Development of Technology for Candy Caramel with Barberry Powder and Sugar Substitute Isomaltitol †
Abstract
1. Introduction
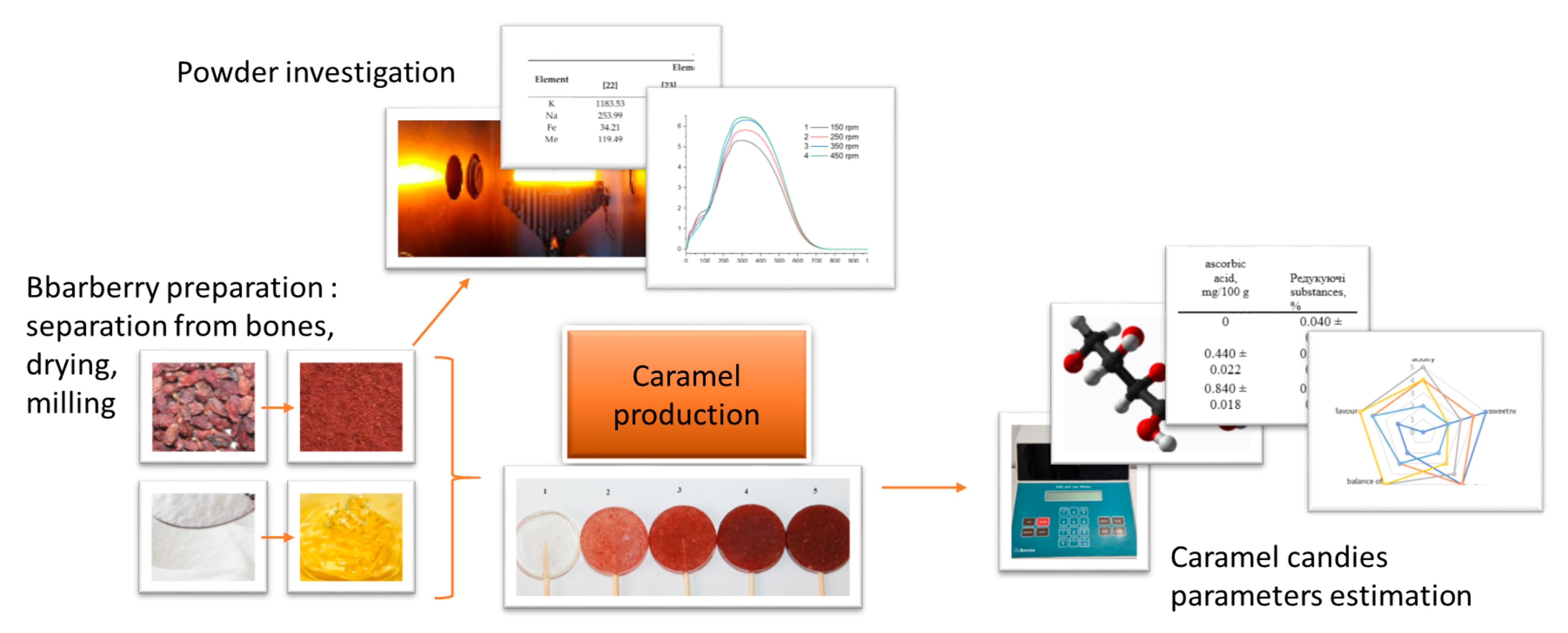
2. Materials and Methods
2.1. Materials
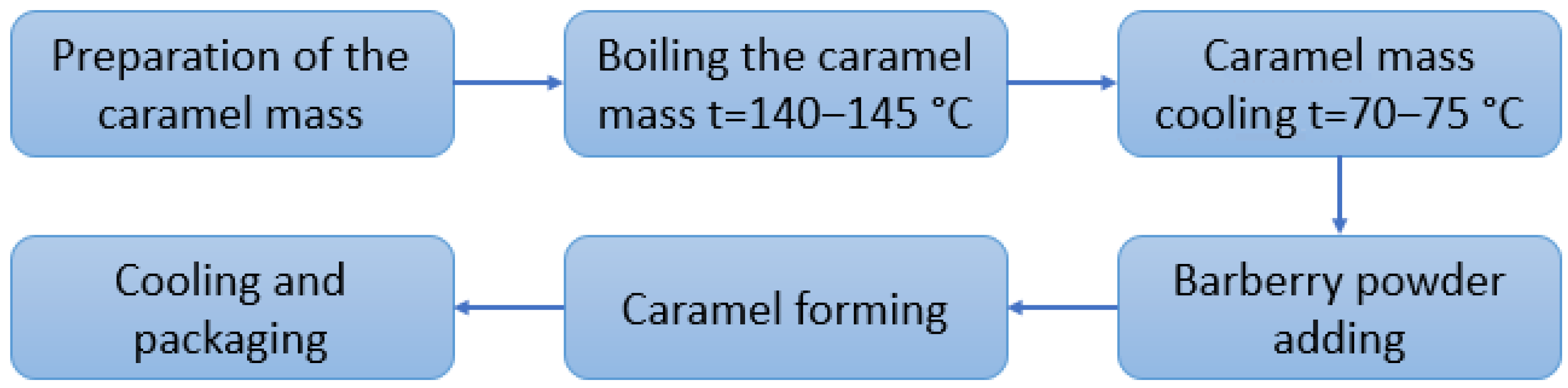
2.2. Sample Preparation
2.3. Methods
3. Results and Discussions
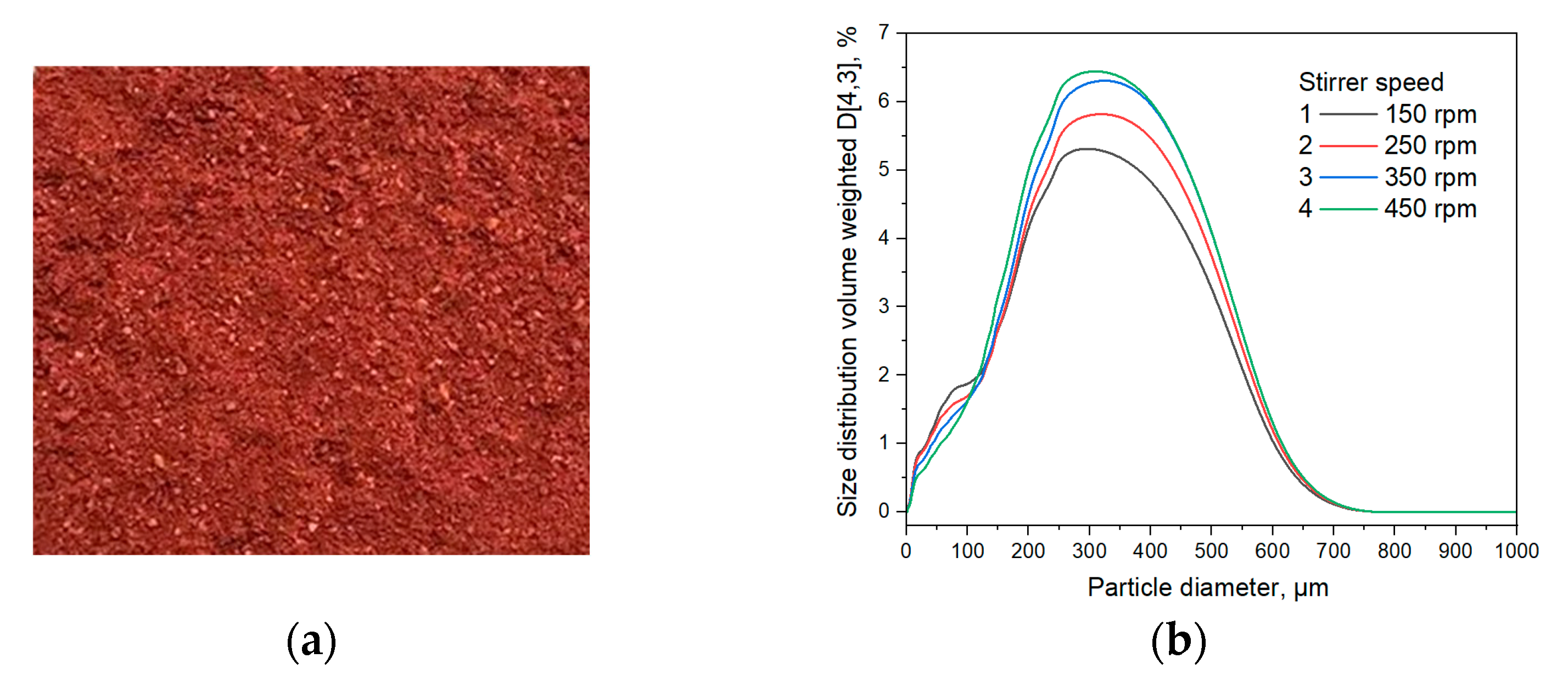
3.1. Physico-Chemical Properties of Barberry
3.2. Physico-Chemical Properties of Candies Containing Barberry
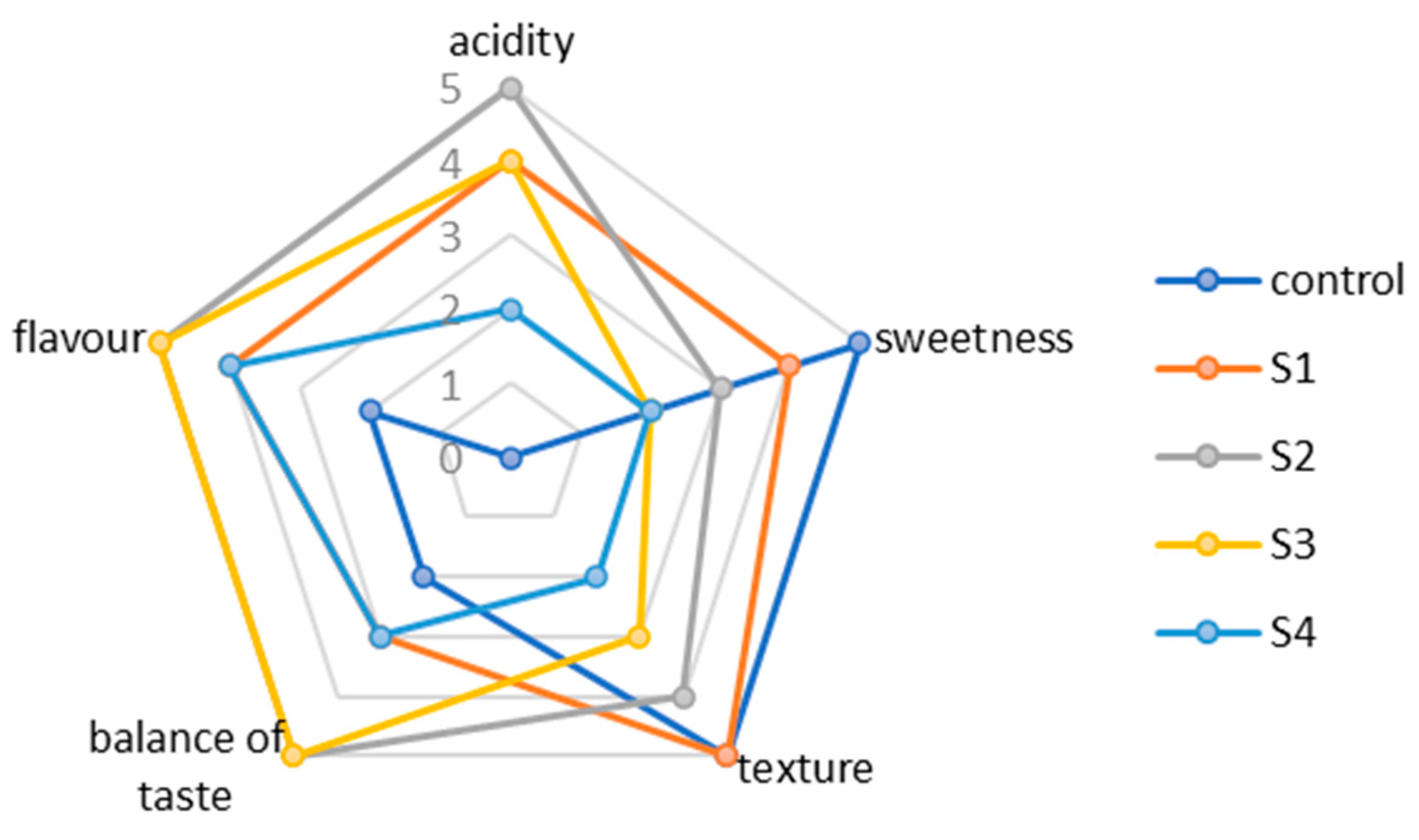
3.3. Sensory Evaluations of Candies Containing Barberry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chitgar, M.F.; Aalami, M.; Maghsoudlou, Y.; Milani, E. Comparative study on the effect of heat treatment and sonication on the quality of barberry (Berberis vulgaris) juice. J. Food Process. Preserv. 2016, 41, 12956. [Google Scholar]
- Natić, M.; Pavlović, A.; Bosco, F.L.; Stanisavljević, N.; Zagorac, D.D.; Akšić, M.F.; Papetti, A. Nutraceutical properties and phytochemical characterization of wild Serbian fruits. Eur. Food Res. Technol. 2019, 245, 469–478. [Google Scholar] [CrossRef]
- Sarraf, M.; Beig-Babaei, A.; Naji-Tabasi, S. Investigating Functional Properties of Barberry Species: An Overview. J. Sci. Food Agric. 2019, 99, 5255–5269. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Langyan, S.; Echeverría, J.; Devkota, H.P.; Tewari, D.; Moosavi, M.A.; Ezzat, S.M.; Perez-Vazquez, A.; Fraga-Corral, M.; Cravotto, G.; et al. Edible fruits and berries as a source of functional polyphenols: Current scene and future perspectives. Phytochem. Rev. 2023, 24, 1197–1225. [Google Scholar] [CrossRef]
- Gundesli, M.; Korkmaz, N.; Okatan, V. Polyphenol content and antioxidant capacity of berries: A review. Int. J. Agric. For. Life Sci. 2019, 3, 350–361. [Google Scholar]
- Andola, H.C.; Rawal, R.S.; Bhatt, I.D. Comparative studies on the nutritive and anti-nutritive properties of fruits in selected Berberis species of West Himalaya, India. Food Res. Int. 2011, 44, 2352–2356. [Google Scholar] [CrossRef]
- Awan, M.; Ali, S.; Ali, A.; Hussain, A.; Ali, M. A comparative study of barberry fruits in terms of its nutritive and medicinal contents from CKNP region, Gilgit-Baltistan, Pakistan. J. Biodivers. Environ. Sci. 2014, 5, 9–17. [Google Scholar]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Kalmarzi, R.N.; Naleini, S.N.; Ashtary-Larky, D.; Peluso, I.; Jouybari, L.; Rafi, A. Anti-Inflammatory and Immunomodulatory Effects of Barberry (Berberis vulgaris) and Its Main Compounds. Oxid. Med. Cell. Longev. 2019, 2019, 6183965. [Google Scholar] [CrossRef]
- Griffin, K. The Usage of Wild Berries and Other Fruits in the Mediaeval and Post-mediaeval Households in Norway. Bot. J. Scotl. 1994, 46, 521–526. [Google Scholar] [CrossRef]
- Salehi, B.; Selamoglu, Z.; Sener, B.; Kilic, M.; Kumar Jugran, A.; de Tommasi, N.; Sinisgalli, C.; Milella, L.; Rajkovic, J.; Flaviana BMorais-Braga, M.; et al. Berberis Plants—Drifting from Farm to Food Applications, Phytotherapy, and Phytopharmacology. Foods 2019, 8, 522. [Google Scholar] [CrossRef]
- Bakmohamadpor, M.; Javadi, A.; Azadmard-Damirchi, S.; Jafarizadeh-Malmiri, H. Effect of barberry (Berberis vulgaris) fruit powder on the quality and shelf life stability of puffed corn extrude. NFS J. 2021, 22, 9–13. [Google Scholar] [CrossRef]
- Aliakbarlu, J.; Ghiasi, S.; Bazargani-Gilani, B. Effect of extraction conditions on antioxidant activity of barberry (Berberis vulgaris L.) fruit extracts. Vet. Res. Forum 2018, 9, 361–365. [Google Scholar]
- Končić, Z.; Kremer, M.; Karlović, D.; Kosalec, I. Evaluation of antioxidant activities and phenolic content of Berberis vulgaris L. and Berberis croatica Horvat. Food Chem. Toxicol. 2010, 48, 2176–2180. [Google Scholar] [CrossRef]
- Nadali, N.; Pahlevanlo, A.; Sarabi-Jamab, M.; Balandari, A. Effect of maltodextrin with different dextrose equivalents on the physicochemical properties of spray-dried barberry juice (Berberis vulgaris L.). J. Food Sci. Technol. 2021, 59, 2855–2866. [Google Scholar] [CrossRef]
- Ebadi, A.; Rezaei, M.; Fatahi, R. Mechanism of seedlessness in Iranian seedless barberry (Berberis vulgaris L. var. asperma). Sci. Hortic. 2010, 125, 486–493. [Google Scholar] [CrossRef]
- Sema, O.; Stabnikova, O.; Sachko, A.; Gubsky, S. Chapter 6: Barberry (Berberis vulgaris L.) As an ingredient for functional food production. In Edible Wild Plants for Functional Food Production; CRC Press: Boca Raton, FL, USA, 2026; in press. [Google Scholar]
- Bourhia, M.; Elmahdaoui, H.; Moussa, S.I.; Ullah, R.; Bari, A. Potential Natural Dyes Food from the Powder of Prickly Pear Fruit Peels (Opuntia spp.) Growing in the Mediterranean Basin under Climate Stress. BioMed Res. Int. 2020, 2020, 7579430. [Google Scholar] [CrossRef]
- Raghu, V.; Platel, K.; Srinivasan, K. Comparison of ascorbic acid content of Emblica officinalis fruits determined by different analytical methods. J. Food Compos. Anal. 2007, 20, 529–533. [Google Scholar] [CrossRef]
- Hebail, F. Determination of Vitamin C Concentration in Various Fresh Orange and Lemon Samples from Janzour Region Using Volumetric Titration. Alq. J. Med. App. Sci. 2024, 7, 1214–1218. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Q. Improving measurement of reducing sugar content in carbonated beverages using Fehling’s reagent. J. Emerg. Investig. 2020, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- ISO 8589:2007/Amd 1:2014; Sensory Analysis―General Guidance for the Design of Test Rooms. International Organization for Standardization: Geneva, Switzerland, 2014. Available online: https://www.iso.org/standard/88371.html (accessed on 6 June 2014).
- Yang, L.; Zhang, Z.; Hu, X.; You, L.; Khan, R.A.A.; Yu, Y. Phenolic contents, organic acids, and the antioxidant and bio activity of wild medicinal berberis plants- as sustainable sources of functional food. Molecules 2022, 27, 2497. [Google Scholar] [CrossRef] [PubMed]
- Çakır, Ö.; Karabulut, A. Comparison of two wild-grown Berberis varieties based on biochemical characterization. J. Food Process. Preserv. 2020, 44, e14844. [Google Scholar] [CrossRef]
- Gurak, P.D.; De Bona, G.S.; Tessaro, I.C.; Marczak, L.D.F. Jaboticaba Pomace Powder Obtained as a Co-product of Juice Extraction: A Comparative Study of Powder Obtained from Peel and Whole Fruit. Food Res. Int. 2014, 62, 786–792. [Google Scholar] [CrossRef]
- Çoban, E.; Karlıdağ, H.; Kutalmış Kutsal, İ. The Influence of Different Ripening Stages, Harvest and Drying Methods on Quality of Unsulfured Sun-Dried Apricots. Food Sci. Technol. 2020, 8, 2397–2404. [Google Scholar] [CrossRef]
- Estrada-Bahena, E.B.; Salazar, R.; Ramírez, M.; Moreno-Godínez, M.E.; Jiménez-Hernández, J.; Romero-Ramírez, Y.; González-Cortázar, M.; Alvarez-Fitz, P. Influence of water activity on physical properties, fungal growth, and ochratoxin A production in dry cherries and green-coffee beans. J. Food Process. Preserv. 2022, 46, e16226. [Google Scholar] [CrossRef]
- Okatan, V.; Colak, A.M. Chemical and phytochemicals content of barberry (Berberis vulgaris L.) fruit genotypes from Sivasli district of Usak province of Western Turkey. Pak. J. Bot. 2019, 51, 165–170. [Google Scholar] [CrossRef]
- Vignesh, A.; Veerakumari, K.P.; Selvakumar, S.; Rakkiyappan, R.; Vasanth, K. Nutritional assessment, antioxidant, anti-inflammatory and antidiabetic potential of traditionally used wild plant, Berberis Tinctoria Lesch. Trends Phytochem. Res. 2021, 5, 71–92. [Google Scholar]
- Stabnikova, O.; Stabnikov, V.; Paredes-López, O. Fruits of wild-grown shrubs for health nutrition. Plant Foods Hum. Nutr. 2024, 79, 20–37. [Google Scholar] [CrossRef]

| Element | Element Content, mg/100 g | ||||
|---|---|---|---|---|---|
| [23] | [24] | [6] | [25] | Ukrainian Barberry | |
| K | 1183.53 | 1274.54 | 392.0–648.9 | 1225/1380 | 1536.94 ± 4.67 |
| Na | 253.99 | 10.92 | 24.0–72.6 | 18/198 | 248.81 ± 2.98 |
| Fe | 34.21 | 10.92 | 12.3–180.8 | 0/10 | 8.19 ± 0.58 |
| Mg | 119.49 | 2.33 | 0.5–5.8 | 58/109 | 75.11 ± 1.23 |
| Ca | 258.73 | 37.40 | 190.2–872.5 | 160/132 | 112.25 ± 5.67 |
| P | 256.04 | 203.95 | 93/109 | ||
| Mn | 0.67 | 2.27 | 1/1 | 1.52 ± 0.12 | |
| Ni | 2.29 | 0.58 | 0.28 ± 0.02 | ||
| Cu | 0.52 | 1.00 | 1.5–2.5 | 0/1 | 1.79 ± 0.15 |
| Zn | 9.47 | 9.01 | 1.6–11.2 | 2/6.4 | 0.92 ± 0.09 |
| Cd | 0.73 | 0 | 0 | ||
| Lollipop Caramel, Sample | Content of Barberry, % | Humidity, % | pH | Titratable acidity, ° (in Terms of Citric Acid) | Ascorbic Acid, mg/100 g | Reducing Substances, % |
|---|---|---|---|---|---|---|
| control | 0 | 0.010 ± 0.002 | 5.09 ± 0.01 | 0.010 ± 0.002 | 0 | 0.040 ± 0.002 |
| S1 | 1 | 0.023 ± 0.001 | 3.45 ± 0.03 | 1.024 ± 0.015 | 0.440 ± 0.022 | 0.038 ± 0.015 |
| S2 | 2.5 | 0.027 ± 0.003 | 3.25 ± 0.02 | 2.752 ± 0.023 | 0.840 ± 0.018 | 0.041 ± 0.003 |
| S3 | 5 | 0.030 ± 0.002 | 3.18 ± 0.01 | 5.504 ± 0.013 | 2.200 ± 0.021 | 0.041 ± 0.002 |
| S4 | 10 | 0.035 ± 0.003 | 3.12 ± 0.02 | 10.176 ± 0.031 | 4.250 ± 0.017 | 0.042 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sema, O.; Aksonova, O.; Sachko, A.; Gubsky, S. Development of Technology for Candy Caramel with Barberry Powder and Sugar Substitute Isomaltitol. Eng. Proc. 2025, 87, 73. https://doi.org/10.3390/engproc2025087073
Sema O, Aksonova O, Sachko A, Gubsky S. Development of Technology for Candy Caramel with Barberry Powder and Sugar Substitute Isomaltitol. Engineering Proceedings. 2025; 87(1):73. https://doi.org/10.3390/engproc2025087073
Chicago/Turabian StyleSema, Oksana, Olena Aksonova, Anastasiia Sachko, and Sergey Gubsky. 2025. "Development of Technology for Candy Caramel with Barberry Powder and Sugar Substitute Isomaltitol" Engineering Proceedings 87, no. 1: 73. https://doi.org/10.3390/engproc2025087073
APA StyleSema, O., Aksonova, O., Sachko, A., & Gubsky, S. (2025). Development of Technology for Candy Caramel with Barberry Powder and Sugar Substitute Isomaltitol. Engineering Proceedings, 87(1), 73. https://doi.org/10.3390/engproc2025087073







