Prediction and Optimisation of Cr (VI) Removal by Modified Cellulose Nanocrystals from Aqueous Solution Using Machine Learning (ANN and ANFIS) †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Modified Cellulose Nanocrystals
2.3. Batch Adsorption
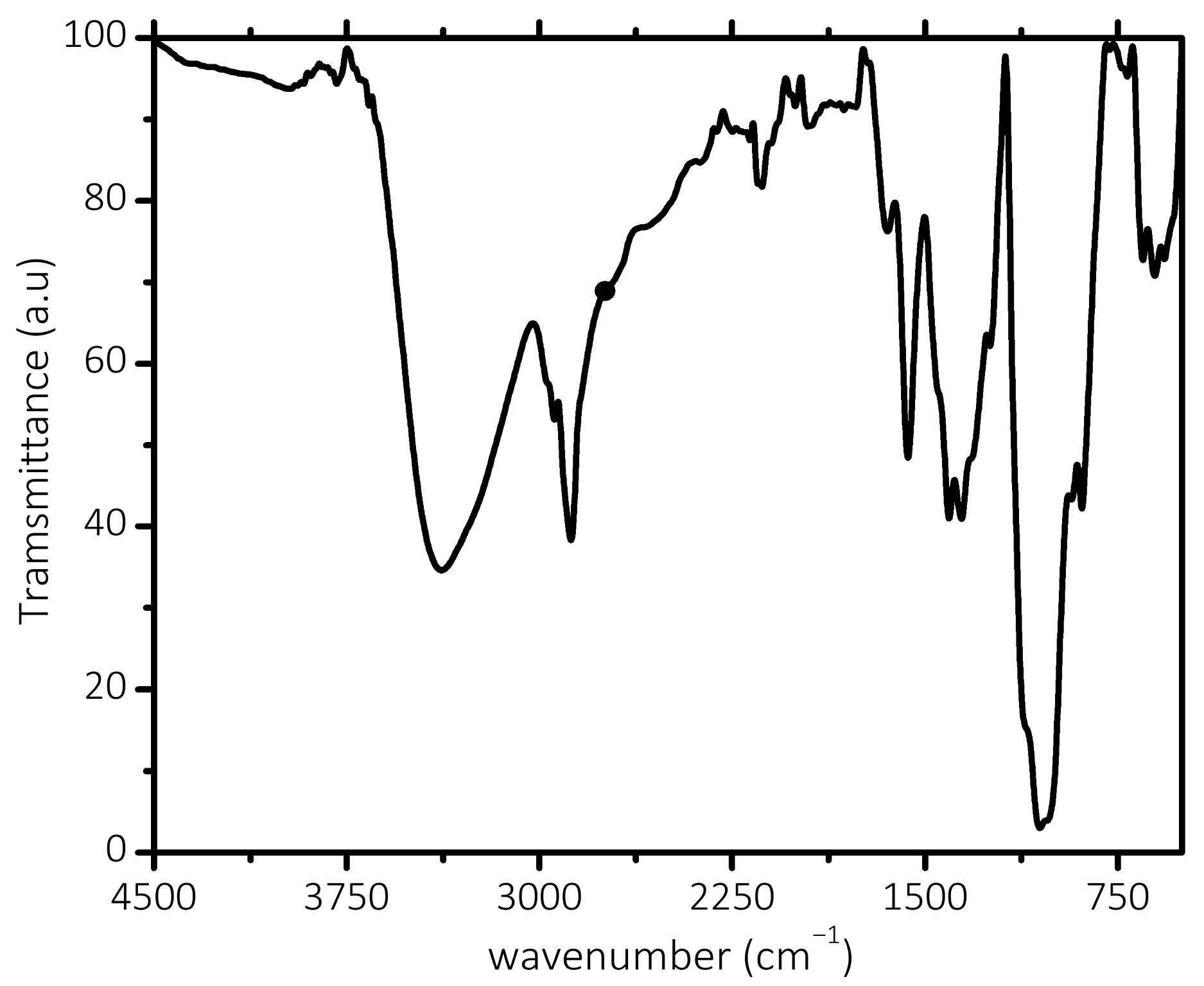
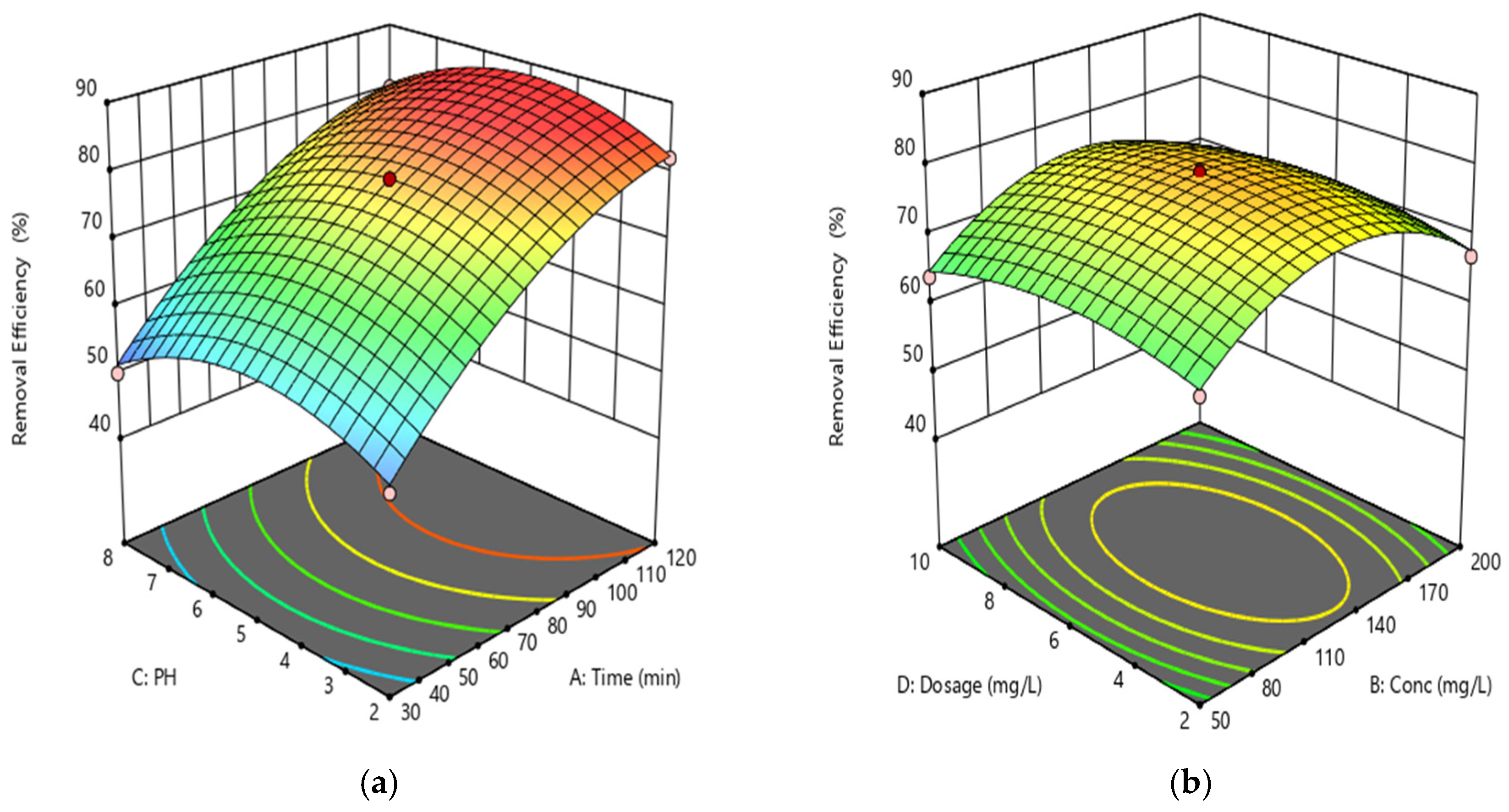
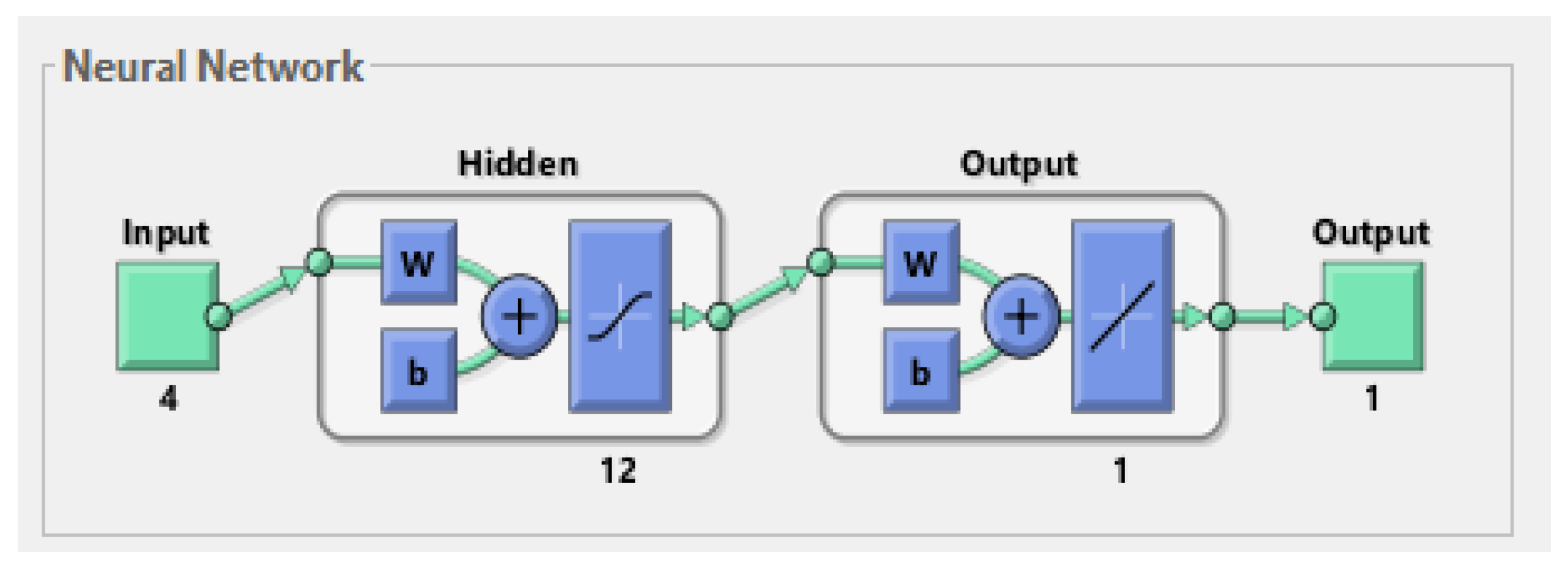
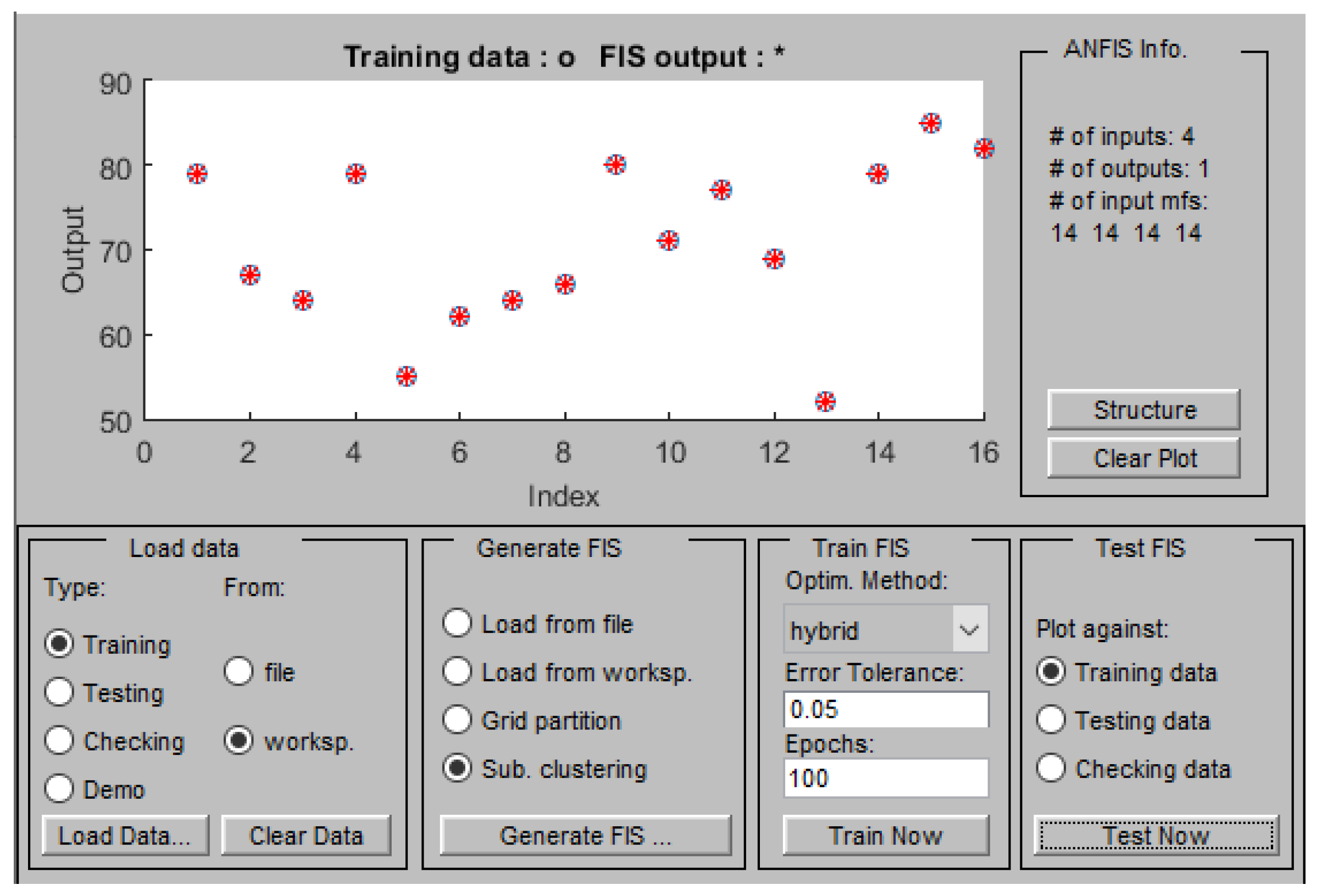
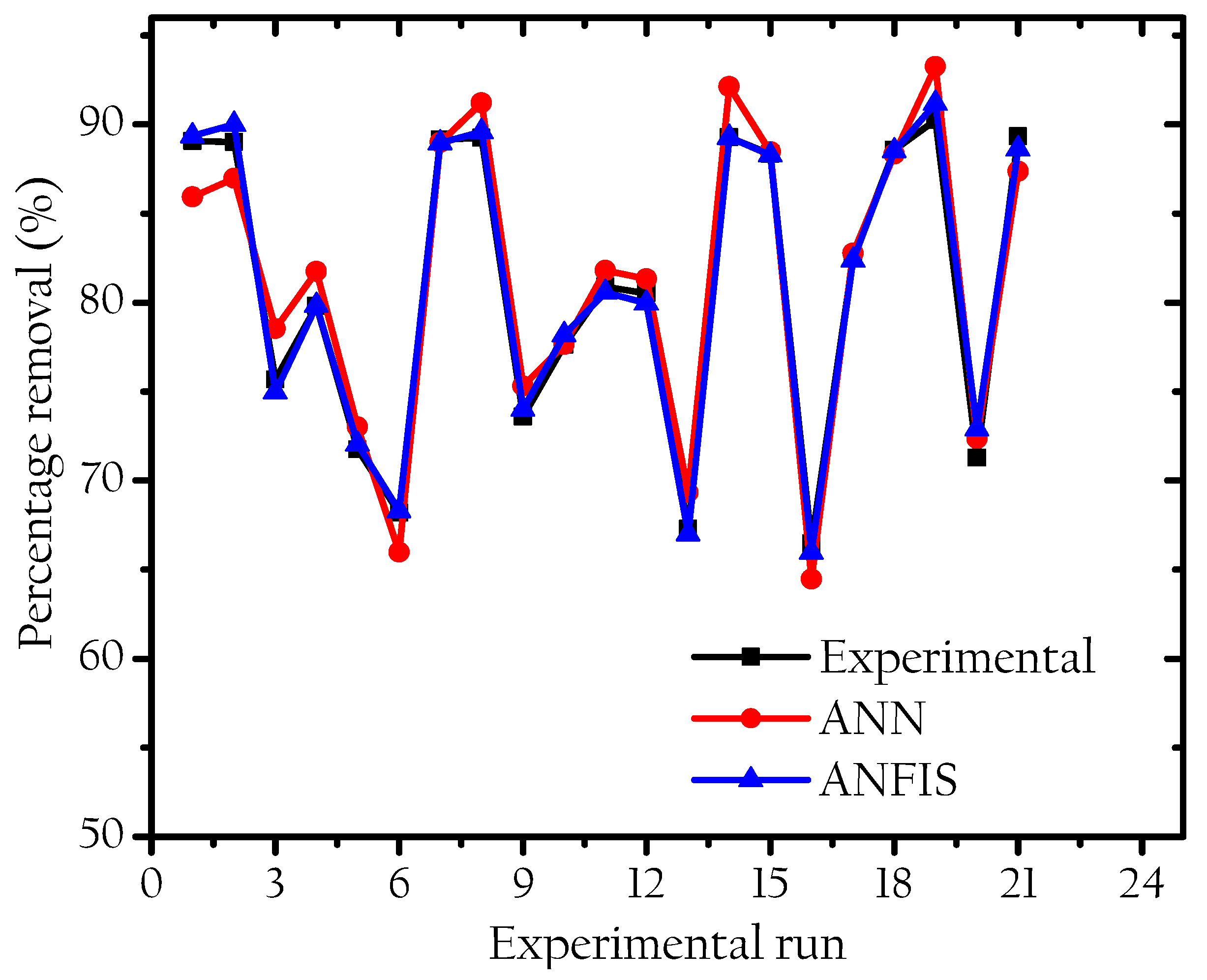
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RMSE | Root means square error |
| AARE | Absolute average relative error |
| ARE | Average relative error |
| MSE | Mean Square Error |
| ANFIS | Adaptive neuro-fuzzy inference system |
| ANN | Artificial neural network |
| LM | Levenberg-Marquardt |
References
- Ling, C.; Wang, Z.; Ni, Y.; Zhu, Z.; Cheng, Z.; Liu, R. Superior adsorption of methyl blue on magnetic Ni–Mg–Co ferrites: Adsorption electrochemical properties and adsorption characteristics. Environ. Prog. Sustain. Energy 2022, 41, e13923. [Google Scholar] [CrossRef]
- Asghar, A.; Jafari, D.; Esfandyari, M.; Ali, S. Prediction of the continuous cadmium removal efficiency from aqueous solution by the packed-bed column using GMDH and ANFIS models. Desalination Water Treat. 2021, 234, 91–101. [Google Scholar] [CrossRef]
- Banza, J.C.; Onyango, M.S. Optmisation of cadmium (II) removal onto biodegradable composite using artificial neural networks and response surface methodology: Quantum chemical performance. EQA-Int. J. Environ. Qual. 2024, 60, 1–17. [Google Scholar]
- Vincent, S.; Kandasubramanian, B. Cellulose nanocrystals from agricultural resources: Extraction and functionalisation. Eur. Polym. J. 2021, 160, 110789. [Google Scholar] [CrossRef]
- Rao, R.A.K.; Rehman, F.; Kashifuddin, M. Removal of Cr (VI) from electroplating wastewater using fruit peel of Leechi (Litchi chinensis). Desalination Water Treat. 2012, 49, 136–146. [Google Scholar] [CrossRef]
- Banza, M.; Rutto, H. Removal of Copper (II) and Lead (II) from hydrometallurgical effluent onto cellulose nanocomposites: Mechanistic and Levenberg-Marquardt in Artificial Neural Network modelling. EQA-Int. J. Environ. Qual. 2023, 54, 19–26. [Google Scholar]
- Yang, Q.; Yang, S.; Tu, C.; Zhu, X.; Guo, Z.; Liu, X. Journal of Environmental Chemical Engineering Amino-functionalised magnetic humic acid nanoparticles for enhanced Pb (II) adsorption: Mechanism analysis and machine learning prediction. J. Environ. Chem. Eng. 2024, 12, 113956. [Google Scholar] [CrossRef]
- Salari, M.; Nikoo, M.R.; Al-Mamun, A.; Rakhshandehroo, G.R.; Mooselu, M.G. Optimizing Fenton-like process, homogeneous at neutral pH for ciprofloxacin degradation: Comparing RSM-CCD and ANN-GA. J. Environ. Manag. 2022, 317, 115469. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Kurniawan, T.A.; Mohyuddin, A.; Othman, M.H.D.; Ali, I.; Abdulkareem-Alsultan, G.; Anouzla, A.; Goh, H.H.; Zhang, D.; Aziz, F.; et al. Rosa damascena waste as biosorbent for co-existing pollutants removal: Fixed-bed column study and ANN modeling. Chem. Eng. Sci. 2024, 293, 120057. [Google Scholar] [CrossRef]
- Ibrahim, A.I.; Vohra, M.S. Novel TiO2@Mg/Fe-LDH for photocatalysis and adsorption of selenium species from wastewater: RSM & ANN/ML modeling. Next Mater. 2025, 8, 100766. [Google Scholar]
- Simsek, S.; Uslu, S.; Simsek, H. Proportional impact prediction model of animal waste fat-derived biodiesel by ANN and RSM technique for diesel engine. Energy 2022, 239, 122389. [Google Scholar] [CrossRef]
- Igwilo, C.N.; Ude, N.C.; Onoh, I.M.; Enekwe, C.B.; Alieze, B.A. RSM, ANN and ANFIS applications in modeling fermentable sugar production from enzymatic hydrolysis of Colocynthis Vulgaris Shrad seeds shell. Bioresour. Technol. Rep. 2022, 18, 101056. [Google Scholar] [CrossRef]
- Banza, M.; Rutto, H. Selective removal of Cr (VI) from hydrometallurgical effluent using modified cellulose nanocrystals (CNCs) with succinic anhydride and ethylenediaminetetraacetic acid: Isotherm, kinetics, and thermodynamic studies. Can. J. Chem. Eng. 2023, 101, 896–908. [Google Scholar] [CrossRef]
- Adebayo, G.B.; Jamiu, W.; Okoro, H.K.; Okeola, F.O.; Adesina, A.K.; Feyisetan, O.A. Kinetics, thermodynamics and isothermal modelling of liquid phase adsorption of methylene blue onto moringa pod husk activated carbon. S. Afr. J. Chem. 2019, 72, 263–273. [Google Scholar] [CrossRef]
- Azadian, M.; Gilani, H.G. Adsorption of Cu2+, Cd2+, and Zn2+ by engineered biochar: Preparation, characterisation, and adsorption properties. Environ. Prog. Sustain. Energy 2023, 42, e14088. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claude, B.J.; Masindi, V.; Sibali, L.L. Prediction and Optimisation of Cr (VI) Removal by Modified Cellulose Nanocrystals from Aqueous Solution Using Machine Learning (ANN and ANFIS). Eng. Proc. 2025, 117, 12. https://doi.org/10.3390/engproc2025117012
Claude BJ, Masindi V, Sibali LL. Prediction and Optimisation of Cr (VI) Removal by Modified Cellulose Nanocrystals from Aqueous Solution Using Machine Learning (ANN and ANFIS). Engineering Proceedings. 2025; 117(1):12. https://doi.org/10.3390/engproc2025117012
Chicago/Turabian StyleClaude, Banza Jean, Vhahangwele Masindi, and Linda L. Sibali. 2025. "Prediction and Optimisation of Cr (VI) Removal by Modified Cellulose Nanocrystals from Aqueous Solution Using Machine Learning (ANN and ANFIS)" Engineering Proceedings 117, no. 1: 12. https://doi.org/10.3390/engproc2025117012
APA StyleClaude, B. J., Masindi, V., & Sibali, L. L. (2025). Prediction and Optimisation of Cr (VI) Removal by Modified Cellulose Nanocrystals from Aqueous Solution Using Machine Learning (ANN and ANFIS). Engineering Proceedings, 117(1), 12. https://doi.org/10.3390/engproc2025117012







