Multimorbidity Burden in Veterans with and Without Type 2 Diabetes Mellitus: A Comparative Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
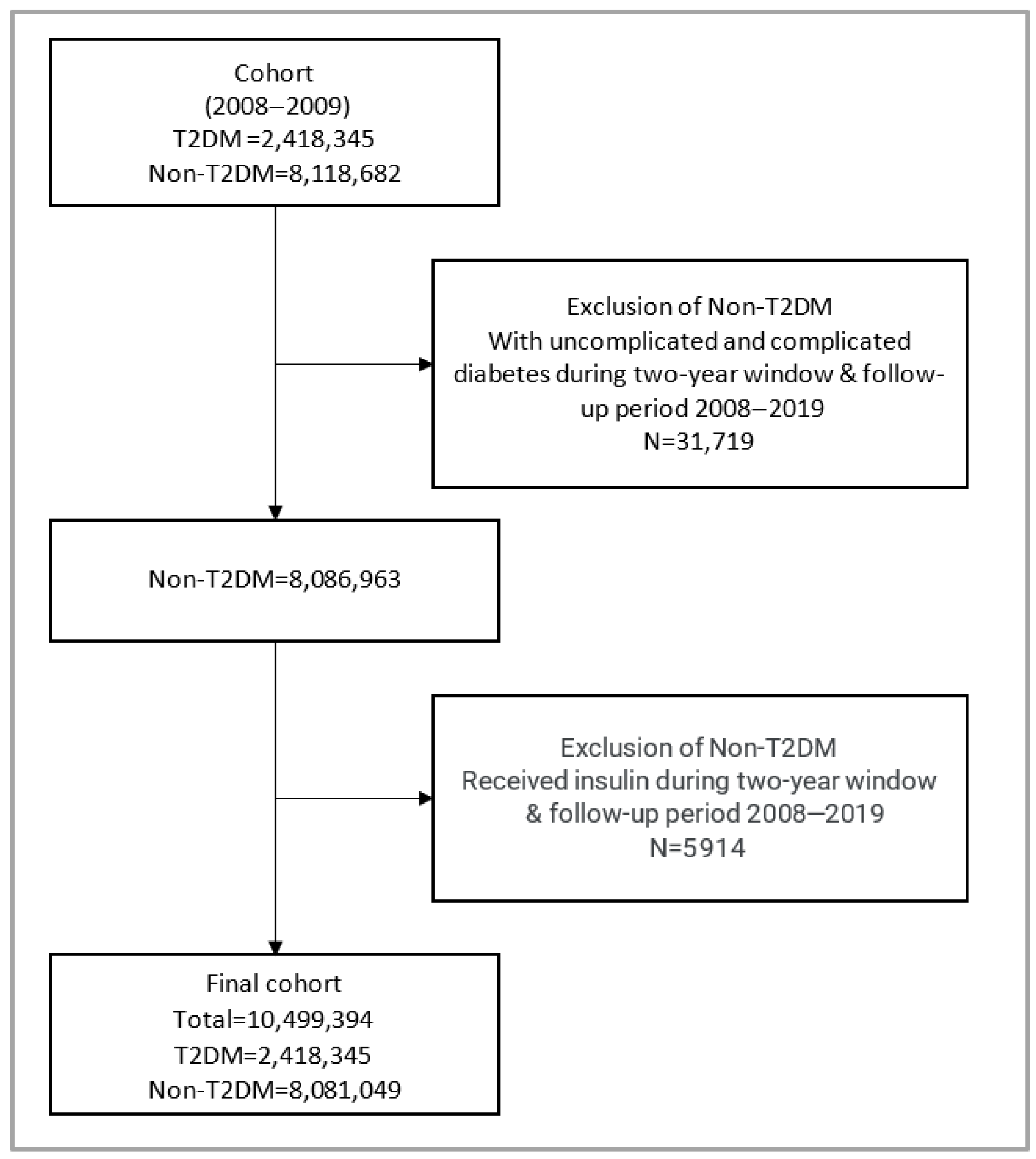
2.2. Study Population
2.3. Exposure and Covariates
2.4. Statistical Analysis
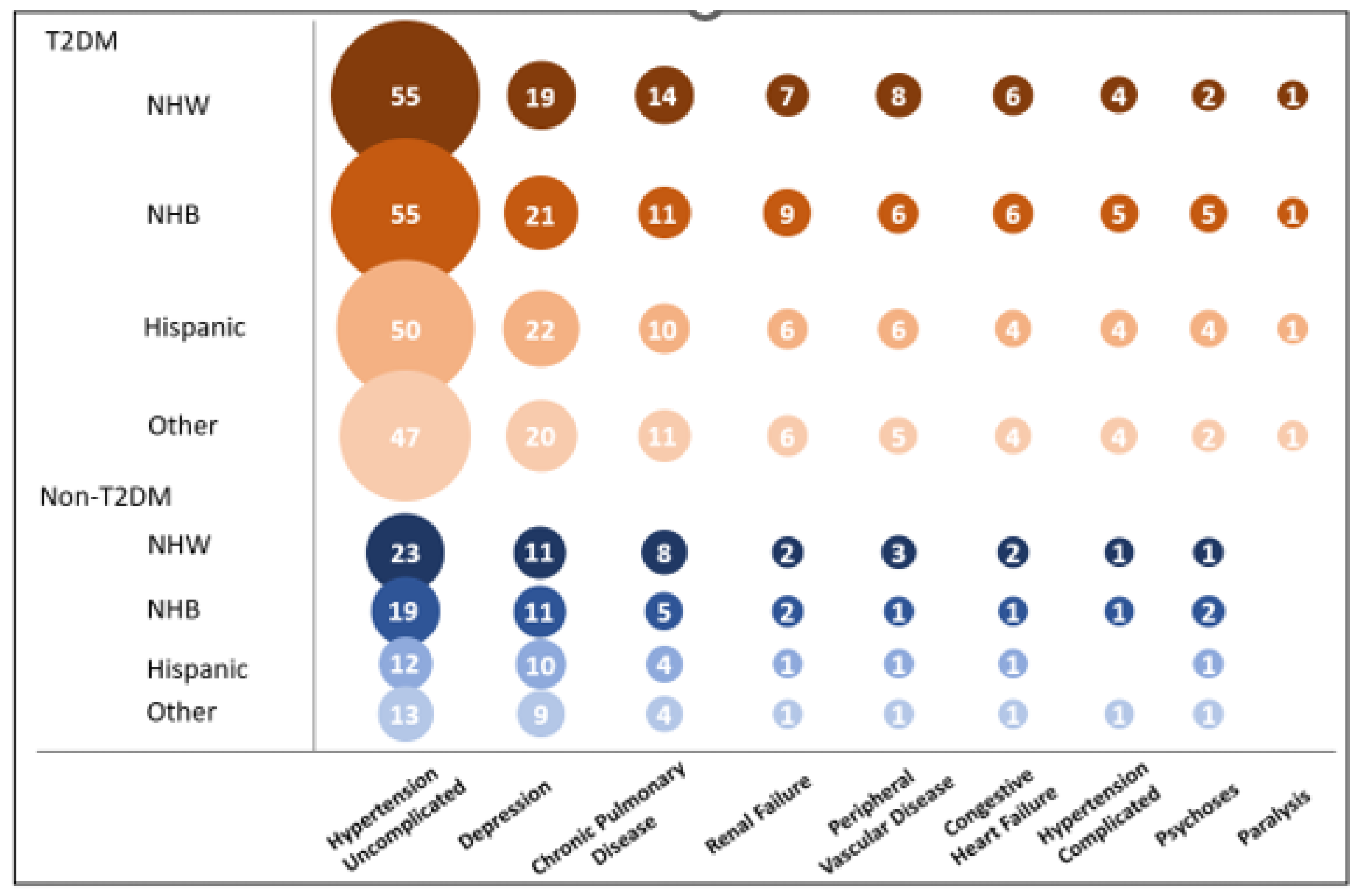
3. Results
Sensitivity Analysis Using Subset of Incident T2DM Cases
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Global Strategy on People-Centred and Integrated Health Services: Interim Report; World Health Organization: Geneva, Switzerland, 2015; Available online: https://apps.who.int/iris/bitstream/handle/10665/155002/WHO_HIS_SDS_2015.6_eng.pdf (accessed on 12 August 2025).
- Pearson-Stuttard, J.; Ezzati, M.; Gregg, E.W. Multimorbidity—A defining challenge for health systems. Lancet Public Health 2019, 4, e599–e600. [Google Scholar] [CrossRef] [PubMed]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, C.; Herrin, J.; Mahajan, S.; Massey, D.; Lu, Y.; Ndumele, C.D.; Drye, E.E.; Krumholz, H.M. Temporal trends in racial and ethnic disparities in multimorbidity prevalence in the United States, 1999–2018. Am. J. Med. 2022, 135, 1083–1092.e14. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Zulman, D.; Scott, J.Y.; Maciejewski, M.L. Costs associated with multimorbidity among VA patients. Med. Care 2014, 52, S31–S36. [Google Scholar] [CrossRef] [PubMed]
- Zulman, D.M.; Chee, C.P.; Wagner, T.H.; Yoon, J.; Cohen, D.M.; Holmes, T.H.; Ritchie, C.; Asch, S.M. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open 2015, 5, e007771. [Google Scholar] [CrossRef] [PubMed]
- National Diabetes Statistics Report Estimates of Diabetes and Its Burden in the United States; Department of Health and Human Services: Washington, DC, USA, 2019.
- Burress, L.A.; Clements, J.M. Racial and Ethnic Disparities in Type 2 Diabetes Complications and In-Hospital Mortality in the United States: A Retrospective Cohort Study. Diabetology 2025, 6, 15. [Google Scholar] [CrossRef]
- Federal Practitioner. Diabetes Mellitus Federal Health Data Trends. Available online: https://www.mdedge.com/fedprac/article/152642/diabetes/diabetes-mellitus-federal-health-data-trends-full (accessed on 12 August 2025).
- Liu, Y.; Sayam, S.; Shao, X.; Wang, K.; Zheng, S.; Wang, L. Prevalence of and Trends in Diabetes Among Veterans, United States, 2005–2014. Prev. Chronic Dis. 2017, 14, E135. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.; Anderson, J.; Boundy, E.; Ferguson, L.; McCleery, E.; Waldrip, K. Mortality Disparities in Racial/Ethnic Minority Groups in the Veterans Health Administration: An Evidence Review and Map. Am. J. Public Health 2018, 108, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.S.; Hoggatt, K.J.; Steers, W.N.; Frayne, S.M.; Huynh, A.K.; Yano, E.M.; Saechao, F.S.; Ziaeian, B.; Washington, D.L. Racial/Ethnic Disparities in Mortality Across the Veterans Health Administration. Health Equity 2019, 3, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Elixhauser, A.; Steiner, C.; Harris, D.R.; Coffey, R.M. Comorbidity measures for use with administrative data. Med. Care 1998, 36, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Habibzadeh, P.; Albrecht, J. Association between race and comorbid conditions among older adults with dementia. J. Clin. Med. 2024, 13, 6368. [Google Scholar] [CrossRef] [PubMed]
- Gebregziabher, M.; Ward, R.C.; Taber, D.J.; Walker, R.J.; Ozieh, M.; Dismuke, C.E.; Axon, R.N.; Egede, L.E. Ethnic and geographic variations in multimorbidty: Evidence from three large cohorts. Soc. Sci. Med. 2018, 211, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Department of Veterans Affairs & Office of Health Equity (U.S.). National Veteran Health Equity Report-FY 2013; Government Printing Office: Washington, DC, USA, 2017.
- van Walraven, C. A comparison of methods to correct for misclassification bias from administrative database diagnostic codes. Int. J. Epidemiol. 2018, 47, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Cui, J. QIC Program and Model Selection in GEE Analyses. Stata J. 2007, 7, 209–220. [Google Scholar] [CrossRef]
- Moffat, K.; Mercer, S.W. Challenges of managing people with multimorbidity in today’s healthcare systems. BMC Fam. Pract. 2015, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.R.; Chandra Das, D.; Sunna, T.C.; Beyene, J.; Hossain, A. Global and regional prevalence of multimorbidity in the adult population in community settings: A systematic review and meta-analysis. EClinicalMedicine 2023, 57, 101860. [Google Scholar] [CrossRef] [PubMed]
- Halbert, C.H.; Armstrong, K.; Gandy, O.H.J.; Shaker, L. Racial differences in trust in health care providers. Arch. Intern. Med. 2006, 166, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.G.; Mitchell, J.A.; Hawkins, J.; Johnson-Lawrence, V. The role of age and multimorbidity in shaping older African American men’s experiences with Patient–Provider communication. Geriatrics 2018, 3, 74. [Google Scholar] [CrossRef] [PubMed]
- Cornell, P.Y.; Halladay, C.W.; Ader, J.; Halaszynski, J.; Hogue, M.; McClain, C.E.; Silva, J.W.; Taylor, L.D.; Rudolph, J.L. Embedding Social Workers In Veterans Health Administration Primary Care Teams Reduces Emergency Department Visits. Health Aff. 2020, 39, 603–612. [Google Scholar] [CrossRef] [PubMed]

| Total | Diabetes | Non-Diabetes | p-Value | |
|---|---|---|---|---|
| Patients (n) | 10,499,394 | 2,418,345 | 8,081,049 | |
| Sex (%) | <0.001 | |||
| Male | 87.40 | 95.77 | 84.88 | |
| Female | 12.60 | 4.23 | 15.12 | |
| Age, years (%) | <0.001 | |||
| <50 | 33.84 | 11.80 | 40.43 | |
| 50–64 | 32.21 | 44.09 | 28.65 | |
| ≥65 | 31.74 | 44.09 | 28.04 | |
| Missing | 2.22 | 0.02 | 2.87 | |
| Race/ethnicity (%) | <0.001 | |||
| NHW | 67.73 | 71.80 | 66.51 | |
| NHB | 14.77 | 18.13 | 13.76 | |
| Hispanic | 5.68 | 6.27 | 5.50 | |
| Other | 2.91 | 3.00 | 2.88 | |
| Missing | 8.91 | 0.79 | 11.34 | |
| ECI (%) | <0.001 | |||
| 0 | 62.63 | 36.79 | 70.37 | |
| 1 | 13.64 | 19.28 | 11.95 | |
| ≥2 | 23.73 | 43.93 | 17.68 | |
| Rurality (%) | <0.001 | |||
| Urban | 67.49 | 63.57 | 68.65 | |
| Rural | 32.23 | 36.20 | 31.04 | |
| Missing | 0.28 | 0.22 | 0.30 | |
| Marital status (%) | <0.001 | |||
| Non-Married | 41.15 | 40.19 | 41.44 | |
| Married | 51.45 | 59.38 | 49.08 | |
| Missing | 7.40 | 0.43 | 9.48 | |
| PCV (%) | ||||
| No | 56.83 | 32.92 | 63.98 | |
| Yes | 43.17 | 67.08 | 36.02 | |
| SCD (≥50%) | <0.001 | |||
| No | 98.42 | 97.41 | 98.72 | |
| Yes | 1.58 | 2.59 | 1.28 | |
| Smoking status (%) | <0.001 | |||
| Non-smoker | 77.10 | 63.71 | 81.11 | |
| Smoker | 22.90 | 36.29 | 18.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frey, L.J.; Gebregziabher, M.; Bishu, K.G.; Youngblood, B.; Obeid, J.S.; Shi, J.; Alba, P.R.; DuVall, S.L.; Blasy, C.D.; Halbert, C.H. Multimorbidity Burden in Veterans with and Without Type 2 Diabetes Mellitus: A Comparative Retrospective Cohort Study. Diabetology 2025, 6, 88. https://doi.org/10.3390/diabetology6090088
Frey LJ, Gebregziabher M, Bishu KG, Youngblood B, Obeid JS, Shi J, Alba PR, DuVall SL, Blasy CD, Halbert CH. Multimorbidity Burden in Veterans with and Without Type 2 Diabetes Mellitus: A Comparative Retrospective Cohort Study. Diabetology. 2025; 6(9):88. https://doi.org/10.3390/diabetology6090088
Chicago/Turabian StyleFrey, Lewis J., Mulugeta Gebregziabher, Kinfe G. Bishu, Brianna Youngblood, Jihad S. Obeid, Jianlin Shi, Patrick R. Alba, Scott L. DuVall, Christopher D. Blasy, and Chanita Hughes Halbert. 2025. "Multimorbidity Burden in Veterans with and Without Type 2 Diabetes Mellitus: A Comparative Retrospective Cohort Study" Diabetology 6, no. 9: 88. https://doi.org/10.3390/diabetology6090088
APA StyleFrey, L. J., Gebregziabher, M., Bishu, K. G., Youngblood, B., Obeid, J. S., Shi, J., Alba, P. R., DuVall, S. L., Blasy, C. D., & Halbert, C. H. (2025). Multimorbidity Burden in Veterans with and Without Type 2 Diabetes Mellitus: A Comparative Retrospective Cohort Study. Diabetology, 6(9), 88. https://doi.org/10.3390/diabetology6090088







