Comparative Adsorption Performance of Chitosan and Iron-Modified Chitosan for the Removal of a Synthetic Textile Dye †
Abstract
1. Introduction
2. Materials and Methods
2.1. Dye Preparation
2.2. Adsorbent Preparation
2.3. Adsorbent Experiments
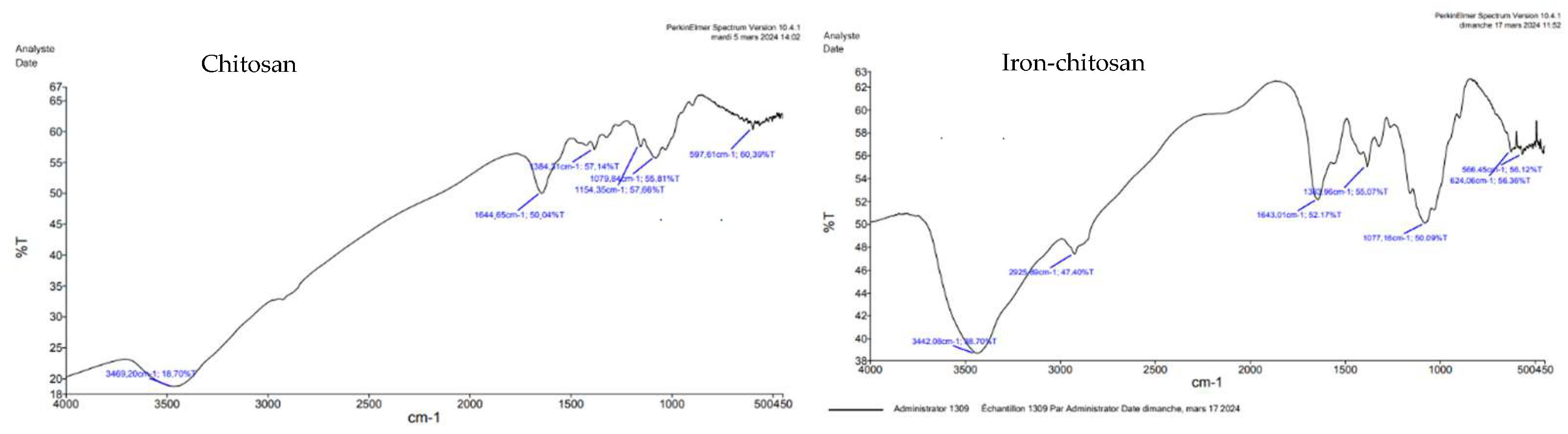
3. Instrumental Analysis
Fourier Transform InfraRed Spectroscopy
4. Results
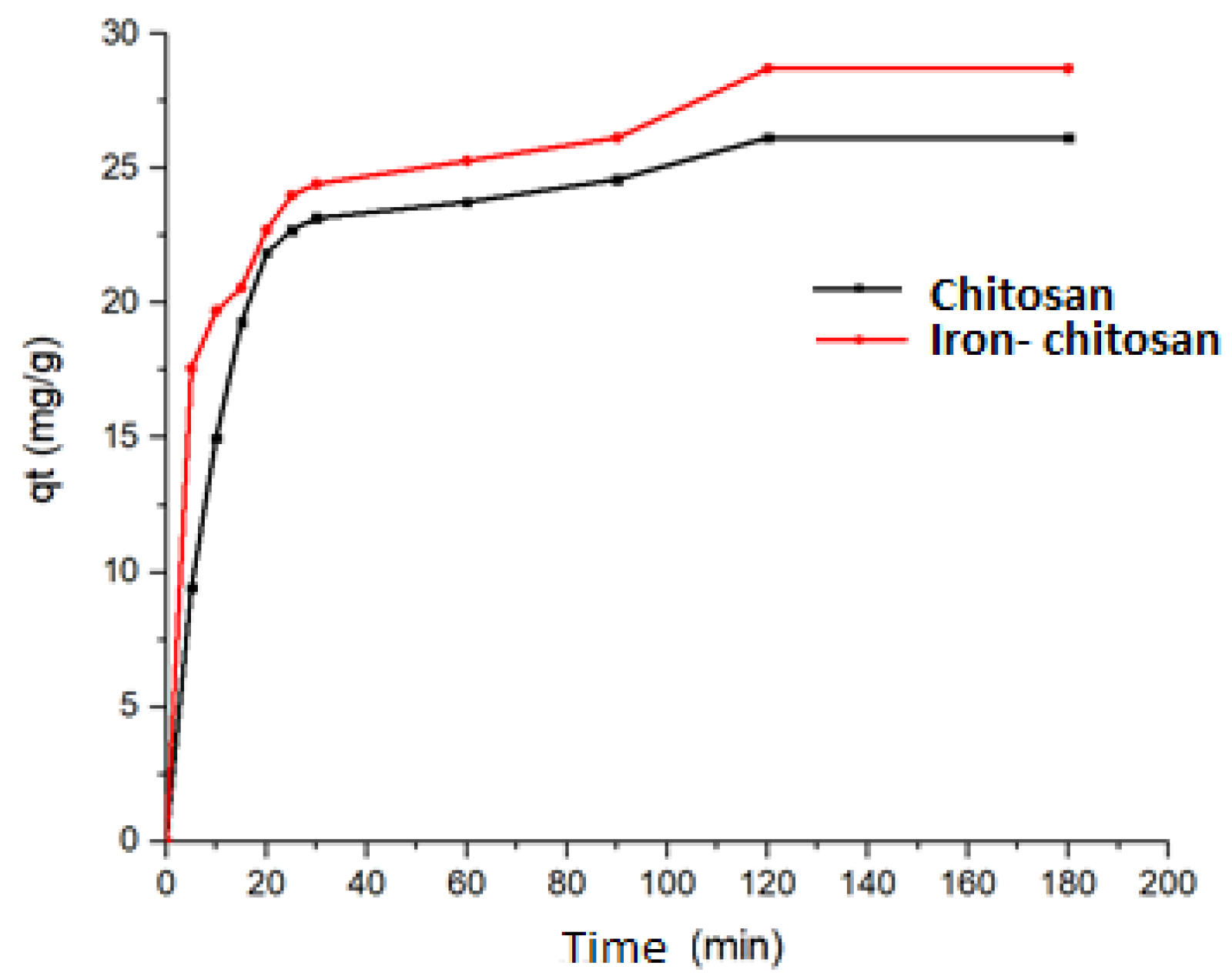
4.1. Adsorption Kinetics
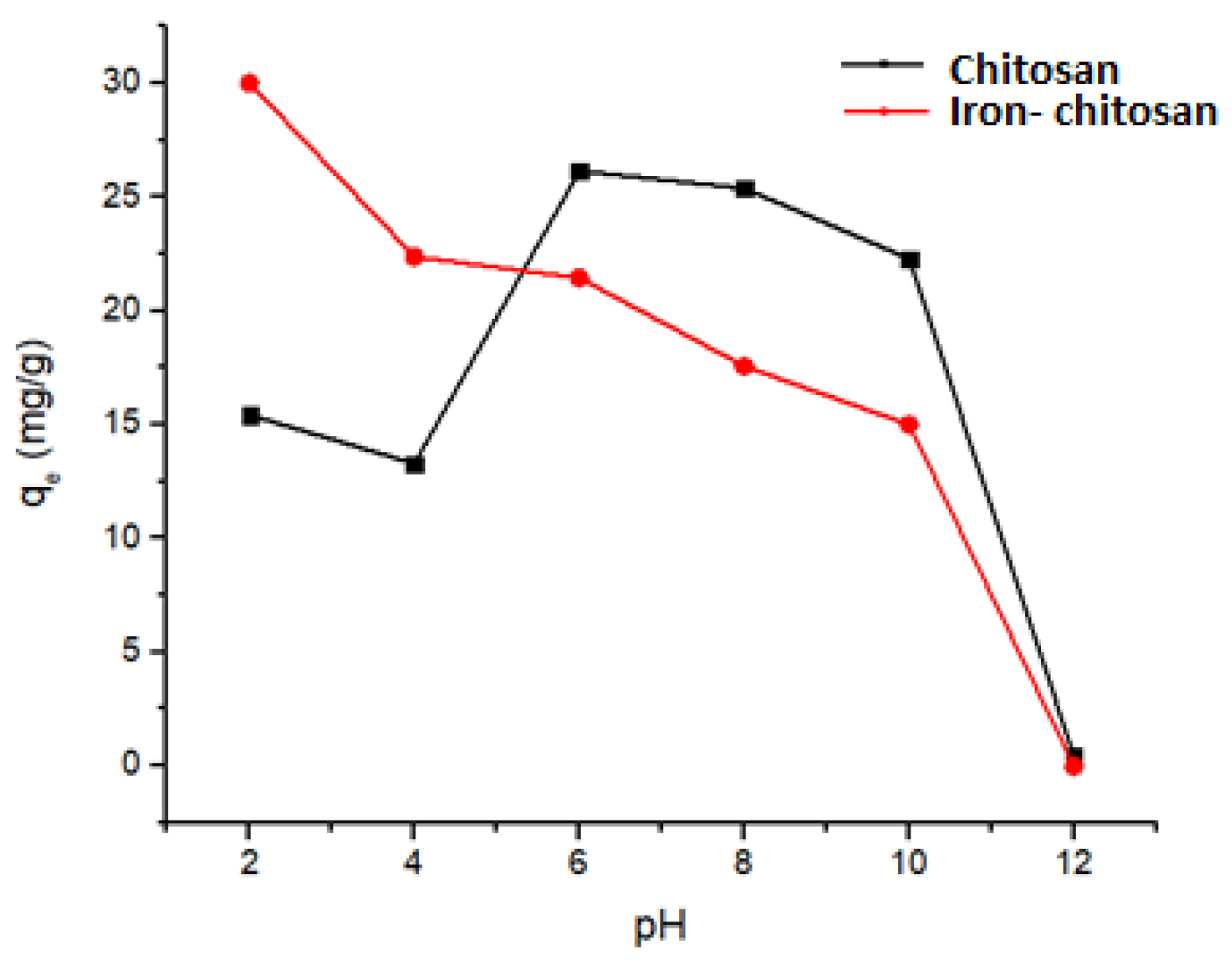
4.2. Influence of the Initial pH on the Adsorption Process
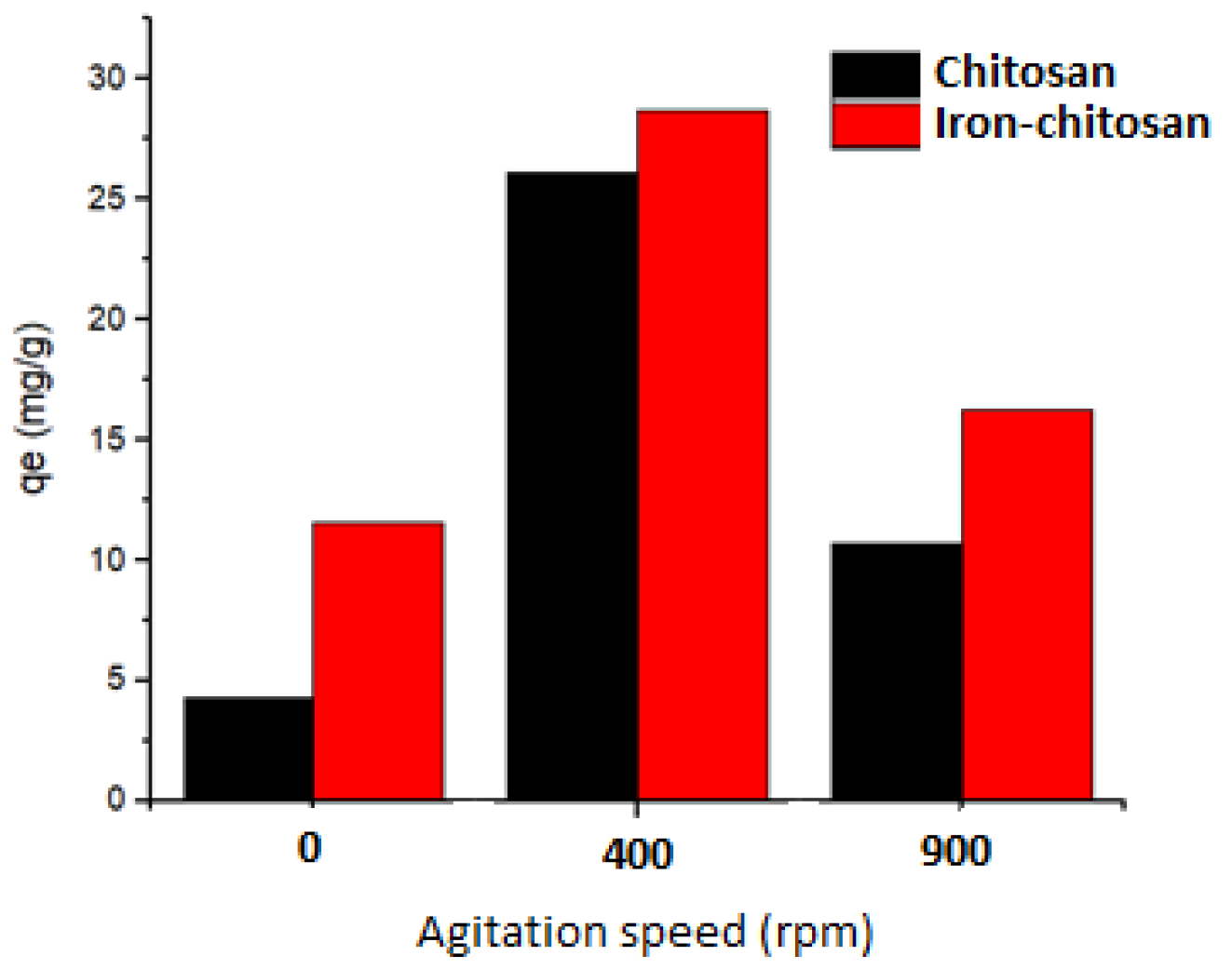
4.3. The Impact of Stirring Rate
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, Y.; Rahman, M.M.; Alam, M.S.; Miah, M.I.; Choudhury, M.S.H.; Alharb, O.A.; Akhtar, P.; Rahman, S.M. Harnessing neural network model with optimization for enhanced ciprofloxacin antibiotic adsorption from contaminated water: A transparent and objective framework. J. Water Process Eng. 2024, 65, 105724. [Google Scholar] [CrossRef]
- Ahmed, Y.; Maya, A.A.S.; Akhtar, P.; Alam, M.S.; AlMohamadi, H.; Islam, M.N.; Alharbi, O.A.; Rahman, S.M. A novel interpretable machine learning and metaheuristic-based protocol to predict and optimize ciprofloxacin antibiotic adsorption with nano-adsorbent. J. Environ. Manage 2024, 370, 122614. [Google Scholar] [CrossRef] [PubMed]
- Rita, K. Textile dyeing industry an environmental hazard. Nat. Sci. 2012, 4, 22–26. [Google Scholar] [CrossRef]
- Akhtar, P.; Ahmed, Y.; Islam, F.; Alam, K.; Mary, M.; Islam, M.Z.; Bhuiyan, M.M.H.; Yaakob, Z. Efficiency of effluent treatment plants and threat to human health and aquatic environment in Bangladesh. Asian. J. Chem. 2016, 28, 60–68. [Google Scholar] [CrossRef]
- Benomara, A.; Guenfoud, F.; Mokhtari, M. Removal of methyl violet 2B by FePO4 as photocatalyst. React. Kinet. Mech. Catal. 2019, 127, 1087–1099. [Google Scholar] [CrossRef]
- Chaari, I.; Fakhfakh, E.; Medhioub, M.; Jamoussi, F. Comparative study on adsorption of cationic and anionic dyes by smectite rich natural clays. J. Mol. Struct. 2019, 1179, 672–677. [Google Scholar] [CrossRef]
- Charola, S.; Yadav, R.; Das, P.; Maiti, S. Fixed-bed adsorption of Reactive Orange 84 dye onto activated carbon prepared from empty cotton flower agro-waste. Sustain. Environ. Res. 2018, 28, 298–308. [Google Scholar] [CrossRef]
- Lansari, I.; Benguella, B.; Kruchinina, N.; Nistratov, A. Adsorption of a textile dye from aqueous solution on natural and modified sawdust. Desalination Water Treat. 2020, 194, 259–268. [Google Scholar] [CrossRef]
- Tizaoui, K.; Benguella, B.; Makhoukhi, B. Selective adsorption of heavy metals (Co2+, Ni2+ and Cr3+) from aqueous solution onto natural marne clay. Desalination Water Treat. 2019, 142, 252–259. [Google Scholar] [CrossRef]
- Rhaman, M.M.; Karim, M.R.; Hyder, M.K.M.Z.; Ahmed, Y.; Nath, R.K. Removal of chromium (VI) from effluent by a magnetic bioadsorbent based on jute stick powder and its adsorption isotherm, kinetics and regeneration study. Water Air Soil Pollut 2020, 231, 164. [Google Scholar] [CrossRef]
- Soares, P.A.; Silva, T.F.C.V.; Manenti, D.R.; Souza, S.M.A.G.U.; Boaventura, R.A.R.; Vilar, V.J.P. Insights into real cotton-textile dyeing wastewater treatment using solar advanced oxidation processes. Environ. Sci. Pollut. Res. 2014, 21, 932–945. [Google Scholar] [CrossRef]
- Ahmed, Y.; Siddiqua, A.A.; Maya, P.; Akhtar, P.; AlMohamadi, H.; Mohammad, A.W.; Ashekuzzaman, S.M.; Agnieszka, O.I.; Udin, M.G. Advancements and challenges in Fenton-based advanced oxidation processes for antibiotic removal in wastewater: From the laboratory to practical applications. J. Environ. Chem. Eng. 2024, 13, 115068. [Google Scholar] [CrossRef]
- Alsbaiee, A.; Smith, B.J.; Xiao, L.; Ling, Y.; Helbling, D.E.; Dichtel, W.R. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 2015, 529, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Jyotishka, N.; Mitali, D.; Adrija, G.; Suprakas, S.R.; Jonathan, T.O.; Basudev, L.; Dipankar, C.; Arpita, A. Chitosan-based adsorbents for remediation of toxic dyes from wastewater: A review on adsorption mechanism, reusability, machine learning based modeling and future perspectives. Int. J. Biol. Macromol. 2025, 311 Pt 2, 143388. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, S.M.; Mukta, R.S.; Mohammed, F.M.; Chowdhury, M.M.R.; Nuruzzaman, K.M. Electrospun Poly(vinyl alcohol)/Chitosan Nanofibers Embedded with a CuO–GO Nanocomposite for pH-Sensitive Adsorption of Heavy Metal Ions and Organic Dyes. ACS Omega 2025, 10, 19294–19313. [Google Scholar]
- Khan, M.N.; Chowdhury, M.; Rahman, M.M. Biobased amphoteric aerogel derived from amine-modified clay-enriched chitosan/alginate for adsorption of organic dyes and chromium (VI) ions from aqueous solution. Mater. Today Sustain. 2021, 13, 100077. [Google Scholar] [CrossRef]
- Liu, C.F.; Sun, R.C.; Zhang, A.P.; Ren, J.L. Preparation of sugarcane bagasse cellulosic phthalate using an ionic liquid as reaction mediu. J. Carbohydr. Polym. 2007, 68, 17–25. [Google Scholar] [CrossRef]
- Hafsa, J.; Smach, M.A.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Antioxidant and antimicrobial proprieties of chitin and chitosan extracted from Parapenaeus Longirostris shrimp shell waste. Ann. Pharm. Fr. 2016, 74, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Musah, M.; Azeh, Y.; Mathew, J.T.; Umar, M.T.; Abdulhamid, Z.; Muhammad, A.I. Adsorption Kinetics and Isotherm Models: A Review. Caliphate J. Sci. Technol. 2022, 4, 20–26. [Google Scholar] [CrossRef]
- Fayoud, N.H.; Tounsi, L.A.; Tahiri, S.; Abderrahmene, A. Etude cinétique et thermodynamique de l’adsorption de bleu de methylene sur les cendres de bois (Kinetic and thermodynamic study of the adsorption of methylene blue on wood ashes). J. Mater. Environ. Sci. 2015, 6, 3295–3306. [Google Scholar]
- Lansari, I.; Benguella, B.; Kruchinina, N.; Nistratov, A. Adsorption of textile dyes from aqueous solution using activated carbon from human hair. React. Kinet. Mech. Catal. 2022, 135, 1891–1903. [Google Scholar] [CrossRef]
- Lansari, I.; Tizaoui, K.; Benguella, B.; Bendimerad, F.A.; Bouchaour, S.M. Optimizing chitosan-based adsorbents for the removal of trisodium ferric complex of N-methyl-1,8-naphtalimide-4-sulfonate. React. Kinet. Mech. Catal. 2025, 138, 4097–4114. [Google Scholar] [CrossRef]
- Lansari, I.; Tizaoui, K.; Benguella, B.; Bensaid, F.Z.; Chekroun, A.; Kruchinina, N.; Nistratov, A. Kinetic study of removal of reactive violet and bemacid yellow from aqueous solution by adsorption onto activated carbon derived from human hair. React. Kinet. Mech. Catal. 2025, 138, 2597–2611. [Google Scholar] [CrossRef]
- Lansari, I.; Benguella, B.; Kruchinina, N.; Nistratov, A. The removal of acid green 4G and anthraquinone orange from aqueous solution using adsorption on activated carbon from human hair. React. Kinet. Mech. Catal. 2022, 135, 987–998. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lansari, I.; Tizaoui, K.; Benguella, B. Comparative Adsorption Performance of Chitosan and Iron-Modified Chitosan for the Removal of a Synthetic Textile Dye. Chem. Proc. 2025, 18, 26. https://doi.org/10.3390/ecsoc-29-26918
Lansari I, Tizaoui K, Benguella B. Comparative Adsorption Performance of Chitosan and Iron-Modified Chitosan for the Removal of a Synthetic Textile Dye. Chemistry Proceedings. 2025; 18(1):26. https://doi.org/10.3390/ecsoc-29-26918
Chicago/Turabian StyleLansari, Imane, Khadidja Tizaoui, and Belkacem Benguella. 2025. "Comparative Adsorption Performance of Chitosan and Iron-Modified Chitosan for the Removal of a Synthetic Textile Dye" Chemistry Proceedings 18, no. 1: 26. https://doi.org/10.3390/ecsoc-29-26918
APA StyleLansari, I., Tizaoui, K., & Benguella, B. (2025). Comparative Adsorption Performance of Chitosan and Iron-Modified Chitosan for the Removal of a Synthetic Textile Dye. Chemistry Proceedings, 18(1), 26. https://doi.org/10.3390/ecsoc-29-26918





