Abstract
Healthcare-associated infections are serious public health problem. Besides antibacterial, antifungal, and antiviral therapies, one potential strategy for breaking the chain of infection transmission is via the installation of antibacterial surfaces. Aluminum is an attractive material for fabricating frequently touched surfaces such as doorknobs, push plate, bedrails, etc. Recently, our research group and others have demonstrated that by utilizing appropriate surface treatment technologies, such as anodization, low-surface-energy passivation, and electrochemical surface modification, on an AA6061-T6 aluminum alloy, aluminum could be rendered antimicrobial. Such surface technologies can be efficient in antimicrobial activities, and they also show advantages in terms of robustness and durability. These novel surfaces have been shown to reduce the microbial burden of clinically relevant pathogens such as Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli.
1. Introduction
Healthcare-associated infections (HCAIs) are serious public health problem [1]. They are the fourth leading cause of death after cancer, heart disease, and stroke [2]. In Canada, more than 200,000 patients contract HCAIs yearly, out of which 8000–12,000 of the cases result in fatality [2]. Besides antibacterial, antifungal, and antiviral therapies, one potential strategy for breaking the chain of HCAI transmission is to make frequently touched surfaces antimicrobial to curb the attachment of pathogens. However, the problems associated with existing antimicrobial solutions, such as a lack of long-term durable antimicrobial coating, the uncontrolled release of antimicrobial agents, and antimicrobial resistance problems [3], have necessitated the need for novel, stable, and durable antimicrobial surfaces in addition to an appropriate cleaning regime for the decontamination of frequently touched surfaces in hygiene-critical environments [4].
Owing to being lightweight and widely recyclable, aluminum (Al) is increasingly becoming the material of choice for engineering constructions. Many Al components are progressively adopted in the fabrication of medical devices [5] and are explored in fabricating frequently touched surfaces such as doorknobs, push plates, bedrails, over-bed tables, and countertops [6]. Aluminum has excellent physical, chemical, and mechanical properties such as a low density, good wear and corrosion resistance, and high elastic modulus and tensile strength [7]. Aluminum is the third most abundant element in the Earth’s crust (after oxygen and silicon) and the most abundant metal on earth. It is a highly reactive metal and exists with a layer of passive aluminum oxide film on its surface. To render aluminum surfaces antimicrobial, this passive oxide layer needs to be modified with the appropriate technology. Our research group and others have demonstrated that by utilizing appropriate surface treatment technologies, aluminum can be rendered antibacterial.
This brief review presents the current progress which has been made in antimicrobial aluminum surface technologies achieved via (i) an anodization process to create a nano-porous topographical pattern, which kills bacteria on contact; (ii) passivating the nano-porous topographical pattern to create superhydrophobic properties to repel the initial attachment of bacteria; and (iii) immobilizing antimicrobial agents on anodized aluminum to kill bacteria on contact.
2. Mechano-Bactericidal Aluminum Surfaces
In nature, many living things, such as insects, plants, sharks, and geckos, have developed protective mechanisms towards pathogens, despite their ability to use complex mechanisms to colonize surfaces [8]. The former, on the other hand, exploit their micro- and nano-topographical features to induce antimicrobial activity. These surfaces that kill bacterial cells via topography are called mechano-bactericidal surfaces [8]. The first mechano-bactericidal surfaces mediated by the nano topography of cicada wings was reported by Ivanova et al. [9]. In this work, the authors showed that by the pure physical contact of P. aeruginosa (gram-ve) bacterium on the topographical rough surface of cicada’s wings, P. aeruginosa died in 30 minutes. As a result, various surfaces on other wings, such as dragonfly and the skin of geckos, and their synthetic analogues, have been fabricated on many substrates, including aluminum alloys [10,11].
Hasan et al. [11] recently fabricated an antiviral aluminum surface using a chemical etching process. The aluminum surface was effective against SARS-CoV-2. In their other study [6], they reported the antimicrobial ability of a topography-mediated aluminum, fabricated using a chemical etching technique against common multi-drug-resistant pathogens such as Pseudomonas aeruginosa (P.A) and Staphylococcus aureus (S.A), and on respiratory viruses such as respiratory syncytial virus (RSV) and rhino virus (RV). While desirable nanoscale topography is achievable, the mechanical property of the nanoscale coating is rather weak [12]. In our research group, Agbe et al. [13] utilized anodization as an alternative surface treatment process to achieve antimicrobial aluminum surfaces with superior antimicrobial properties and mechanical robustness. In that work, a one-step hard aluminum anodization process was employed and anodization parameters, such as electrolytes concentration, current density, and anodization time, were optimized to obtain desirable surface morphology (with diameters 151 ± 37 nm), which was effective in inactivating 100% Escherichia-coli (E. coli) bacteria (Figure 1 and Figure 2).

Figure 1.
Representative images of colonies of E. coli in contact with as-received aluminum from 1 to 4 h (A–C); 3 wt.% H3PO4 anodized aluminum coupon-3HP40 from 1 to 4 h (D–F); and the reference copper samples from 1 to 4 h (G–I). Reprinted with permission from ACS Biomaterials Science & Engineering 8(3) (2022) 1087–1095. Copyright (2022) American Chemical Society.
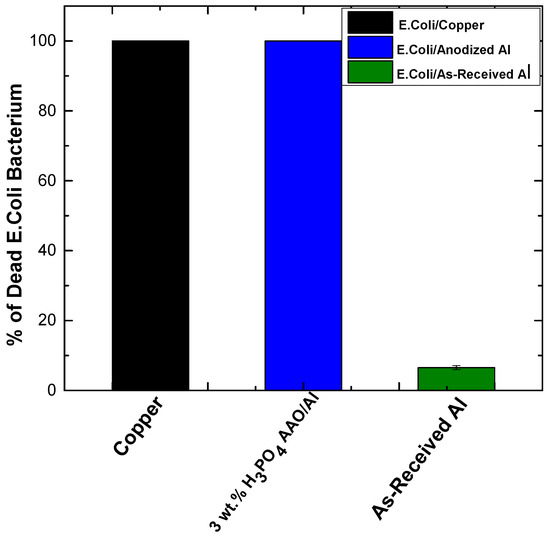
Figure 2.
Bactericidal efficiency of E. coli bacteria on 3 wt.% H3PO4 anodized aluminum 3HP40 compared to control samples (as-received aluminum and copper) under 1 h of contact. Data represent three independent experiments. Reprinted with permission from ACS Biomaterials Science & Engineering 8(3) (2022) 1087–1095. Copyright (2022) American Chemical Society.
3. Superhydrophobic Antimicrobial Aluminum Surfaces
Scientists have discovered a unique water roll-off property in several plants, such as lotus leaves. These plants utilize their surface micro-nanoscale structures to adapt and inhibit colonization as an evolutionary surviving strategy [14]. The micro-nanostructures are made of long chain palmitic (hexadecanoic) and stearic (octadecanoic) fatty acids [15], which render them superhydrophobic. Inspired by nature, scientists have now fabricated superhydrophobic surfaces in the lab by patterning micro-nano structures, followed by passivation with low-surface-energy molecules. Various strategies such as photolithography, sol–gel, plasma etching, anodization, and chemical etching [16] have been used to fabricate superhydrophobic surfaces, which find wide applications as self-cleaning, anticorrosion, drag-reduction, and anti-biofouling surfaces of different components [17]. Of particular interest is in an anti-biofouling application, as superhydrophobic surfaces have the potential to prevent initial bacterial attachment and subsequent biofilm formation.
However, superhydrophobic surfaces fail under long-term exposure in humid environments [18]. Thus, incorporating bactericides in superhydrophobic surfaces provides the promise of not only integrating anti-biofouling and antibacterial properties, but also improving their longevity. Chung et al. [19] fabricated silver-perfluorodecanethiolate coatings on a silicon wafer with both superhydrophobic and antibacterial properties via the precipitation method, using perfluorodecanethiol and silver as fluorinated and metal–thiolate complex precursors, respectively. In another work, Wang et al. [20] fabricated superhydrophobic diamond films with both antibacterial and anti-biofouling properties using both hot filament chemical vapor deposition and a sol–gel perfluorodecyltrichlorosilane process. In spite of significant effort being made for the use of superhydrophobic coatings in the application of antibacterial surfaces, the bacterial-repellent performance was rather low [21,22]. Furthermore, the applicability of such coating is limited due to degradation with time and a lack of stability.
Novel superhydrophobic coatings, which anchor low-surface-energy molecules on aluminum substrates for enhanced durability and stability, appear increasingly attractive. In a recent work by Agbe et al. [23], a novel coating of silver–polymethyhydrosiloxane (Ag-PMHS) nanocomposites (anchored on anodized Al) was fabricated via a sol–gel process for antibacterial and anti-biofouling applications. The Ag-PMHS nanocomposite coatings (anchored within an anodized Al oxide (anodized aluminum oxide)), led to enhanced adhesion, durability, and stability (Figure 3). The superhydrophobic Ag-PMHS nanocomposite coatings (achieved by combined effects of nano-rough porous anodized aluminum-Ag-PMHS nanocomposites, and low-surface-energy passivation), resulted in bacterial adhesion reductions of 99.0 %, 99.5 %, and 99.3 % for Pseudomonas aeruginosa (P.A.), E.coli, and Staphylococcus aureus (S.A.), respectively (Figure 4).

Figure 3.
Digital images of scratch test based on American Standard Test Method (ASTM D 3359-02) showing the adhesion of Ag–PMHS nanocomposite coatings with a Ag+/Si–H molar ratio of 50:2 on: (A) as-received Al; (B) AAO/Al (AgP-NcAAO); (C) 0.4% w/v silicone incorporated in AgP–NcAAO (04Sil-AgP–NcAAO); and (D) 04Sil-AgP–NcAAO in 90 days of immersion (04Sil-AgP–NcAAO-90D). Reprinted with permission from ACS Applied Bio Materials 3 (2020) 4062–4073. Copyright (2020) American Chemical Society.
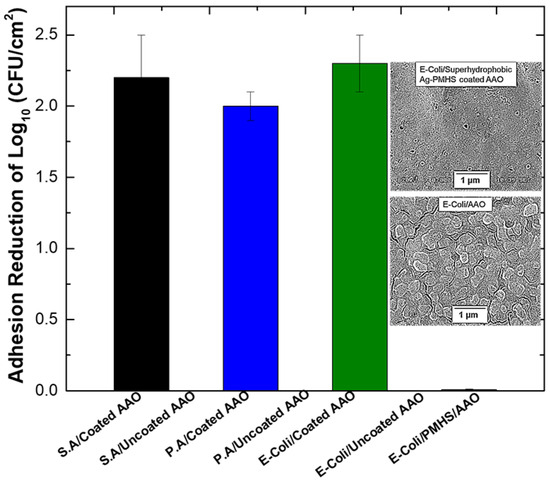
Figure 4.
Adhesion reduction in bacteria (S.A, P.A, or E-coli) on superhydrophobic AAO/Al sample (04Sil-AgP–NcAAO) and control samples (AAO/Al and PMHS/AAO/Al). Data represent multiple independent experiments. Inset: SEM micrograph of E-coli biofilm on superhydrophobic AAO/Al sample (Top) and control sample (AAO/Al) (Bottom). Reprinted with permission from ACS Applied Bio Materials 3 (2020) 4062–4073. Copyright (2020) American Chemical Society.
4. Hydrophilic Antimicrobial Aluminum Surfaces
Hydrophilic antimicrobial coatings are surfaces with a contact angle between 10° and 90°. Like superhydrophobic antimicrobial surfaces, the fabrication of hydrophilic antibacterial surfaces involves physical, chemical, and biological processes. These surfaces are particularly useful for orthopedic and medical implant devices as they enhance protein conditioning and cell tissue integration [24]. Various hydrophilic antimicrobial agents such as TiO2, ZnO, Ag, and Cu nanoparticles are commonly fabricated on metals and other substrates. However, silver-based hydrophilic antibacterial coatings fabricated via an electrochemical reduction process are further discussed.
As a widely used engineering material (for the fabrication of frequently touched surfaces), aluminum has become one of the generally used substrates for the electrodeposition of metallic coatings such as silver. For the electrodeposition of silver on anodized aluminum oxide (AAO/Al), an AC power source is recommended [25,26]. In a typical AC electrodeposition process, the barrier oxide layer acts as a rectifying p-n junction source. Therefore, AAO/Al templates can be used directly as the cathode during an electrodeposition process. Thus, the barrier layer becomes preferentially conductive during the cathodic half-cycle. This rectifying property allows for a reduction in metal ions in the pore while decreasing the oxidation rate of the deposited metal [26]. Chi et al. [25] deployed AC electrodeposition to deposit Ag for an antimicrobial application. Their results showed a 95% antimicrobial efficiency for E. coli, P. aeruginosa, Streptococcus faecalis, and S. aureus. On the other hand, for DC silver electrodeposition, it is necessary to separate the AAO/Al templates from the base aluminum metal by chemical etching, followed by the metallization of the former [27]; while DC silver electrodeposition on aluminum is feasible, in practice, it can be challenging due to the increased complexity of the overall electrodeposition process [28].
However, a novel approach was recently reported by Agbe et al. [29]. In their work, a two-step electrochemical process was deployed to fabricate Ag3PO4 nanoparticles on anodized aluminum oxide for antimicrobial applications. Ag+ ion was first reduced to Ag0 metal at a reduction process, while the metallic Ag0 was oxidized again into Ag+ ion. The antimicrobial properties of the Ag3PO4-coated anodized aluminum (Ag3PO4/AAO/Al) resulted in 100% E. coli bacteria inactivation (Figure 5).
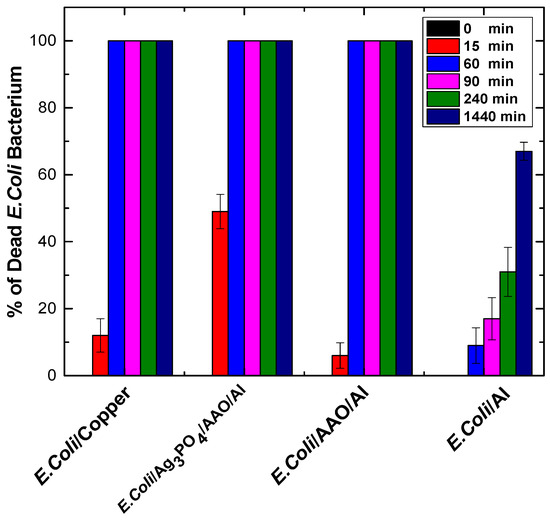
Figure 5.
Antimicrobial activity of E. coli bacteria on Ag3PO4-coated anodized AL (Ag3PO4/AAO/Al) relative to the control samples (copper, AAO/Al, and as-received aluminum) under different contact times. Reprinted with permission from Surface and Coatings Technology 428 (2021) 127892. Copyright (2021) Elsevier B.V.
5. Conclusions
Multidrug-resistance pathogens such as Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli can survive on frequently touched surfaces, such as doorknobs, push plates, over-bed tables, and bed rails, in hygiene-critical environment. Such surfaces can become potential reservoirs for the transmission of healthcare-associated infections. Antimicrobial coatings have been recognized as an important strategy for reducing the microbial burden and breaking the chain of infections. Recent advances in antimicrobial aluminum surface technologies have demonstrated that the utilization of novel surface engineering strategies, such as anodization, low-surface-energy passivation, and electrochemical surface modification of an AA6061-T6 aluminum alloy, has proved to be efficient in antimicrobial activities, and there are also advantages in terms of robustness and durability. These novel surfaces have shown the ability to reduce the microbial burden of clinically relevant and healthcare-associated pathogens such as Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli.
Author Contributions
Conceptualization, H.A., D.K.S. and X.-G.C.; formal analysis, H.A.; writing—original draft preparation, H.A.; writing—review and editing, supervision, D.K.S. and X.-G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schmidt, M.G. The role of antimicrobial surfaces in hospitals to reduce healthcare-associated infections (HAIs). In Decontamination in Hospitals and Healthcare; Walker, J., Ed.; Woodhead Publishing: Cambridge, UK, 2020; pp. 259–299. [Google Scholar]
- MacLaurin, A.; Amaratunga, K.; Couris, C.; Frenette, C.; Galioto, R.; Hansen, G.; Happe, J.; Neudorf, K.; Pelude, L.; Quach, C.; et al. Measuring and Monitoring Healthcare-Associated Infections: A Canadian Collaboration to Better Understand the Magnitude of the Problem. Healthc. Q. 2020, 22, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Adlhart, C.; Verran, J.; Azevedo, N.F.; Olmez, H.; Keinänen-Toivola, M.M.; Gouveia, I.; Melo, L.F.; Crijns, F. Surface modifications for antimicrobial effects in the healthcare setting: A critical overview. J. Hosp. Infect. 2018, 99, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Cole, M. Exploring the hand hygiene competence of student nurses: A case of flawed self assessment. Nurse Educ. Today 2009, 29, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Jain, S.; Padmarajan, R.; Purighalla, S.; Sambandamurthy, V.K.; Chatterjee, K. Multi-scale surface topography to minimize adherence and viability of nosocomial drug-resistant bacteria. Mater Des. 2018, 140, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Xu, Y.; Yarlagadda, T.; Schuetz, M.; Spann, K.; Yarlagadda, P.K. Antiviral and Antibacterial Nanostructured Surfaces with Excellent Mechanical Properties for Hospital Applications. ACS Biomater. Sci. Eng. 2020, 6, 3608–3618. [Google Scholar] [CrossRef] [PubMed]
- Lumley, R. (Ed.) Fundamentals of Aluminium Metallurgy: Production, Processing and Applications; Woodhead Publishing: Cambridge, UK, 2011. [Google Scholar]
- Lin, N.; Berton, P.; Moraes, C.; Rogers, R.; Tufenkji, N. Nanodarts, nanoblades, and nanospikes: Mechano-bactericidal nanostructures and where to find them. Adv. Colloid Interface Sci. 2018, 252, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Webb, H.K.; Truong, V.K.; Pogodin, S.; Baulin, V.A.; Watson, G.S.; Watson, J.A.; Crawford, R.J.; Ivanova, E.P. Selective bactericidal activity of nanopatterned superhydrophobic cicada Psaltoda claripennis wing surfaces. Appl. Microbiol. Biotechnol. 2013, 97, 9257–9262. [Google Scholar] [CrossRef]
- Dickson, M.N.; Liang, E.I.; Rodriguez, L.A.; Vollereaux, N.; Yee, A.F. Nanopatterned polymer surfaces with bactericidal properties. Biointerphases 2015, 10, 021010. [Google Scholar] [CrossRef]
- Hasan, J.; Pyke, A.; Nair, N.; Yarlagadda, T.; Will, G.; Spann, K.; Yarlagadda, P.K.D.V. Antiviral Nanostructured Surfaces Reduce the Viability of SARS-CoV-2. ACS Biomater. Sci. Eng. 2020, 6, 4858–4861. [Google Scholar] [CrossRef]
- Shi, H.; Yu, M.; Liu, J.; Rong, G.; Du, R.; Wang, J.; Li, S. Effect of alkaline etching on microstructure and anticorrosion performance of anodic film on Al-Mg-Si alloy. Corros. Sci. 2020, 169, 108642. [Google Scholar] [CrossRef]
- Agbe, H.; Sarkar, D.K.; Chen, X.-G. Anodized Aluminum Surface with Topography-Mediated Antibacterial Properties. ACS Biomater. Sci. Eng. 2022, 8, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Roy, A.; Chatterjee, K.; Yarlagadda, P. Mimicking insect wings: The roadmap to bioinspiration. ACS Biomater. Sci. Eng. 2019, 5, 3139–3160. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.; Nguyen, S.; Webb, H.; Hasan, J.; Truong, V.; Lamb, R.; Duan, X.; Tobin, M.; Mahon, P.; Crawford, R. Molecular organization of the nanoscale surface structures of the dragonfly Hemianax papuensis wing epicuticle. PLoS ONE 2013, 8, e67893. [Google Scholar] [CrossRef]
- Safaee, A.; Sarkar, D.; Farzaneh, M. Superhydrophobic properties of silver-coated films on copper surface by galvanic exchange reaction. Appl. Surf. Sci. 2008, 254, 2493–2498. [Google Scholar] [CrossRef]
- Brassard, J.-D.; Sarkar, D.; Perron, J. Synthesis of monodisperse fluorinated silica nanoparticles and their superhydrophobic thin films. ACS Appl. Mater. Interfaces 2011, 3, 3583–3588. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, J.; Hao, L.; Yegin, Y.; Bae, M.; Ulugun, B.; Taylor, T.; Scholar, E.; Cisneros-Zevallos, L.; Oh, J. Dual-Functional, Superhydrophobic Coatings with Bacterial Anticontact and Antimicrobial Characteristics. ACS Appl. Mater. Interfaces 2020, 12, 21311–21321. [Google Scholar] [CrossRef]
- Chung, J.-S.; Kim, B.; Shim, S.; Kim, S.-E.; Sohn, E.-H.; Yoon, J.; Lee, J.-C. Silver-perfluorodecanethiolate complexes having superhydrophobic, antifouling, antibacterial properties. J. Colloid Interface Sci. 2012, 366, 64–69. [Google Scholar] [CrossRef]
- Wang, T.; Huang, L.; Liu, Y.; Li, X.; Liu, C.; Handschuh-Wang, S.; Xu, Y.; Zhao, Y.; Tang, Y. Robust Biomimetic Hierarchical Diamond Architecture with a Self-Cleaning, Antibacterial, and Antibiofouling Surface. ACS Appl. Mater. Interfaces 2020, 12, 24432–24441. [Google Scholar] [CrossRef]
- Meier, M.; Dubois, V.; Seeger, S. Reduced bacterial colonisation on surfaces coated with silicone nanostructures. Appl. Surf. Sci. 2018, 459, 505–511. [Google Scholar] [CrossRef]
- Crick, C.; Ismail, S.; Pratten, J.; Parkin, I. An investigation into bacterial attachment to an elastomeric superhydrophobic surface prepared via aerosol assisted deposition. Thin Solid Films 2011, 519, 3722–3727. [Google Scholar] [CrossRef]
- Agbe, H.; Sarkar, D.; Chen, X.; Faucheux, N.; Soucy, G.; Bernier, J.-L. Silver-polymethylhydrosiloxane nanocomposite coating on anodized aluminum with superhydrophobic and antibacterial properties. ACS Appl. Bio Mater. 2020, 3, 4062–4073. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2018, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Chi, G.; Yao, S.; Fan, J.; Zhang, W.; Wang, H. Antibacterial activity of anodized aluminum with deposited silver. Surf. Coat. Technol. 2002, 157, 162–165. [Google Scholar] [CrossRef]
- Stepniowski, W.; Moneta, M.; Karczewski, K.; Michalska-Domanska, M.; Czujko, T.; Mol, J.; Buijnsters, J. Fabrication of copper nanowires via electrodeposition in anodic aluminum oxide templates formed by combined hard anodizing and electrochemical barrier layer thinning. J. Electroanal. Chem. 2018, 809, 59–66. [Google Scholar] [CrossRef]
- Ghaddar, A.; Gieraltowski, J.; Gloaguen, F. Effects of anodization and electrodeposition conditions on the growth of copper and cobalt nanostructures in aluminum oxide films. J. Appl. Electrochem. 2009, 39, 719–725. [Google Scholar] [CrossRef]
- Alkire, R.; Gogotsi, Y.; Simon, P. Nanostructured Materials in Electrochemistry; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Agbe, H.; Sarkar, D.; Chen, X. Electrochemically synthesized silver phosphate coating on anodized aluminum with superior antibacterial properties. Surf. Coat. Technol. 2021, 428, 127892. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).