Supramolecular Assemblies Driven by N-H…O and O-H…O Hydrogen Bonding Interactions: Experimental and Theoretical Investigation into the Supramolecular Architectures of Dihydropyrimidin-2(1H)-ones †
Abstract
1. Introduction
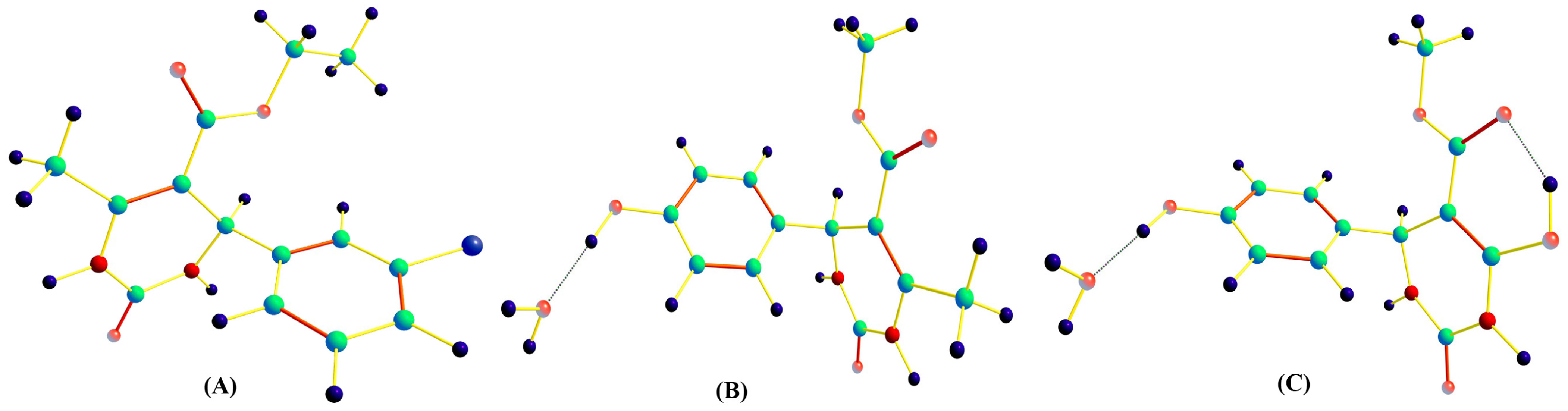
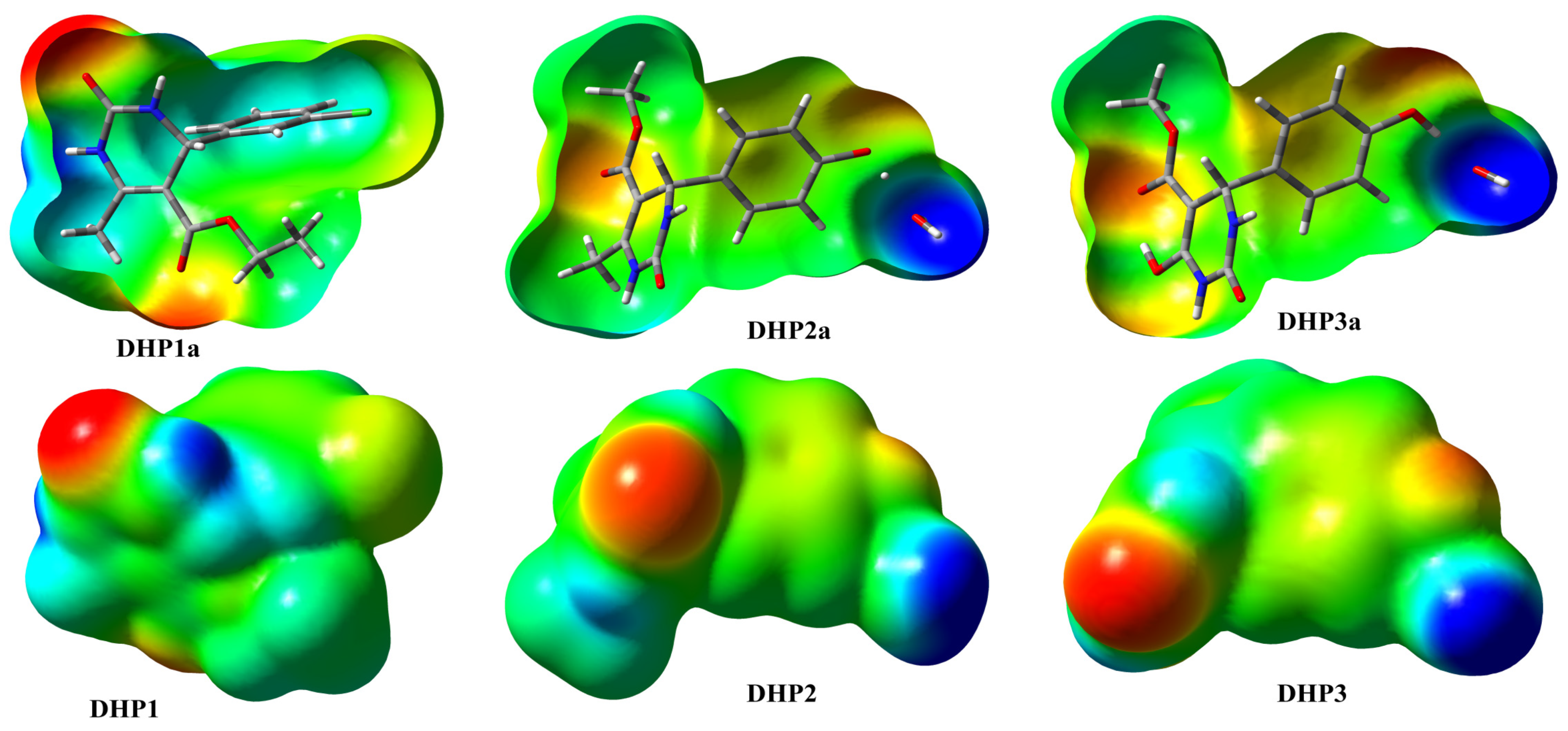
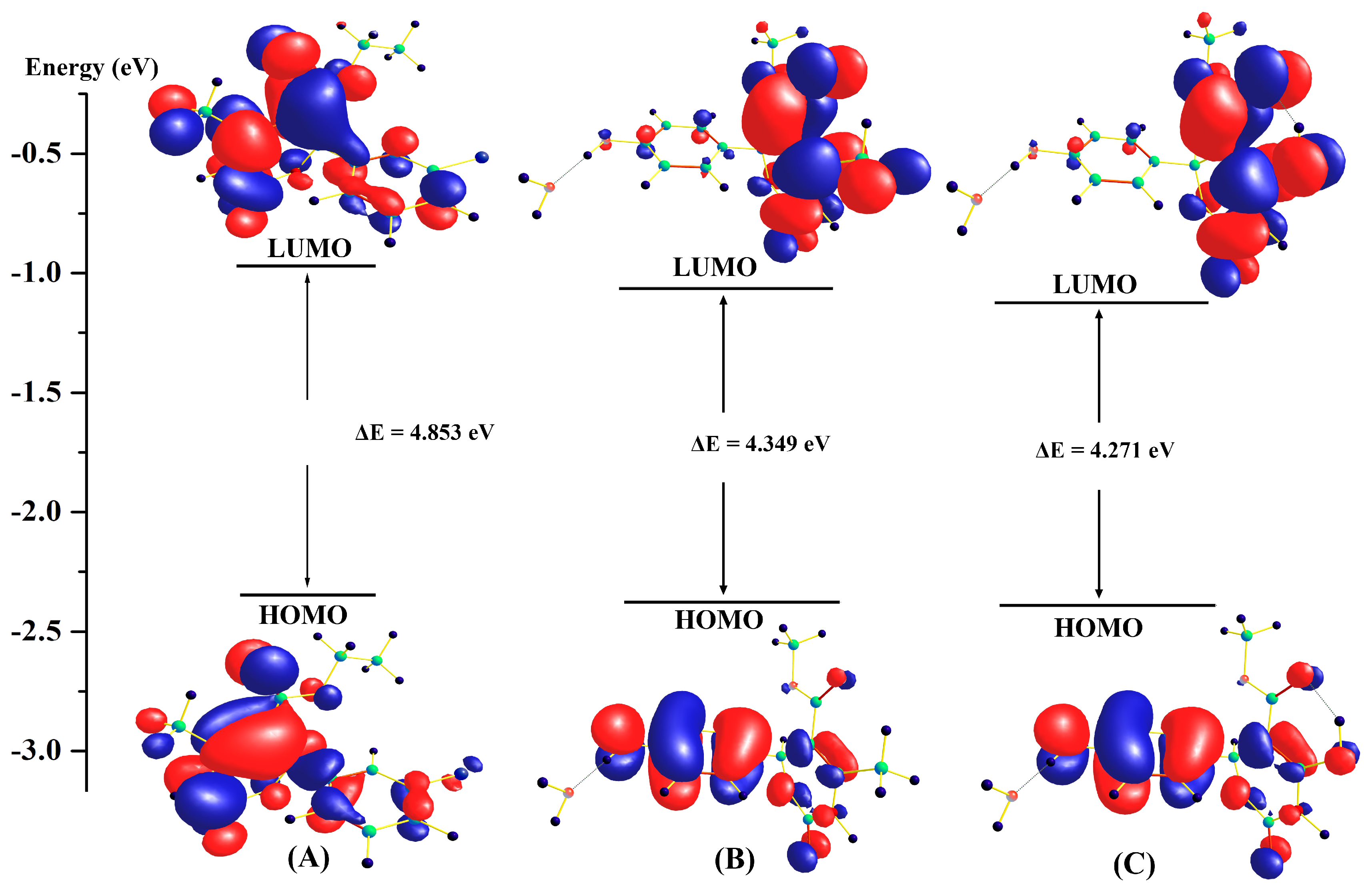
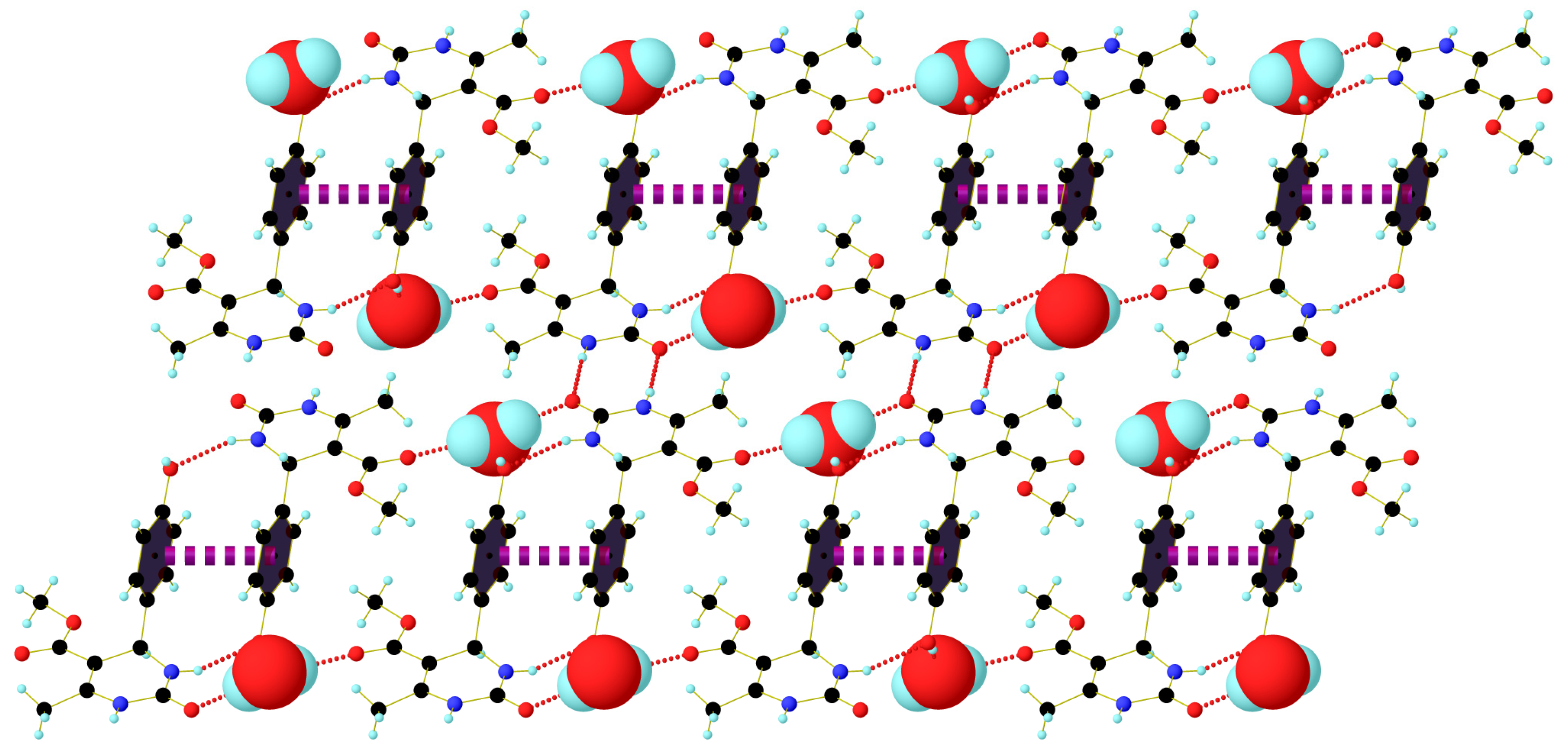
2. Results and Discussion
3. Experimental
3.1. Computational Studies
3.2. CSD Search
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lundberg, D.J.; Brown, C.M.; Bobylev, E.O.; Oldenhuis, N.J.; Alfaraj, Y.S.; Zhao, J.; Kevlishvili, I.; Kulik, H.J.; Johnson, J.A. Nested non-covalent interactions expand the functions of supramolecular polymer networks. Nat. Commun. 2024, 15, 3951. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.M. Supramolecular chemistry. Science 1993, 260, 1762–1763. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Urban, M.W. Self-healing of polymers via supramolecular chemistry. Adv. Mater. Interfaces 2018, 5, 1800384. [Google Scholar] [CrossRef]
- Mukherjee, A. Building upon Supramolecular Synthons: Some Aspects of Crystal Engineering. Cryst. Growth Des. 2015, 15, 3076–3085. [Google Scholar] [CrossRef]
- Tian, J.; Han, X.; Yang, H.R.; Liu, J.; Chen, J.-F.; Wei, T.-B.; Yao, H.; Qu, W.-J.; Shi, B.; Lin, Q. Hydrogen-bonded supramolecular polymer networks for effective removal of perchlorate in water via clustered hydrogen-bonding. Nat. Commun. 2025, 16, 6481. [Google Scholar] [CrossRef] [PubMed]
- Lugger, S.J.D.; Houben, S.J.A.; Foelen, Y.; Debije, M.G.; Schenning, A.P.H.J.; Mulder, D.J. Hydrogen-bonded supramolecular liquid crystal polymers: Smart materials with stimuli-responsive, self-healing, and recyclable properties. Chem. Rev. 2022, 122, 4946–4975. [Google Scholar] [CrossRef] [PubMed]
- Prins, L.J.; Reinhoudt, D.N.; Timmerman, P. Noncovalent synthesis using hydrogen bonding. Angew. Chem. Int. Ed. 2001, 40, 2382–2426. [Google Scholar] [CrossRef]
- Concellón, A.; Blasco, E.; Martinez-Felipe, A.; Martínez, J.L.; Sícs, I.; Ezquerra, T.A.; Nogales, A.; Pinol, M.; Oriol, L. Light-responsive self-assembled materials by supramolecular post-functionalization via hydrogen bonding of amphiphilic block copolymers. Macromolecules 2016, 49, 7825–7836. [Google Scholar] [CrossRef]
- Feringán, B.; Romero, P.; Serrano, J.L.; Gimenez, R.; Sierra, T. Supramolecular columnar liquid crystals formed by hydrogen bonding between a clicked star-shaped s-triazine and benzoic acids. Chem. Eur. J. 2015, 21, 8859–8866. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, B.; Behbahani, F.K. Recent developments in the synthesis and applications of dihydropyrimidin-2(1H)-ones and thiones. Mol. Divers. 2018, 22, 405–446. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O. Biologically active dihydropyrimidones of the Biginelli-type a literature survey. Eur. J. Med. Chem. 2000, 35, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Rani, J.W.S.; Kathing, C.; Singh, N.G.; Nongrum, R.; Rahman, N.; Kharmawlong, G.; Nongkhlaw, R. One-pot synthesis of 3, 4-dihydropyrimidin-2(1H)-ones catalysed by [BMIM] Br under solvent-free condition. Bulg. Chem. Commun. 2016, 48, 3–10. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurbah, S.D. Supramolecular Assemblies Driven by N-H…O and O-H…O Hydrogen Bonding Interactions: Experimental and Theoretical Investigation into the Supramolecular Architectures of Dihydropyrimidin-2(1H)-ones. Chem. Proc. 2025, 18, 28. https://doi.org/10.3390/ecsoc-29-26921
Kurbah SD. Supramolecular Assemblies Driven by N-H…O and O-H…O Hydrogen Bonding Interactions: Experimental and Theoretical Investigation into the Supramolecular Architectures of Dihydropyrimidin-2(1H)-ones. Chemistry Proceedings. 2025; 18(1):28. https://doi.org/10.3390/ecsoc-29-26921
Chicago/Turabian StyleKurbah, Sunshine Dominic. 2025. "Supramolecular Assemblies Driven by N-H…O and O-H…O Hydrogen Bonding Interactions: Experimental and Theoretical Investigation into the Supramolecular Architectures of Dihydropyrimidin-2(1H)-ones" Chemistry Proceedings 18, no. 1: 28. https://doi.org/10.3390/ecsoc-29-26921
APA StyleKurbah, S. D. (2025). Supramolecular Assemblies Driven by N-H…O and O-H…O Hydrogen Bonding Interactions: Experimental and Theoretical Investigation into the Supramolecular Architectures of Dihydropyrimidin-2(1H)-ones. Chemistry Proceedings, 18(1), 28. https://doi.org/10.3390/ecsoc-29-26921





