1. Introduction
Crude oil, a vital resource since the 19th century, is being depleted rapidly due to excessive exploitation driven by global industrialization [
1]. To address this, research suggests that biofuels derived from biomass could offer a sustainable alternative. Biofuels have the potential to be carbon-neutral and emit fewer greenhouse gases than fossil fuels. Lignocellulosic biomass, composed of cellulose, hemicellulose, and lignin, is particularly promising for biofuel production [
2].
Hydrothermal liquefaction (HTL) is a promising method for converting biomass into biocrude oil, operating at temperatures of 200 to 450 °C and pressures of 4 to 22 MPa. HTL eliminates over 50% of biomass oxygen, producing biocrude oil with superior quality and increased heating value compared with pyrolysis-derived oils. HTL is not affected by feedstock moisture levels, making it versatile and efficient [
3].
Canada aims to increase the use of renewable fuels to reduce carbon emissions, with waste-to-energy technologies offering opportunities for transforming waste into valuable energy resources. Thermochemical conversion technologies like direct combustion, gasification, and HTL can efficiently convert biomass into heat, syngas, and bio-oil, respectively [
4].
This study aims to investigate the impact of process parameters on the yield of biocrude via the HTL of pulp and paper mill residues, which are classified as forest residue and lignocellulosic biomass. Furthermore, the impact of three different catalytic materials i.e., zeolite (HZSM-5), gamma-alumina (γ-Al2O3), and activated carbon on the HTL reaction are analyzed.
2. Materials and Methods
Synthetic feedstocks were utilized in replicating real biomass (pulp and paper mill residues). Microcrystalline cellulose, xylose, and alkali lignin were used to represent the cellulose, hemicellulose, and lignin fractions of pulp and paper mill residues. Dichloromethane was used as the recovery solvent. The catalysts employed are gamma-alumina (γ-Al2O3), zeolite (HZSM-5), and activated carbon.
The feedstock is made up of biomass and solvent (water). The percentages of the three components were obtained from a chemical analysis of the pulp and paper sludge obtained from the Kraft lignin process performed by Migneault et al. [
5]. These are given as follows: cellulose—59.32%, hemicellulose—11.6%, and lignin—29.08%.
The biomass introduced varies for each run. The corresponding volume of water (solvent) is added to achieve the required feedstock concentration. The feedstock is subsequently sonicated and introduced into the reactor vessel. Nitrogen gas (N
2) gas is used for pressure-leak testing and to create an inert reaction environment. For catalytic runs, 1g of catalyst is added and the reaction is initiated. The residence time starts when the temperature reaches 98% of the set temperature. When the residence time has elapsed, the reactor cools down to room temperature, and the HTL products are collected. The yields of the different phases are determined using the following equations:
As shown in
Table 1, in this study, a face-centered central composite design (CCD) was utilized to design the experimental runs while the response surface methodology (RSM) approach was employed for optimizing the response (
(
%)) [
6].
The characterization techniques employed for the catalysts used in these research work include N2 Physisorption Analysis (Brunauer–Emmett–Teller (BET) surface area measurement), and Power X-ray Diffraction (XRD).
3. Results and Discussions
3.1. Effects of Reaction Conditions on Biocrude Yield
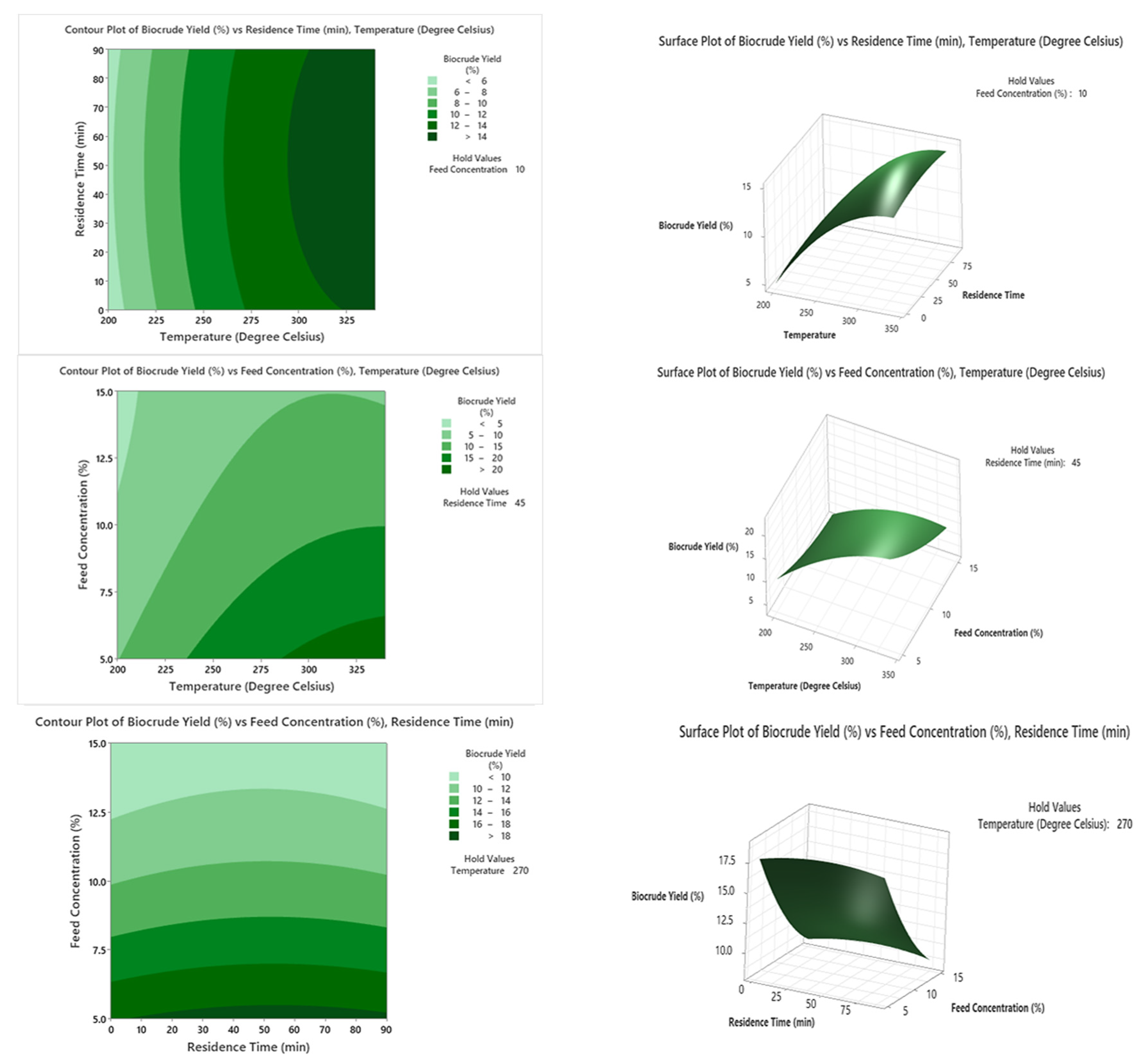
The contour and three-dimensional (3D) surface shown in
Figure 1 show the impacts of the reaction parameters on the biocrude yield. From the experimental runs carried out, a maximum yield of 21.5% was obtained.
The results show that an increase in reaction temperature leads to an increase in biocrude yield during the HTL of pulp and paper mill residues. The higher the temperature, the greater the biomass hydrolysis rate, and this consequently leads to increased biocrude formation.
The perusal of the impact of feed concentration on biocrude yield revealed that an inverse relationship exists. This is due to enhanced biomass hydrolysis taking place when more volume of solvent is added. In HTL, water has a dual role—reactant and catalyst. Consequently, an increase in the amount of water in the feedstock results in a corresponding increase in biocrude formation.
From the investigations, it was observed that an increase in residence time in the HTL reaction led to a greater period of interaction between the reactants and, thus, an increase in biocrude yield. It was also noticed that residence time has a lower effect on biocrude yield compared with temperature and feed concentration.
3.2. Optimization via RSM
3.2.1. Fitting of the Model and Analysis of Variance (ANOVA)
Utilizing Minitab Statistical Software [version Minitab® 21.4.1 (64-bit)], the acquired data underwent fitting procedures, highlighting the quadratic model as the most appropriate among the various options. The ANOVA findings underscored the statistical significance of the quadratic model, which is evident from the p-value of below 0.0001 and F-value of 42.69.
3.2.2. Optimization of Process Parameters (Non-Catalytic Optimization)
The optimization process was conducted using the response surface methodology (RSM) tool included in Minitab Statistical Software [version Minitab® 21.4.1 (64-bit)]. The optimum reaction parameters obtained were as follows: temperature: 340 °C; residence time: 56 min; and feed concentration: 5%, with a corresponding optimum biocrude yield of 22.84%. Upon running the optimization function on the software, experimental validation was carried out using these optimum conditions to validate the biocrude yield predicted. An experimental biocrude yield of 21.72% was obtained, proving that the predicted model is an excellent fit for the HTL process.
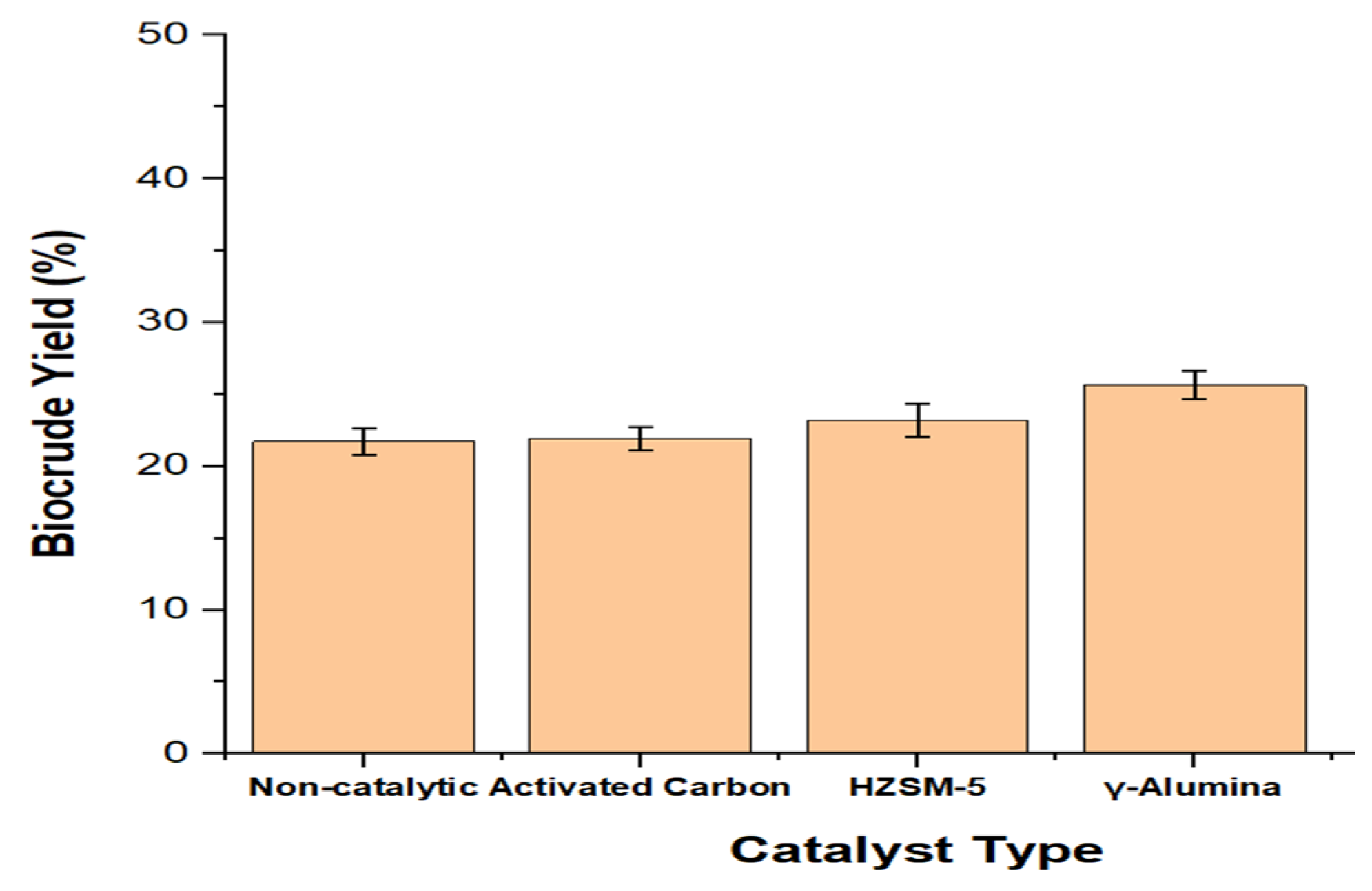
3.2.3. Catalyst Performance Evaluation (Catalytic Optimization)
The biocrude yield was optimized using three (3) commercial catalysts: gamma-alumina (γ-Al
2O
3), zeolite (HZSM-5), and activated charcoal under the optimum conditions (temperature: 340 °C, feed concentration: 5%, and residence time: 56 min) for non-catalytic HTL. One (1) gram of each catalyst was adopted for all the runs. From the results obtained in
Figure 2, the γ-alumina catalyst produced the highest improvement in biocrude yield, followed by HZSM-5 and activated charcoal.
3.3. Characterization Results
3.3.1. N2 Physisorption (BET) Analysis
Comparing the surface areas of the three catalysts reveals that activated carbon possesses the highest surface area, about 412.7 m2/g, amongst the catalysts. This is followed by γ-alumina (311.62 m2/g) and HZSM-5 (283.0 m2/g). While large surface areas should provide more sites for a reaction to take place and lead to improved yields, there are other important catalyst properties that improve/inhibit the biocrude yields. These are discussed in subsequent sections.
For pore size, the results show that all three catalysts are mesoporous (i.e., 2–50 nm pore size—as classified by IUPAC) in nature [
7], with γ-alumina possessing the largest pore size of 4.52 nm, while activated carbon and HZSM-5 have pore sizes of 4.07 nm and 3.10 nm, respectively. The assessment of the pore volumes reveals that activated carbon has the largest pore volume (0.420 cm
3/g). This is followed by γ-alumina (0.338 cm
3/g) and HZSM-5 (0.220 cm
3/g).
BET analysis was also performed on the spent catalysts for comparative analysis (
Table 2). A reduction in surface area and pore volume was observed for the three catalysts. This is due to the biochar occupying a considerable portion of the catalysts’ surfaces. For the pore size, a general increase in the pore sizes of the catalysts was observed after the reaction.
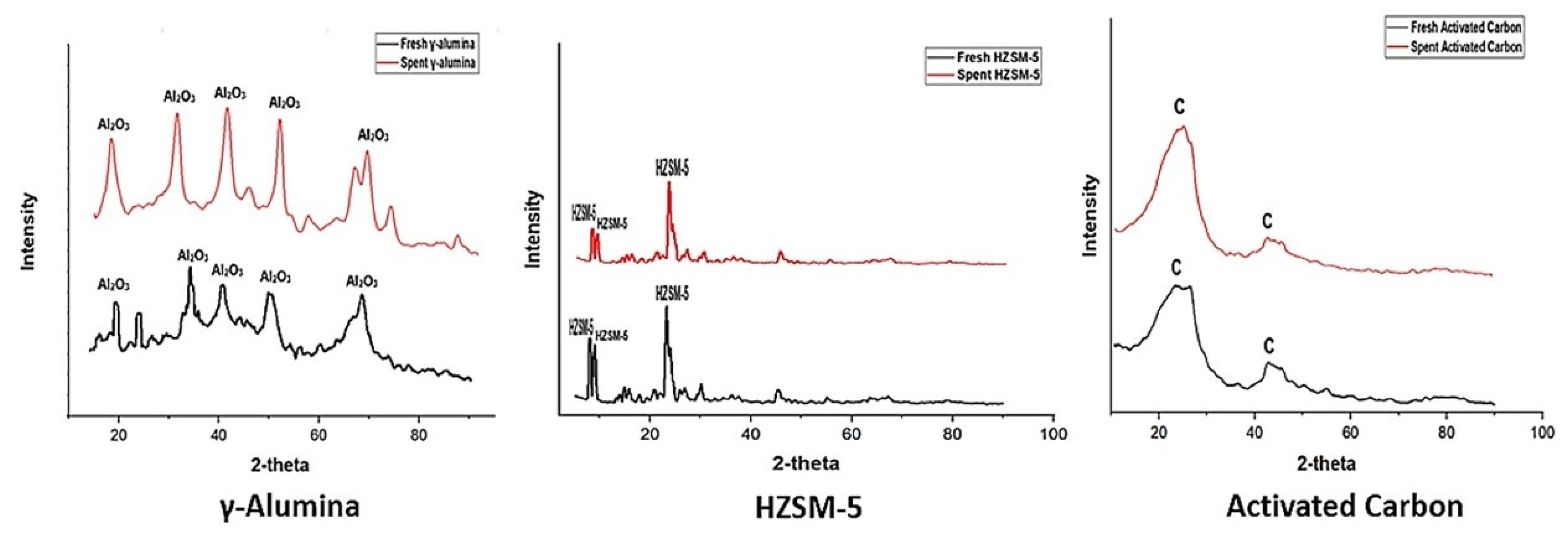
3.3.2. X-Ray Diffraction (XRD)
The γ-alumina sample has a high level of crystallinity based on the clear and discernible reflections observed at the 2θ angles of 18°, 35°, 40°, 50°, and 68° in its X-ray diffraction pattern (
Figure 3). The random distribution of aluminum ions inside the tetrahedral sites of the spinel lattice is believed to be the cause of these reflections, as stipulated by Pathak [
8] and Olusola et al. [
9]. The diffractograms obtained from the analysis of HZSM-5 exhibit distinct diffraction patterns, indicating the crystalline structure of the catalyst. Prominent and characteristic peaks are observed at 8°, 9°, and 24°. This trend was opined by Liu et al. [
10]. Lacking a distinct peak, the very wide diffraction peaks of activated carbon show that it is essentially amorphous. The two (2) broad peaks can be observed at 2θ = 24° and 43°. Jiang et al. [
11] and Perego & Peratello [
12] corroborated this observation in their research.
The crystallinity of the catalysts reduces after the HTL reaction. This can be observed in the plots below, which reveal fewer prominent peaks in the diffractograms for the spent catalysts compared with the fresh catalysts.
3.4. Catalyst Performance–Characteristic Correlations
The relationship between the properties of catalysts and their performance in producing biocrude yields can be observed through specific trends and phenomena, as demonstrated in recent experimental data and illustrated in
Figure 4.
It has been observed that there is a direct correlation between the size of the catalyst crystallites and the biocrude yield, with smaller crystallite sizes of γ-alumina leading to increased yields. This increase can be attributed to the fact that smaller crystallites provide a larger surface area relative to their volume, enhancing the catalytic activity and thereby improving the conversion efficiency of biomass into biocrude.
Similarly, the crystallinity of the catalysts plays a crucial role in determining the yield of biocrude. An increase in crystallinity, which refers to the degree of structural order within the catalyst, typically enhances the catalyst’s stability and its effectiveness in facilitating the biomass conversion process. Consequently, γ-alumina, which possesses the highest degree of crystallinity, produced the highest biocrude yield.
Conversely, no clear trend was identified concerning the effects of pore-size-to-pore-volume ratio and the Brunauer–Emmett–Teller (BET) surface areas on the biocrude yield. This inconsistency might stem from the fact that the catalysts under consideration do not originate from the same parent material, which implies that intrinsic material properties could overshadow the impacts of physical structure variations like pore dimensions and surface area.
Thus, the performance of catalysts in biocrude production is significantly influenced by the compatibility of the catalyst material with the specific type of biomass used. In this context, γ-alumina (gamma-alumina) catalysts have shown promise. The selectivity of γ-alumina, which refers to its ability to catalyze specific reactions preferentially, plays a pivotal role in its effectiveness. When paired appropriately with the biomass feedstock, γ-alumina catalysts can significantly enhance the yield and quality of biocrude, underscoring the importance of tailored catalyst selection to optimize the output of renewable fuel production processes.
4. Conclusions
From the outcomes of the research work conducted, the following key conclusions can be drawn:
Pulp and paper mill residues, the byproducts generated from the pulp and paper manufacturing process, have emerged as a viable resource for the production of biocrude. These residues, primarily consisting of lignocellulosic materials, have shown significant potential for conversion into renewable energy sources through appropriate chemical processes.
Through systematic experimentation, it was determined that a temperature of 340 °C, a feed concentration of 5%, and a residence time of 56 min are the optimum parameters for the HTL process aimed at converting these residues into biocrude.
Among the variety of catalysts evaluated in this study, γ-Al2O3 (gamma-alumina) was found to be the most effective, yielding approximately 26% biocrude. This high yield can be attributed to several intrinsic properties, such as its selectivity, high degree of crystallinity, and acidity.
Gamma-alumina is a suitable catalyst precursor for the effective conversion of pulp and paper mill residues into biocrude due to its acidic properties.
Author Contributions
Conceptualization, methodology, data curation, writing—original draft preparation: T.A. and M.Y.; writing—review and editing: P.R.; writing—review and editing, formal analysis, funding acquisition: H.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was funded by Mitacs through an industrial partnership with Enoverra Energy & Environment (Mitacs Accelerate contract (IT28806). The financial support from both organizations is greatly appreciated.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We would like to thank our industrial partner, Energy & Environment, and its founder, Mukesh Kapila, P.Eng., for their financial support and industrial mentorship. The authors also extend their gratitude to CETRI, University of Regina, for providing the research infrastructure to carry out this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yusuf, M.; Ibrahim, H. Introduction to Biofuel Production: A Step Towards Sustainable Energy. In Emerging Sustainable Technologies for Biofuel Production; Shah, M., Deka, D., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 1–14. [Google Scholar]
- Abioye, K.J.; Harun, N.Y.; Sufian, S.; Yusuf, M.; Jagaba, A.H.; Ekeoma, B.C.; Kamyab, H.; Sikiru, S.; Waqas, S.; Ibrahim, H. A review of biomass ash related problems: Mechanism, solution, and outlook. J. Energy Inst. 2024, 112, 101490. [Google Scholar] [CrossRef]
- Chisti, Y. Chapter 1—Introduction to algal fuels. In Biomass, Biofuels, Biochemicals; Pandey, A., Chang, J.-S., Soccol, C.R., Lee, D.-J., Chisti, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–31. [Google Scholar]
- Rosha, P.; Ibrahim, H. Technical feasibility of biomass and paper-mill sludge co-gasification for renewable fuel production using Aspen Plus. Energy 2022, 258, 124883. [Google Scholar] [CrossRef]
- Migneault, S.; Koubaa, A.; Riedl, B.; Nadji, H.; Deng, J.; Zhang, T. Binderless fiberboard made from primary and secondary pulp and paper sludge. Wood Fiber Sci. 2011, 43, 180–193. [Google Scholar]
- Khan, A.A.; Zaidi, S.; Qureshi, F.; Yusuf, M.; Al-Kahtani, A.A.; Kamyab, H.; Gupta, M.; Pandit, B.; Gill, H.S.; Ibrahim, H. Response surface optimization and support vector regression modeling of microwave-assisted essential oil extraction from cumin seeds. Ind. Crops Prod. 2024, 208, 117895. [Google Scholar] [CrossRef]
- Asefa, T.; Dubovoy, V. Ordered Mesoporous/Nanoporous Inorganic Materials via Self-Assembly. In Comprehensive Supramolecular Chemistry II; Atwood, J.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 157–192. [Google Scholar] [CrossRef]
- Pathak, K.D. Catalytic Conversion of Glycerol to Value-Added Liquid Chemicals. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, November 2005. [Google Scholar]
- Olusola, O.J.; Sudip, M. Temperature programme reduction (TPR) studies of cobalt phases in-alumina supported cobalt catalysts. J. Pet. Technol. Altern. Fuels 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Liu, Q.; Wen, D.; Yang, Y.; Fei, Z.; Zhang, Z.; Chen, X.; Tang, J.; Cui, M.; Qiao, X. Enhanced catalytic performance for light-olefins production from chloromethane over hierarchical porous ZSM-5 zeolite synthesized by a growth-inhibition strategy. Appl. Surf. Sci. 2018, 435, 945–952. [Google Scholar] [CrossRef]
- Jiang, L.; Yan, J.; Hao, L.; Xue, R.; Sun, G.; Yi, B. High rate performance activated carbons prepared from ginkgo shells for electrochemical supercapacitors. Carbon N. Y. 2013, 56, 146–154. [Google Scholar] [CrossRef]
- Perego, C.; Peratello, S. Experimental methods in catalytic kinetics. Catal. Today 1999, 52, 133–145. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).