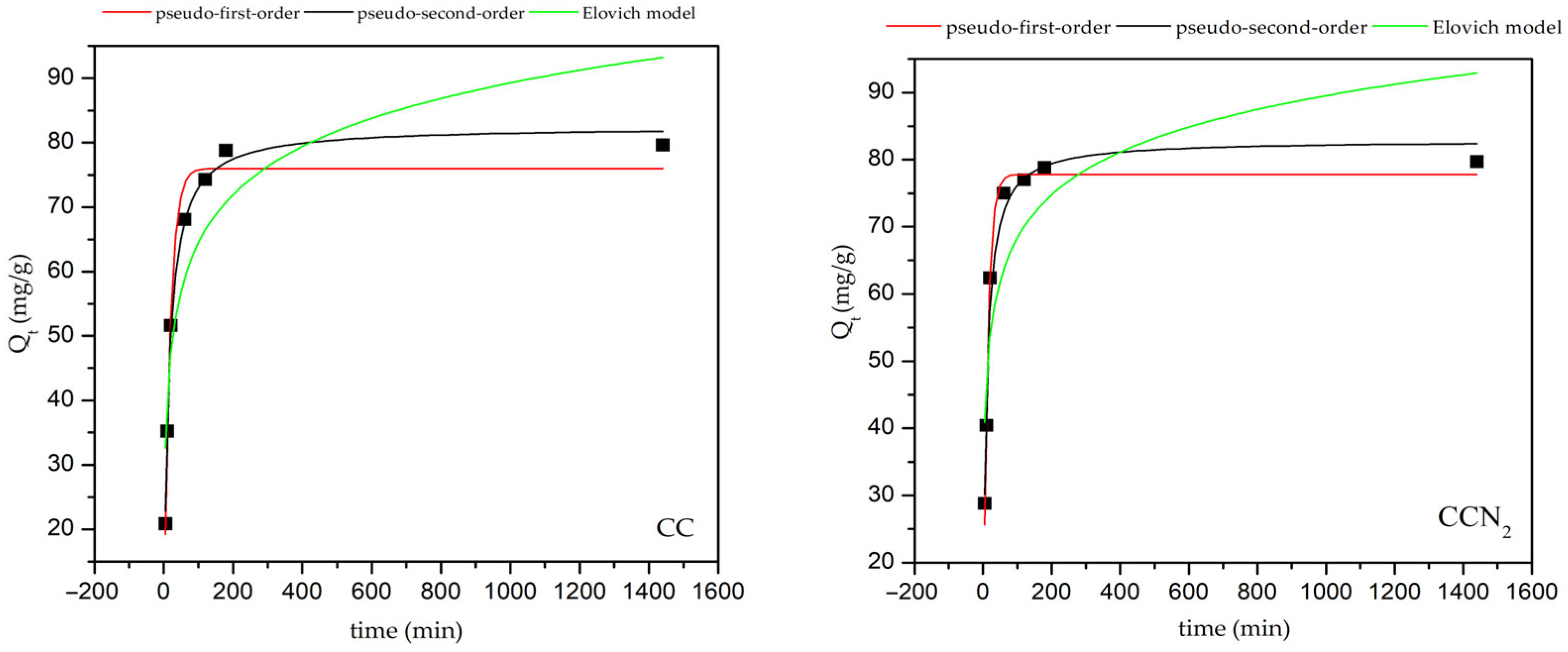
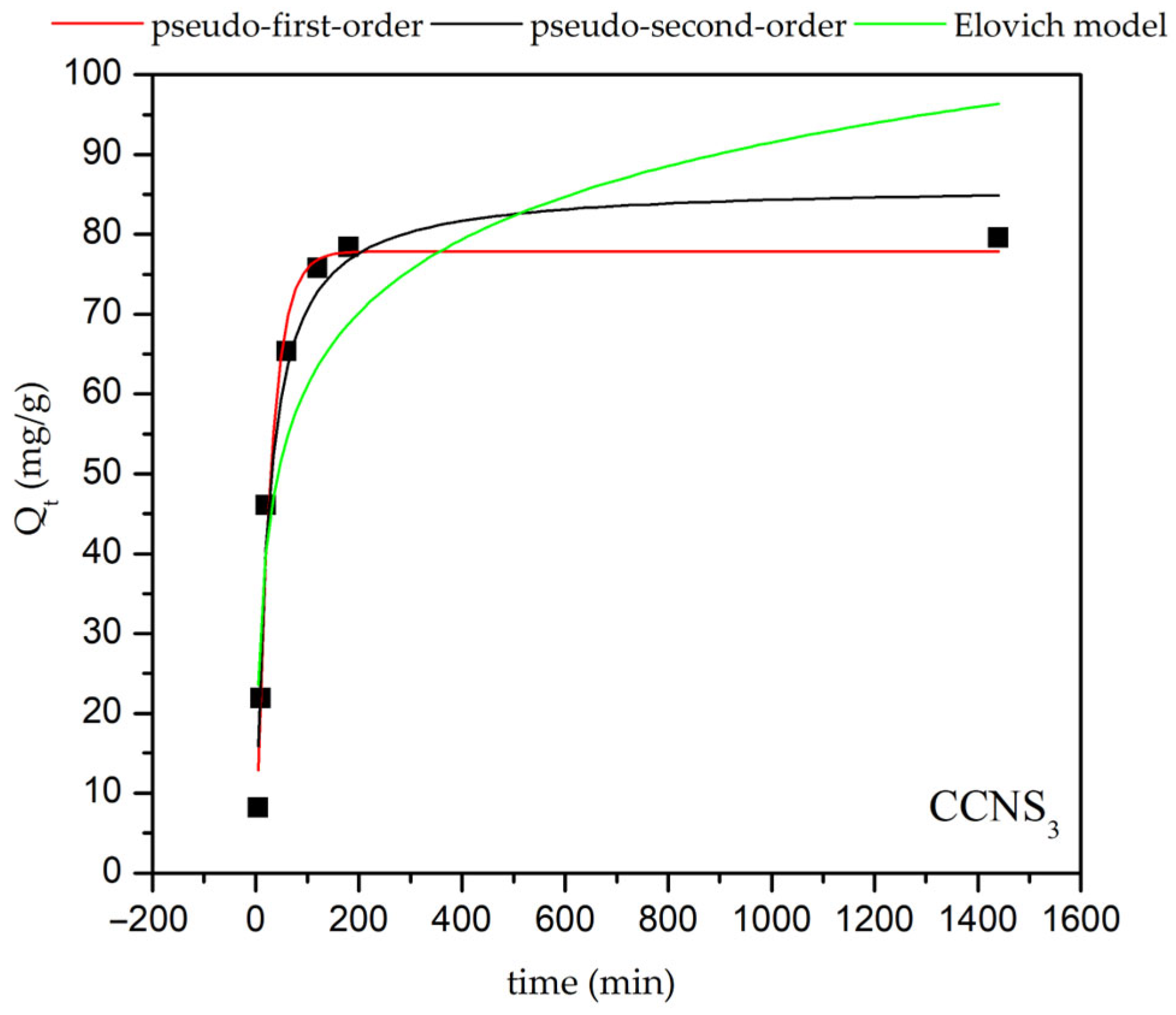
| Pseudo-First-Order Kinetic Models | |
| non linear | linear |
| Pseudo-Second-Order Kinetic Models | |
| non linear | (4)linear |
| Elovich model | |
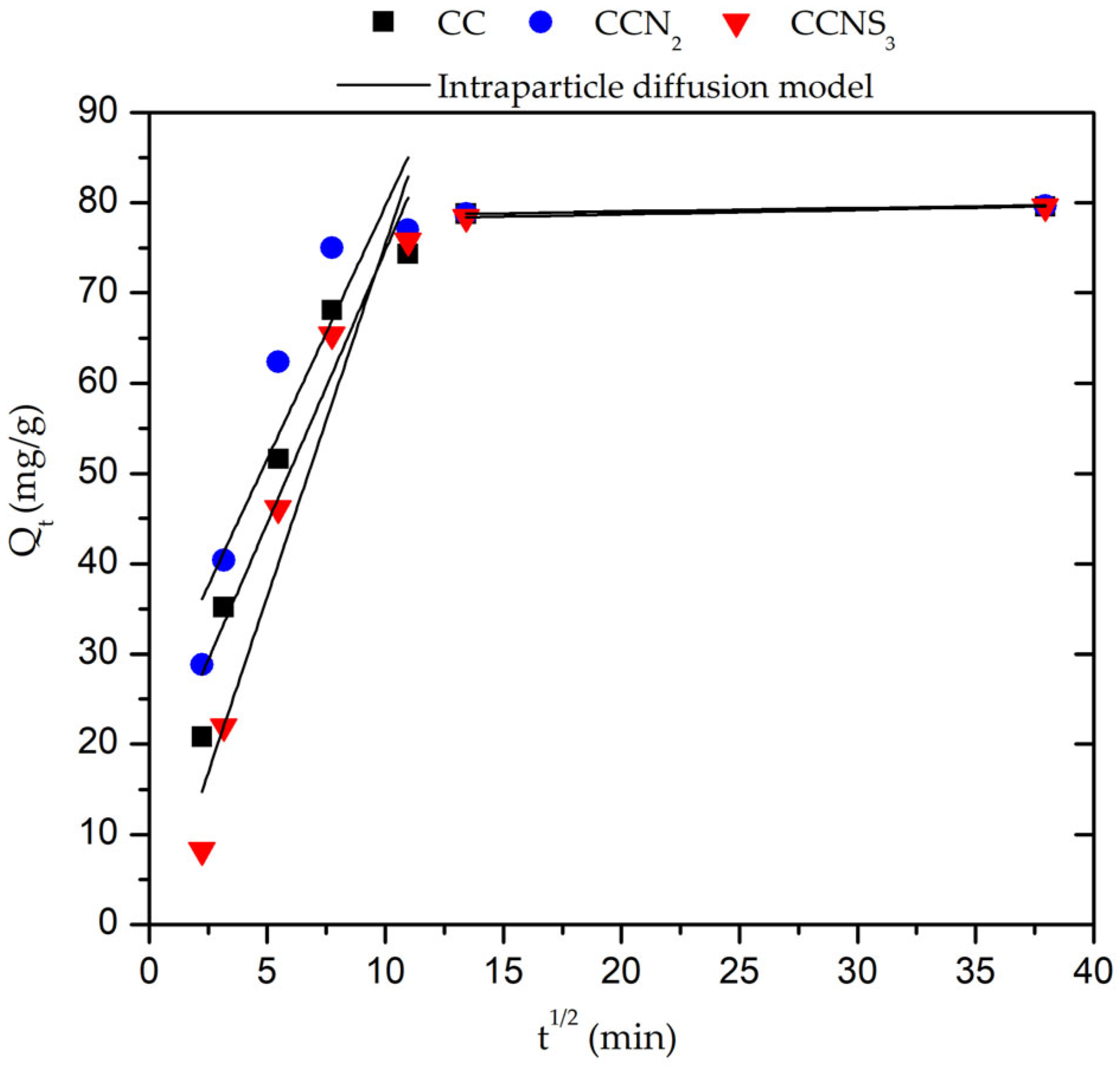
| Intraparticle diffusion model | |
| Cryogel | pseudo-first-Order | pseudo-second-order | Elovich Model | ||||||
| non-linear | non-linear | ||||||||
| linear | linear | ||||||||
| R2 | k1, g/mg min | qe calc, mg/g | R2 | k2, g/mg min | qe calc, mg/g | R2 | α, mg/g min | β, g/mg | |
| CC | 0.9774 | 0.0584 | 75.98 | 0.9941 | 0.0765 | 82.51 | 0.7846 | 42.03 | 0.09 |
| 0.9561 | 0.0214 | 3.92 | 1.0000 | 0.0012 | 80.00 | ||||
| CCN2 | 0.9878 | 0.0799 | 77.78 | 0.9750 | 0.1143 | 82.88 | 0.6953 | 155.79 | 0.11 |
| 0.9033 | 0.0286 | 3.68 | 1.0000 | 0.0021 | 80.00 | ||||
| CCNS3 | 0.9814 | 0.0362 | 77.84 | 0.9629 | 0.045 | 86.19 | 0.7589 | 13.00 | 0.07 |
| 0.9898 | 0.0278 | 4.25 | 0.9994 | 0.00064 | 80.65 | ||||
| Cryogel | R1 | ki1 (mg/gmin1/2) | C1 (mg/g) | R2 | ki2 (mg/gmin1/2) | C2 (mg/g) |
|---|---|---|---|---|---|---|
| CC | 0.9597 | 6.055 | 14.18 | 0.8947 | 0.033 | 78.36 |
| CCN2 | 0.9295 | 5.611 | 23.53 | 0.8187 | 0.037 | 78.31 |
| CCNS3 | 0.9707 | 2.736 | 7.81 | 0.9231 | 0.049 | 77.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bracanović, I.; Kalijadis, A.; Simić, M.; Krstić, A. Adsorption of Methylene Blue from an Aqueous Solution by Carbon Materials: A Kinetic Study. Eng. Proc. 2025, 99, 19. https://doi.org/10.3390/engproc2025099019
Bracanović I, Kalijadis A, Simić M, Krstić A. Adsorption of Methylene Blue from an Aqueous Solution by Carbon Materials: A Kinetic Study. Engineering Proceedings. 2025; 99(1):19. https://doi.org/10.3390/engproc2025099019
Chicago/Turabian StyleBracanović, Ivan, Ana Kalijadis, Miloš Simić, and Aleksandar Krstić. 2025. "Adsorption of Methylene Blue from an Aqueous Solution by Carbon Materials: A Kinetic Study" Engineering Proceedings 99, no. 1: 19. https://doi.org/10.3390/engproc2025099019
APA StyleBracanović, I., Kalijadis, A., Simić, M., & Krstić, A. (2025). Adsorption of Methylene Blue from an Aqueous Solution by Carbon Materials: A Kinetic Study. Engineering Proceedings, 99(1), 19. https://doi.org/10.3390/engproc2025099019






