Abstract
Generally, cheap and environmentally friendly biosorbent materials attract the attention of researchers and become the focus of research. Cellulose and hemicellulose come to the fore among biosorbent materials in biosorption processes. However, lignin is also a very abundant and underutilized biopolymer resource that can be preferred for biosorbent production. Lignin is an amorphous phenolic biopolymer with a structurally three-dimensional branched network structure. This biopolymer has advantages such as being available in large quantities, as well as high selectivity and sorption capacity. However, one of the biggest disadvantages is that lignin exhibits a heterogeneous structure for the balanced production of biosorbents. The structural behavior of lignin depends largely on the source and the processing conditions from which it is isolated. Today, although the use of lignin-based biosorbents is increasing, only 5% of the available lignin globally is used. This review summarized the world’s current trends, perspectives, and recent developments in lignin-based biosorbents in terms of all properties of lignin.
1. Introduction
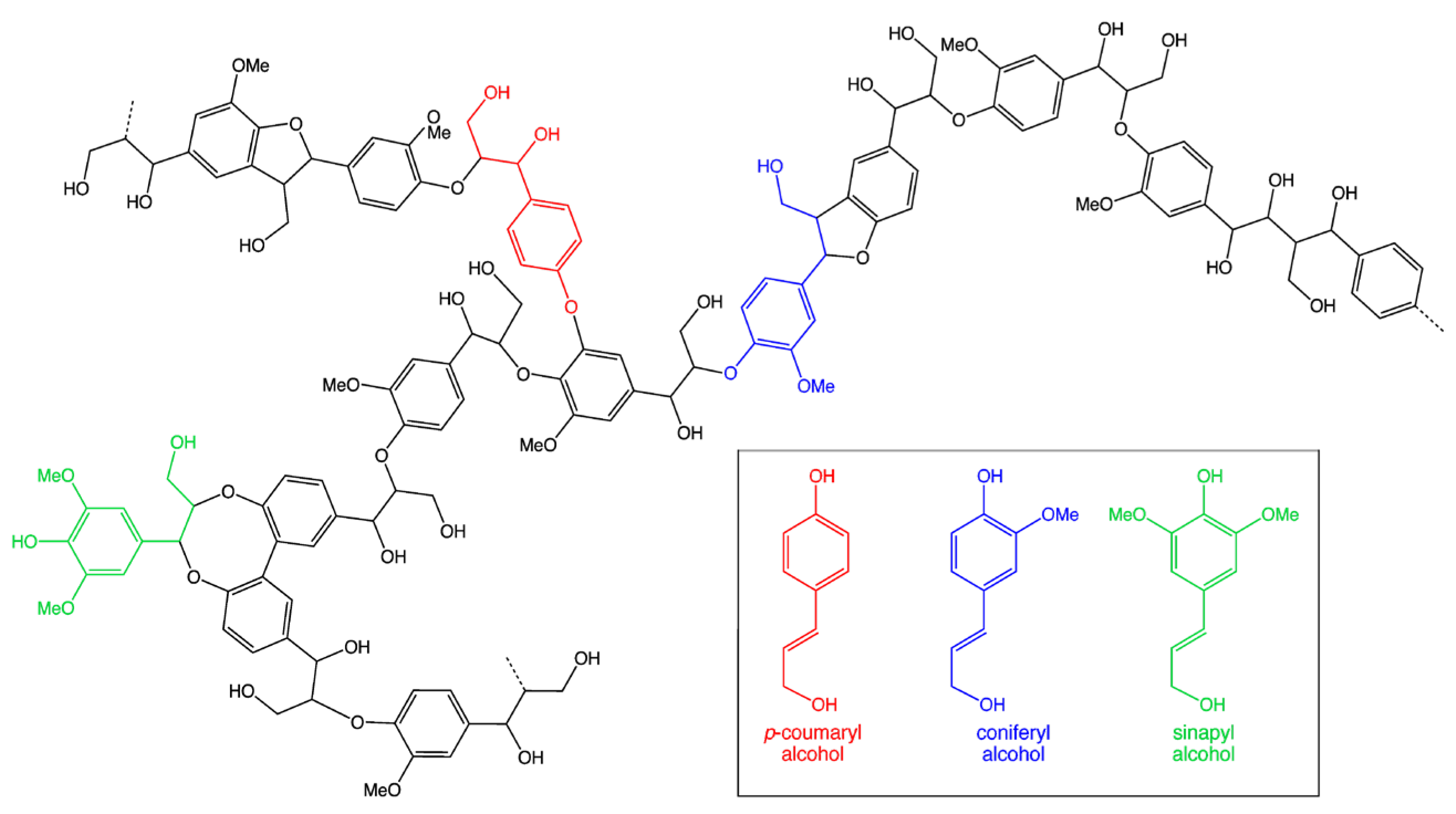
The term lignin, used by A.P. Candolle (Swedish botanist) in 1819, is the second-most important natural polymer in the world after cellulose, meaning “lignum (wood)” in Latin [1]. It has amorphous properties and exhibits a complex molecular structure compared to other natural polymers (Figure 1). The functional bonds and chemical structure of lignin vary depending on the material from which it is obtained. As an important component of lignocellulosic material, lignin has specific properties such as regeneration, biocompatibility, unique aromatic structure, and various functional groups, which has made it more attractive [2]. Approximately 170 billion tons of lignocellulosic material is synthesized by photosynthetic methods every year in the world. Among these, lignin ranks second, with approximately 60 billion tons produced [3]. According to these data, the average lignin content among lignocellulosic waste is approximately 35%.
Figure 1.
Chemical structure of lignin.
In terms of synthesis and quantity, lignin is the most preferred natural terrestrial polymer after cellulose, hemicellulose, and chitin. Lignin, mostly obtained from plant-based products, is one of the most abundant polymers containing a complex polyaromatic structure, controlling water conduction in the cell wall and providing mechanical support to the plant [4,5]. Due to its complex structure, 1–2% of lignin can be used [6]. The source and synthesis of lignin change the quality of lignin, and this also affects its application areas. There are four common processes for lignin production. These include sulfite, Kraft, soda and organosolv pulping [7]. The produced lignin material is currently used as an additive in the construction, feed, and food sectors [8]. The lignin types that are synthesized (lignosulfonates, Kraft lignin) show biological antibacterial [9], and antifungal properties [4]. Lignin is a natural source of phenolic compounds [10]. Materials containing lignin have attracted attention from both different industries and academic research over the years [5]. In this review, bibliometric analysis of recent studies and current approaches on the use of lignin as a biosorbent for the removal of different pollutants from aqueous systems due to their harmful properties and intense presence in receptive environments has been made.
2. Material and Methods
“Web of Science Core Collection; Science Direct, Springer, Wiley, Taylor & Francois, Scopus” (Clarivate Analytics®, Boston, MA, USA) and “Google Scholar” (Googleplex, Mountain View, CA, USA) were the databases used in this study. Bibliometric analysis was performed based on these databases. First, a general search was performed using the keywords “adsorption”, “biosorption”, “adsorbent”, “biosorbent”, and “different pollutant” in the basic search tool. For this research, the search has been narrowed down to specific keywords. In this context, the keywords “lignocellulosic”, “lignin”, “lignocellulose biomass”, “agricultural waste”, “food waste”, “cellulose”, and “hemicellulose” were researched to cover the last years.
3. Result and Discussion
Lignin (C18H13N3Na2O8S2), which is a lignocellulosic material, is the most abundant and renewable resource in the world. In particular, its compatibility with modifications makes it easier to serve as a raw material in the field of green chemistry. In recent years, lignin obtained from agricultural, food, and plant waste has been evaluated as a biosorbent material.
3.1. Natural Lignin Chemical Properties
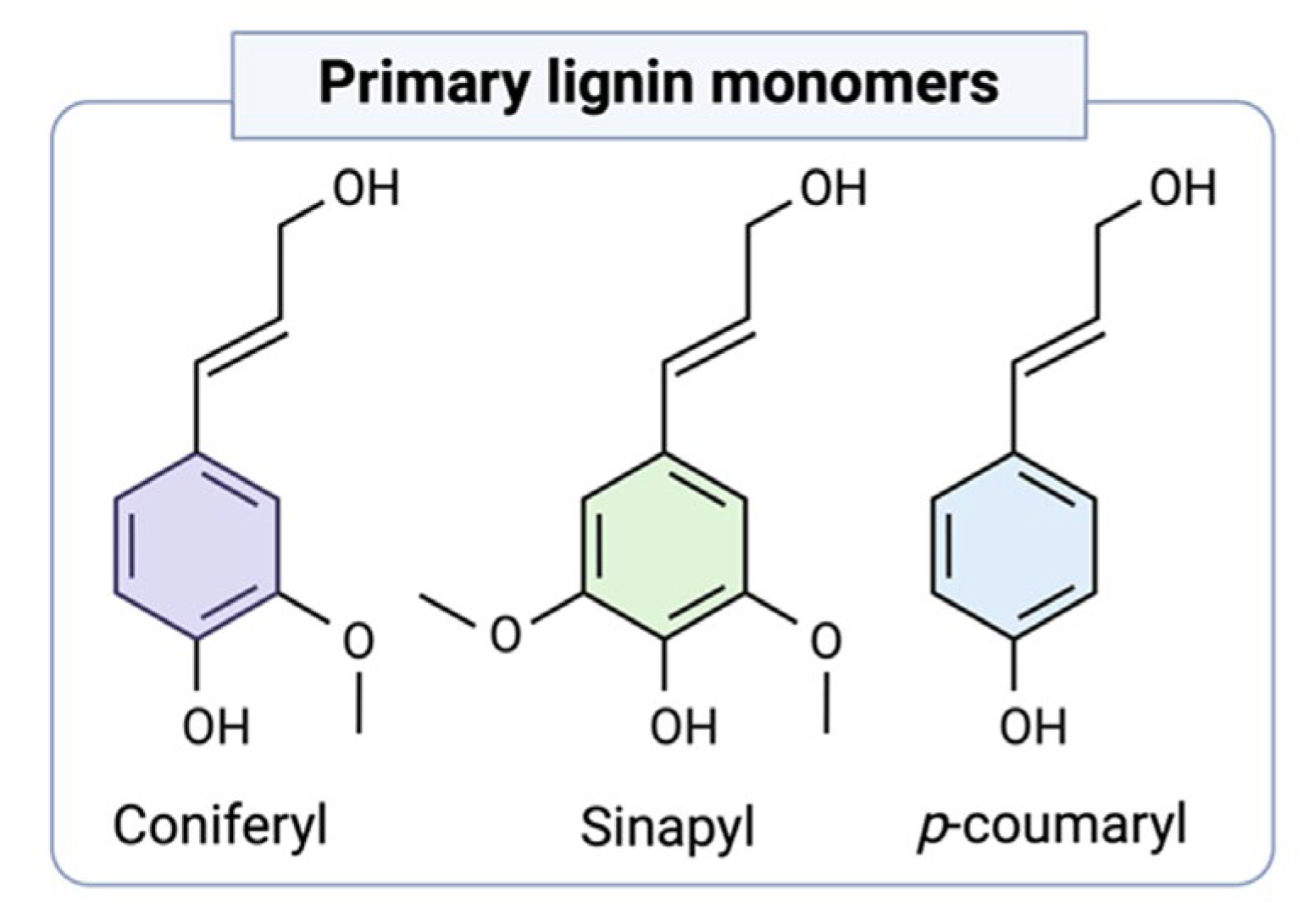
Lignin is the most abundant biopolymer in the biosphere after cellulose and hemicellulose. Therefore, it is necessary to understand the complex structure of lignin to better understand the possible lignin applications. Lignin, which is formed by the combination of 4-hydroxyphenylpropanoids, is defined as a complex phenolic polymer containing coniferyl, sinapyl, and p-coumaryl alcohols [10] (Figure 2). Lignin is a kind of complex organic polymer with a natural aromatic ring structure, mainly consisting of C (60%), H (6%), O (30%), and small amounts of N elements [3].
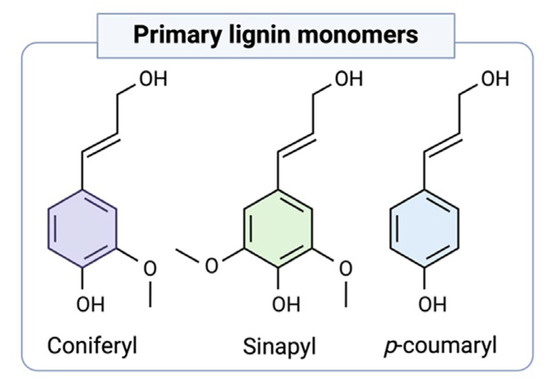
Figure 2.
Primary lignin monolignols [10].
During the synthesis process, C-C bond and β-O-4 bond (equivalent to 50% of total bonds) [10]. Lignin is structurally more reactive than natural polymer species. Its structure contains methtoxyl (R−O−CH3), carboxyl (R−COOH), carbonyl (C=O), hydroxyl (-OH), and some aldehyde groups (-RCHO). The presence and amounts of these functional groups vary depending on the synthesis of lignin and the main material synthesized [10]. Various chemical and physical approaches have been proposed to modify lignin. These modification methods generally include replacing the hydroxyl groups of lignin with hydrophobic groups [5]. The molecular weight of lignin is a fundamental factor affecting the obtaining of high value-added products from lignin [1]. The main process discovered for the evaluation of lignin is its conversion into aromatic compounds through depolymerization [11]. The main functional groups in lignin characterization, according to FTIR data, are listed in Table 1 [12]. These groups play an active role, especially in the adsorption process.

Table 1.
Functional analysis of lignin based on the FITIR [12].
3.2. Classification of Lignin
Lignin, in the structure of materials evaluated as garbage and waste, is emerging as a smart and effective material today. First of all, it is necessary to understand the complex structure of lignin to better understand possible lignin applications. Lignin is an aromatic biopolymer consisting of phenyl propane units. The classification of lignin varies according to the separation and synthesis method performed according to the type of material. In the separation process, the bonds between lignin and carbohydrates are broken by methods such as Klasson, Komarov, Willstätter, Purves, etc. Lignin is classified into 4 different groups according to the separation methods [13].
- Sulfur containing: Kraft lignin (KL), lignosulfonates (LS);
- Sulfur free: Soda lignin (SL), organosolv lignin (OL), steam-explosion lignin (BL);
- Other types of lignin: Ground wood lignin (MWL), hydrolysis lignin (HL), pyrolysis lignin (PL);
- New generation (environmentally friendly): Ionic liquid lignin (IL), deep eutectic lignin (DÖÇL) [1].
Lignosulfonates are water-soluble polyelectrolyte polymers with hydrophobic (aromatic) and hydrophilic (sulfonated group) structures [14]. They are produced using bases such as sulfur dioxide as well as calcium, ammonium, magnesium, and sodium hydroxide. In particular, α- and β-ether bonds are broken. Lignosulfonate production has been reported as 1.8 million tons per year and constitutes 90% of total commercial lignin. Different functional groups in the structure of lignosulfonates (hydroxyl-, carboxylic- and sulfur-containing groups) provide lignosulfonates with superior wettability, absorbency, and colloidal properties [15]. Kraft lignin was patented by Carl F. Dahl in 1879 [1]. Kraft delignification process, in which hydroxyl groups are activated by cleaving the -aryl bonds of lignin, takes place at three different temperatures (150 °C, 150–170 °C, >170 °C). Then, lignin recovery is achieved at pH 5 [10]. Kraft lignin has a hydrophobic structure, and modification is required to improve this property. It has a molecular weight of 200 to 200,000 g/mol [16] and a dry matter ash content of <3%. Although it constitutes 95% of lignin produced worldwide, its application area (electricity generation) is limited [10]. The organosolv process involves the synthesis of lignin from raw material by filtration and drying using a mixture of different organic solvents (formic or acetic acid and ethanol). This type of lignin has a higher degree of purity than other types due to the minimum carbohydrate and ash content. It is hydrophobic like Kraft lignin and has a low molecular weight (500 to 5000 mol/g). The most common organosolv process is the Alcell (ethanol extracted) method [10]. The soda lignin type, which dates back to 1845, is obtained without using sulfur-containing chemicals. It is carried out by adding NaOH at 160–170 °C. Plant-based leafy tree woods are generally used. SL is difficult to obtain due to the high carboxylic acid in the structure of the raw material. The molecular weights of SL vary between 840 g/mol and 6820 g/mol. The raw material is treated with steam at 180–230 °C within 1–20 min. The lignocellulosic structure is decomposed, and the steam-explosion lignin is separated by washing. BL lignin has a low molecular weight and is better dissolved in organic solvents. Nowadays, materials with lignocellulosic structure such as food wastes are abundant in receiving environments, easily available, and of low cost [5,10]. In particular, they are preferred as adsorbent materials in the adsorption process in wastewater treatment.
3.3. Use of Biosorbent
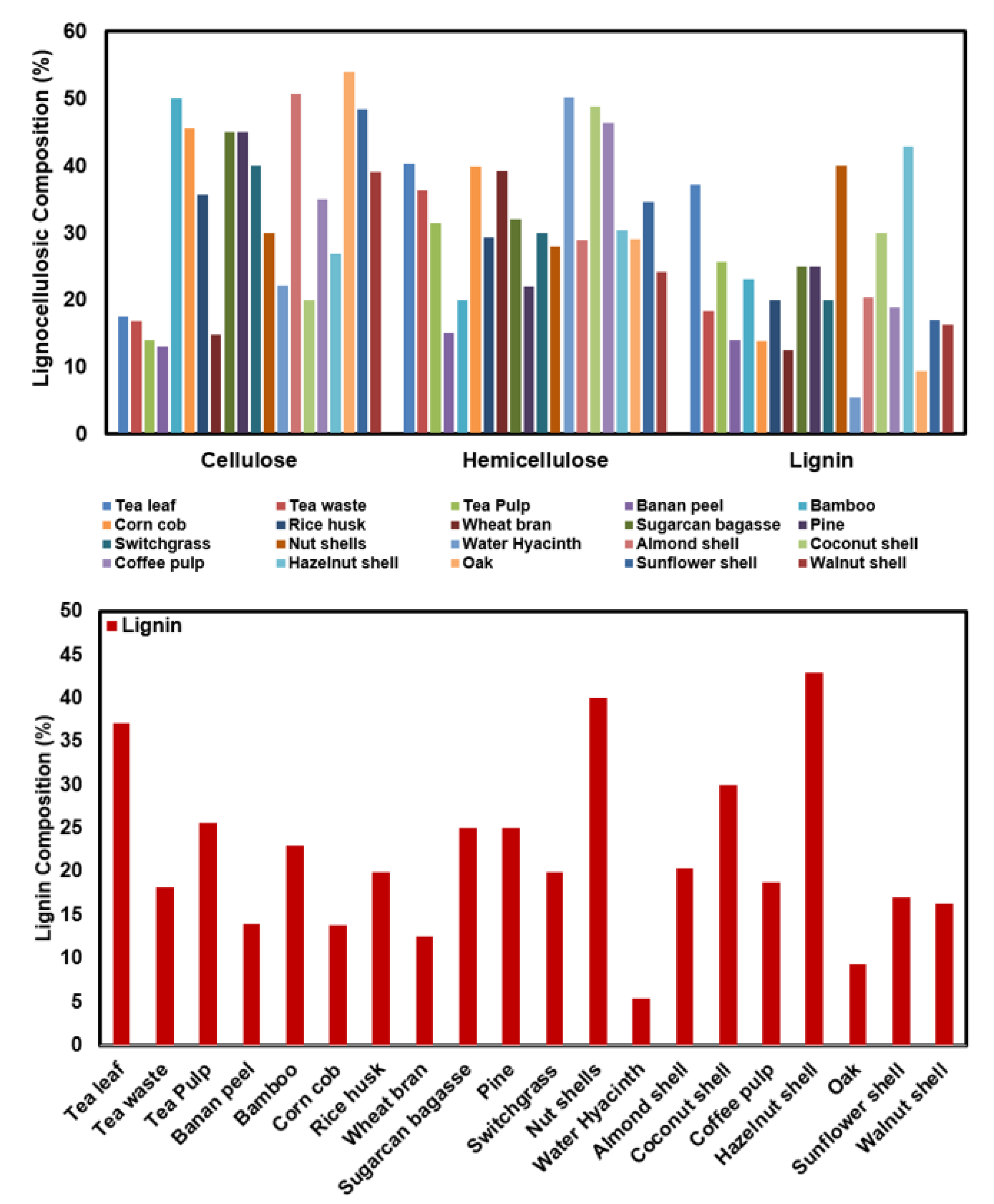
Most agricultural food wastes are disposed of by directly throwing them into the trash or burning them across all countries of the world. This situation particularly affects the use of natural resources and supports the formation of secondary pollution (problems caused by decomposition) [17]. Therefore, it is necessary to evaluate the components in the content of these materials, which are considered as garbage. In this context, the use of biosorbents is the most effective method in the biosorption process. Lignin is around 20–30% of the structure of various plant-based wastes [17]. Among the lignin types, lignosulfonates, in particular, have more suitable qualities for biosorbent use [18,19]. Lignin is suitable for biosorbent use due to its easy availability, wide source, low cost, and easy modification properties. In particular, functional groups are one of the effective factors in biosorption, and the functional groups of lignin are sufficient for biosorbent [20,21]. Due to its heterogeneous structure, effective adsorption or biosorption capacity can be achieved with modifications. Approximately 90% of the forms found in dry and raw lignocellulosic material consist of cellulose (35–55% wt), hemicellulose (20–40% wt), and lignin (10–25% wt) (Figure 3) [22,23,24].
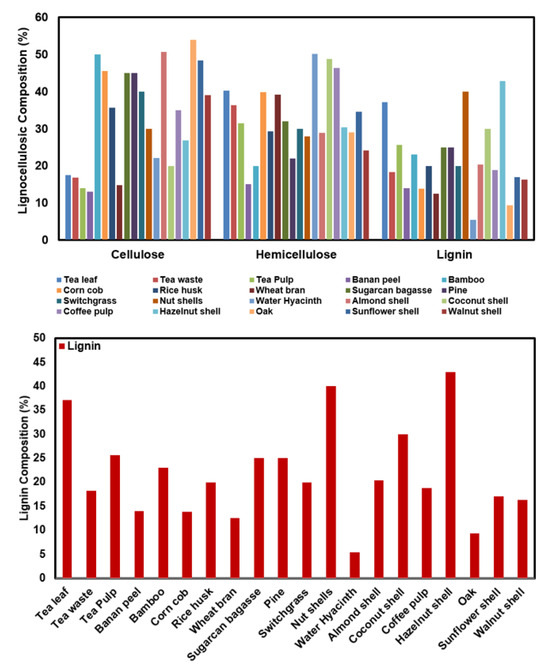
Figure 3.
Composition of some lignocellulosic materials [22,23,24].
4. Conclusions
These lignins, which are transformed into various forms, have attracted attention for their potential applications in areas ranging from agriculture and biomedicine to food science and pharmacology. The use of lignin-based materials and composites has already become an important focus area and increasing research and development efforts have been carried out over the years. Considering its near-zero cost and simplicity, lignin production from food waste is both economical and efficient. These wastes should not be considered as waste, but their applicability in treatment and other sectors should be emphasized. The lignin material evaluated in this study is not only used for biosorption but is also used as a raw material in the energy, food, and cosmetics sectors, and is evaluated as an active ingredient in the animal nutrition and pharmaceutical industries. In the future, more intensive use of waste with the waste disposal approach is aimed, especially in water treatment and soil improvement.
Author Contributions
Conceptualization, methodology, formal analysis, investigation, resources, writing—original draft preparation, writing—review and editing, visualization, H.Ç., T.B., İ.Ş. and Ş.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gülsoy, S.K.; Pekgözlü Kılıç, A. Lignin. İn Classification and Molecule Weight; Serüven Publishing: Ankara, Türkiye, 2023; pp. 131–153. ISBN 978-625-6760-55-4. [Google Scholar]
- Wang, T.; Jiang, M.; Yu, X.; Niu, N.; Chen, L. Application of lignin adsorbent in wastewater treatment: A Review. Sep. Purif. Technol. 2022, 302, 122116. [Google Scholar] [CrossRef]
- Shao, L.; Liu, N.; Wang, Z.; Zhan, P.; Zhang, L.; Wu, Z. Functional Lignin-based Polymers: Isolation, Synthetic Methods and High-valued Applications. ChemistrySelect 2023, 8, e202301633. [Google Scholar] [CrossRef]
- Jha, A.; Kumar, A. Deciphering the Role of Sodium Lignosulfonate against Candida spp. as Persuasive Anticandidal Agent. Int. J. Biol. Macromol. 2018, 107, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, M.; Mukherjee, S.; Baniasadi, H.; Abidnejad, R.; Mujtaba, M.; Lipponen, J.; Seppala, J.; Rojas, J. Lignin beyond the status quo: Recent and emerging composite applications. Green Chem. 2024, 26, 593–630. [Google Scholar] [CrossRef] [PubMed]
- Gosselink, R.J.A.; De Jong, E.; Guran, B.; Abächerli, A. Co-Ordination Network for Lignin—Standardisation, Production andApplications Adapted to Market Requirements (EUROLIGNIN). Ind. Crops Prod. 2004, 20, 121–129. [Google Scholar] [CrossRef]
- Berlin, A.; Balakshin, M. Industrial Lignins. In Bioenergy Research: Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 315–336. ISBN 978-0-444-59561-4. [Google Scholar]
- Lora, J. Industrial Commercial Lignins: Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 225–241. ISBN 978-0-08-045316-3. [Google Scholar]
- Dong, X.; Dong, M.; Lu, Y.; Turley, A.; Jin, T.; Wu, C. Antimicrobial and Antioxidant Activities of Lignin from Residue of Corn Stover to Ethanol Production. Ind. Crops Prod. 2011, 34, 1629–1634. [Google Scholar] [CrossRef]
- Reyes, D.C.; Ma, Z.; Romero, J.J. The Antimicrobial Properties of Technical Lignins and Their Derivatives—A Review. Polymers 2024, 16, 2181. [Google Scholar] [CrossRef]
- Cheng, X.; Plma, B.; Zhao, H.; Zhang, H.; Wang, J.; Chen, Z.; Hu, J. Photoreforming for Lignin Upgrading: A Critical Review. ChemSusChem 2023, 16, e202300675. [Google Scholar] [CrossRef] [PubMed]
- Ruthran, V.B.; Barmaan, P.; Kada, R.; Kumar, A. Lignin-based adsorbent for effective removal of toxic heavy metals from wastewater. Emergent Mater. 2022, 5, 923–943. [Google Scholar]
- Torres, L.A.Z.; Woiciechowski, A.L.; de Andrade Tanobe, V.O.; Karp, S.G.; Lorenci, L.C.G.; Faulds, C.; Soccol, C.R. Lignin as a potential source of high-added value compounds: A review. J. Clean. Product. 2020, 263, 121499. [Google Scholar] [CrossRef]
- Xu, C.; Ferdosian, F. Conversion of Lignin into Bio-Based Chemicals and Materials. In Green Chemistry and Sustainable Technology; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-662-54959-9. [Google Scholar]
- Gonçalves, S.; Ferra, J.; Paiva, N.; Martins, J.; Carvalho, L.H.; Magalhães, F.D. Lignosulphonates as an Alternative to Non-Renewable Binders in Wood-Based Materials. Polymers 2021, 13, 4196. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Kondor, A.; Sakuraba, Y.; Rojas, O.J.; Ago, M. Surface energy properties of lignin particles studied by inverse gas chromatography and interfacial adhesion in polyester composites with electromagnetic transparency. Cellulose 2022, 29, 2961–2973. [Google Scholar] [CrossRef]
- Kainth, S.; Sharma, P.; Pandey, O.P. Green sorbents from agricultural wastes: A review of sustainable adsorption materials. Appl. Surf. Sci. Adv. 2024, 19, 100562. [Google Scholar] [CrossRef]
- Supanchaiyamat, N.; Jetsrisuparb, K.; Knijnenburg, J.T.N.; Tsang, D.C.W.; Hunt, A.J. Lignin materials for adsorption: Current trend, perspectives and opportunities. Bioresour. Technol. 2019, 272, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xue, K.; Wang, B.; Ren, W.; Sun, D.; Shao, C.; Sun, R. Advances in lignin-based biosorbents for sustainable wastewater treatment. Bioresour. Technol. 2024, 395, 130347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y.; Wang, D.; Yu, D.; Wu, C. Lignin-based adsorbents for heavy metals. Ind. Crops Prod. 2023, 193, 116119. [Google Scholar] [CrossRef]
- Santander, P.; Butter, B.; Oyarce, E.; Yanez, M.; Xiao, L.P.; Sanchez, J. Lignin-based adsorbent materials for metal ion removal from wastewater: A review. Ind. Crops Prod. 2021, 167, 113510. [Google Scholar] [CrossRef]
- Maia, L.C.; Soares, L.C.; Gurgel, L.V.A. A review on the use of lignocellulosic materials for arsenic adsorption. J. Environ. Manage. 2021, 288, 112397. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jiang, B.; Chen, H.; Wu, W.; Wu, S.; Jin, Y.; Xiao, H. Recent advances in understanding the efects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol. Biofuels 2021, 14, 205. [Google Scholar] [CrossRef] [PubMed]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Chemistry and specialty industrial applications of lignocellulosic biomass. Waste Biomass Valori. 2021, 12, 2145–2169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).