The Role of Adenosine Signaling in Obesity-Driven Type 2 Diabetes: Revisiting Mechanisms and Implications for Metabolic Regulation
Abstract
1. Introduction
2. Obesity and Type 2 Diabetes: The Role of Inflammation
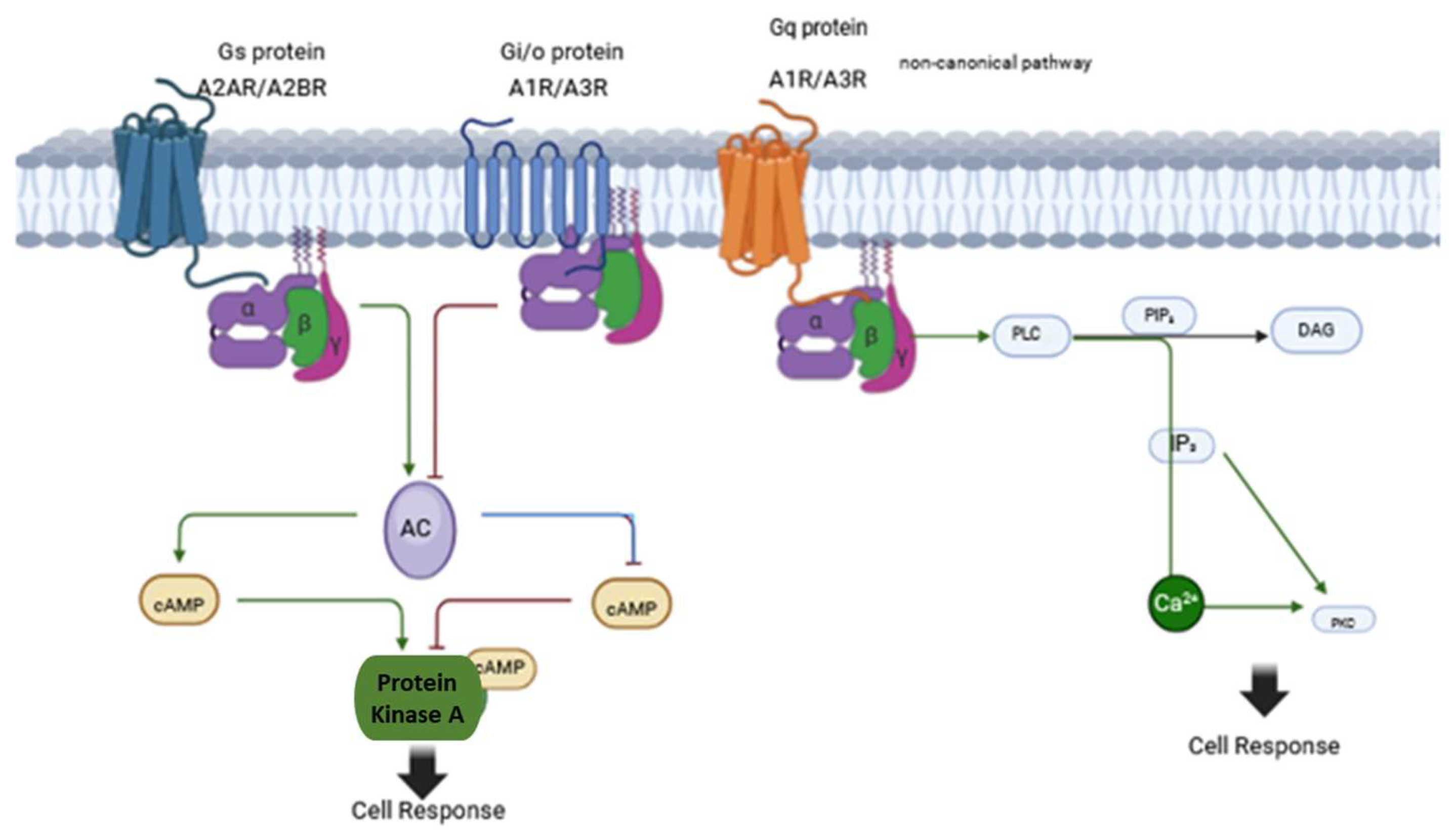
3. Adenosine System
4. The Adenosine System in Metabolic Regulation
4.1. The Role of Adenosine System in Pancreas
4.2. The Role of Adenosine System in the Liver
4.3. The Role of Adenosine System in Adipose Tissue
4.3.1. Brown Adipose Tissue
4.3.2. White Adipose Tissue
4.4. The Role of Adenosine in the Skeletal Muscle Metabolism
4.5. The Role of Adenosine in the Hypothalamic Regulation of Whole-Body Energy Metabolism
| Tissue | Primary Adenosine Receptors | Main Functions | Metabolic Effects | References |
|---|---|---|---|---|
| Pancreas | A1, A2A, A2B, A3 | Regulates insulin and glucagon secretion | - Suppresses Glucagon release - Inhibits insulin release under low glucose concentrations | [60,61,63,66,67,71] |
| Liver | A1, A2A, A2B | Regulates gluconeogenesis, glycogenolysis, lipid metabolism, and inflammation | - Suppresses or promotes hepatic glucose production (context-dependent) - Modulates insulin sensitivity and steatosis | [74,75,78,79] |
| White Adipose Tissue | A1, A2A, A2B | Controls lipolysis, adipogenesis, and inflammation | - A1R inhibits lipolysis and improves insulin action - Increase leptin secretion - A2AR/2BR reduce inflammation and promote M2 macrophages | [89,90,94,96] |
| Brown Adipose Tissue | A2A | Promotes thermogenesis, lipid oxidation, and energy expenditure | - A2AR activation enhances UCP1 expression and mitochondrial activity - Increases energy dissipation via heat | [84,85,86,87] |
| Skeletal Muscle | A1, A2A, A2B, A3 | Regulates insulin sensitivity, and glucose uptake | - Enhances mitochondrial oxidative metabolism, - Decreases senescence biomarkers - Potentiates insulin sensitivity | [88,100,101] |
| Hypothalamus | A1, A2A | Controls appetite, sympathetic tone, and whole-body energy balance | - A1R suppresses appetite via POMC/NPY modulation - Inactivation of AgRP neurons - Induces NREM sleep | [59,104,105,107] |
4.6. The Role of Adenosine Receptors in Immune System Regulation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC | Adenylate cyclase |
| ADP | Adenosine diphosphate |
| AMP | Adenosine monophosphate |
| ATP | Adenosine triphosphate |
| A1R | Adenosine 1 Receptor |
| A2AR | Adenosine 2A Receptor |
| A2BR | Adenosine 2B Receptor |
| A3R | Adenosine 3 Receptor |
| AGRP | Agouti-related peptide |
| ATGL | Adipose triglyceride lipase |
| BAT | Brown Adipose Tissue |
| cAMP | Cyclic Adenosine monophosphate |
| CCL2 | Monocyte chemoattractant protein-1 |
| CNS | Central Nervous System |
| CSF | cerebrospinal fluid |
| DAG | Diacylglycerol |
| FGF21 | Fibroblast growth factor 21 |
| HFD | High Fat Diet |
| HSL | Hormone-sensitive lipase |
| IL1β | Interleukin 1 beta |
| IL6 | Interleukin 6 |
| IL10 | Interleukin 10 |
| IP3 | inositol trisphosphate |
| IRS-1 | insulin receptor substrate 1 |
| IRS-2 | insulin receptor substrate 2 |
| JNK | c-Jun N-terminal kinase |
| KO | Knock Out |
| MCP-1 | Monocyte chemoattractant protein-1 |
| NOD | Non-obese diabetic |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| POMC | Pro-opiomelanocortin |
| PKA | Protein kinase A |
| PLC | Phospholipase C |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| SREBP-1 | Sterol regulatory element-binding protein 1 |
| T1D | Type 1 Diabetes |
| T2D | Type 2 Diabetes |
| TNF-α | Tumor Necrosis Factor-alpha |
| UCP-1 | Uncoupling protein 1 |
| WAT | White adipose tissue |
References
- World Obesity Federation. World Obesity Atlas 2023; World Obesity Federation: London, UK, 2023. [Google Scholar]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and diagnostic criteria of clinical obesity. Lancet. Diabetes Endocrinol. 2025, 13, 221–262. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Seeley, R.J.; Zeltser, L.M.; Drewnowski, A.; Ravussin, E.; Redman, L.M.; Leibel, R.L. Obesity Pathogenesis: An Endocrine Society Scientific Statement. Endocr. Rev. 2017, 38, 267–296. [Google Scholar] [CrossRef]
- Blüher, M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr. Opin. Lipidol. 2010, 21, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Are metabolically healthy obese individuals really healthy? Eur. J. Endocrinol. 2014, 171, R209–R219. [Google Scholar] [CrossRef] [PubMed]
- Mongraw-Chaffin, M.; Foster Meredith, C.; Anderson Cheryl, A.M.; Burke Gregory, L.; Haq, N.; Kalyani Rita, R.; Ouyang, P.; Sibley Christopher, T.; Tracy, R.; Woodward, M.; et al. Metabolically Healthy Obesity, Transition to Metabolic Syndrome, and Cardiovascular Risk. JACC 2018, 71, 1857–1865. [Google Scholar] [CrossRef]
- Bell, J.A.; Kivimaki, M.; Hamer, M. Metabolically healthy obesity and risk of incident type 2 diabetes: A meta-analysis of prospective cohort studies. Obes. Rev. 2014, 15, 504–515. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. Summary of Revisions: Standards of Care in Diabetes—2024. Diabetes Care 2023, 47, S5–S10. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Chen, J.F.; Masino, S.A.; Vaugeois, J.M. Actions of adenosine at its receptors in the CNS: Insights from knockouts and drugs. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 385–412. [Google Scholar] [CrossRef]
- Haskó, G.; Cronstein, B.N. Adenosine: An endogenous regulator of innate immunity. Trends Immunol. 2004, 25, 33–39. [Google Scholar] [CrossRef]
- Vincenzi, F.; Pasquini, S.; Contri, C.; Cappello, M.; Nigro, M.; Travagli, A.; Merighi, S.; Gessi, S.; Borea, P.A.; Varani, K. Pharmacology of Adenosine Receptors: Recent Advancements. Biomolecules 2023, 13, 1387. [Google Scholar] [CrossRef] [PubMed]
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P.; et al. Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Mao, J.; Yuan, Y.; Luo, P.; Wang, G.; Zhou, S. Features, functions, and associated diseases of visceral and ectopic fat: A comprehensive review. Obesity 2025, 33, 825–838. [Google Scholar] [CrossRef]
- Wallenius, V.; Wallenius, K.; Ahrén, B.; Rudling, M.; Carlsten, H.; Dickson, S.L.; Ohlsson, C.; Jansson, J.O. Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 2002, 8, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Investig. 1995, 95, 2409–2415. [Google Scholar] [CrossRef]
- Ioffe, E.; Moon, B.; Connolly, E.; Friedman, J.M. Abnormal regulation of the leptin gene in the pathogenesis of obesity. Proc. Natl. Acad. Sci. USA 1998, 95, 11852–11857. [Google Scholar] [CrossRef]
- Vaisse, C.; Halaas, J.L.; Horvath, C.M.; Darnell, J.E., Jr.; Stoffel, M.; Friedman, J.M. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat. Genet. 1996, 14, 95–97. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Côté, M.; Mauriège, P.; Bergeron, J.; Alméras, N.; Tremblay, A.; Lemieux, I.; Després, J.P. Adiponectinemia in visceral obesity: Impact on glucose tolerance and plasma lipoprotein and lipid levels in men. J. Clin. Endocrinol. Metab. 2005, 90, 1434–1439. [Google Scholar] [CrossRef]
- Harman-Boehm, I.; Blüher, M.; Redel, H.; Sion-Vardy, N.; Ovadia, S.; Avinoach, E.; Shai, I.; Klöting, N.; Stumvoll, M.; Bashan, N.; et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: Effect of regional adiposity and the comorbidities of obesity. J. Clin. Endocrinol. Metab. 2007, 92, 2240–2247. [Google Scholar] [CrossRef]
- Barbosa, P.; Pinho, A.; Lázaro, A.; Paula, D.; Campos, J.C.; Tralhão, J.G.; Pereira, M.J.; Paiva, A.; Laranjeira, P.; Carvalho, E. High percentage of immune Th1 and Tc1 cells infiltrating visceral adipose tissue in people with obesity. Obes. Res. Clin. Pract. 2024, 18, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zeng, Q.; Xie, L.; Zhang, H.; Hu, W.; Xiao, L.; Zhou, H.; Wang, F.; Xie, W.; Song, J.; et al. Adipose stem cells control obesity-induced T cell infiltration into adipose tissue. Cell Rep. 2024, 43, 113963. [Google Scholar] [CrossRef]
- Memon, R.A.; Fuller, J.; Moser, A.H.; Smith, P.J.; Grunfeld, C.; Feingold, K.R. Regulation of putative fatty acid transporters and Acyl-CoA synthetase in liver and adipose tissue in ob/ob mice. Diabetes 1999, 48, 121–127. [Google Scholar] [CrossRef]
- Fabbrini, E.; deHaseth, D.; Deivanayagam, S.; Mohammed, B.S.; Vitola, B.E.; Klein, S. Alterations in fatty acid kinetics in obese adolescents with increased intrahepatic triglyceride content. Obesity 2009, 17, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J. Hyperinsulinemia and Its Pivotal Role in Aging, Obesity, Type 2 Diabetes, Cardiovascular Disease and Cancer. Int. J. Mol. Sci. 2021, 22, 25–29. [Google Scholar] [CrossRef]
- Esser, N.; Utzschneider, K.M.; Kahn, S.E. Early beta cell dysfunction vs insulin hypersecretion as the primary event in the pathogenesis of dysglycaemia. Diabetologia 2020, 63, 2007–2021. [Google Scholar] [CrossRef] [PubMed]
- Mehran, A.E.; Templeman, N.M.; Brigidi, G.S.; Lim, G.E.; Chu, K.-Y.; Hu, X.; Botezelli, J.D.; Asadi, A.; Hoffman, B.G.; Kieffer, T.J.; et al. Hyperinsulinemia Drives Diet-Induced Obesity Independently of Brain Insulin Production. Cell Metab. 2012, 16, 723–737. [Google Scholar] [CrossRef]
- Kim, J.Y.; Nasr, A.; Tfayli, H.; Bacha, F.; Michaliszyn, S.F.; Arslanian, S. Increased Lipolysis, Diminished Adipose Tissue Insulin Sensitivity, and Impaired β-Cell Function Relative to Adipose Tissue Insulin Sensitivity in Obese Youth With Impaired Glucose Tolerance. Diabetes 2017, 66, 3085–3090. [Google Scholar] [CrossRef]
- Kasher-Meron, M.; Youn, D.Y.; Zong, H.; Pessin, J.E. Lipolysis defect in white adipose tissue and rapid weight regain. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E185–E193. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Budavari, A.; Murray, D.; Spiegelman, B.M. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J. Clin. Investig. 1994, 94, 1543–1549. [Google Scholar] [CrossRef]
- Eguchi, J.; Kong, X.; Tenta, M.; Wang, X.; Kang, S.; Rosen, E.D. Interferon regulatory factor 4 regulates obesity-induced inflammation through regulation of adipose tissue macrophage polarization. Diabetes 2013, 62, 3394–3403. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Deyoung, S.M.; Saltiel, A.R. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E166–E174. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Bai, Y.; Wang, G.; Song, Q.; Guo, C.; Zhang, L.; Wang, Q. Delivery of Adipose-Derived Stem Cells Attenuates Adipose Tissue Inflammation and Insulin Resistance in Obese Mice Through Remodeling Macrophage Phenotypes. Stem Cells Dev. 2015, 24, 2052–2064. [Google Scholar] [CrossRef] [PubMed]
- Zeyda, M.; Farmer, D.; Todoric, J.; Aszmann, O.; Speiser, M.; Györi, G.; Zlabinger, G.J.; Stulnig, T.M. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int. J. Obes. 2007, 31, 1420–1428. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef]
- Valdearcos, M.; Douglass, J.D.; Robblee, M.M.; Dorfman, M.D.; Stifler, D.R.; Bennett, M.L.; Gerritse, I.; Fasnacht, R.; Barres, B.A.; Thaler, J.P.; et al. Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility. Cell Metab. 2017, 26, 185–197.e183. [Google Scholar] [CrossRef]
- de Paula, G.C.; Brunetta, H.S.; Engel, D.F.; Gaspar, J.M.; Velloso, L.A.; Engblom, D.; de Oliveira, J.; de Bem, A.F. Hippocampal Function Is Impaired by a Short-Term High-Fat Diet in Mice: Increased Blood-Brain Barrier Permeability and Neuroinflammation as Triggering Events. Front. Neurosci. 2021, 15, 734158. [Google Scholar] [CrossRef]
- Valdearcos, M.; Robblee, M.M.; Benjamin, D.I.; Nomura, D.K.; Xu, A.W.; Koliwad, S.K. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014, 9, 2124–2138. [Google Scholar] [CrossRef]
- Souza, G.F.; Solon, C.; Nascimento, L.F.; De-Lima-Junior, J.C.; Nogueira, G.; Moura, R.; Rocha, G.Z.; Fioravante, M.; Bobbo, V.; Morari, J.; et al. Defective regulation of POMC precedes hypothalamic inflammation in diet-induced obesity. Sci. Rep. 2016, 6, 29290. [Google Scholar] [CrossRef] [PubMed]
- Morari, J.; Anhe, G.F.; Nascimento, L.F.; de Moura, R.F.; Razolli, D.; Solon, C.; Guadagnini, D.; Souza, G.; Mattos, A.H.; Tobar, N.; et al. Fractalkine (CX3CL1) is involved in the early activation of hypothalamic inflammation in experimental obesity. Diabetes 2014, 63, 3770–3784. [Google Scholar] [CrossRef]
- Lieu, L.; Chau, D.; Afrin, S.; Dong, Y.; Alhadeff, A.L.; Betley, J.N.; Williams, K.W. Effects of metabolic state on the regulation of melanocortin circuits. Physiol. Behav. 2020, 224, 113039. [Google Scholar] [CrossRef] [PubMed]
- Dunwiddie, T.V.; Masino, S.A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001, 24, 31–55. [Google Scholar] [CrossRef]
- Peleli, M.; Carlstrom, M. Adenosine signaling in diabetes mellitus and associated cardiovascular and renal complications. Mol. Asp. Med. 2017, 55, 62–74. [Google Scholar] [CrossRef]
- Pasquini, S.; Contri, C.; Borea, P.A.; Vincenzi, F.; Varani, K. Adenosine and Inflammation: Here, There and Everywhere. Int. J. Mol. Sci. 2021, 22, 7685. [Google Scholar] [CrossRef]
- Boison, D.; Yegutkin, G.G. Adenosine Metabolism: Emerging Concepts for Cancer Therapy. Cancer Cell 2019, 36, 582–596. [Google Scholar] [CrossRef] [PubMed]
- van Calker, D.; Müller, M.; Hamprecht, B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J. Neurochem. 1979, 33, 999–1005. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Arslan, G.; Halldner, L.; Kull, B.; Schulte, G.; Wasserman, W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch. Pharmacol. 2000, 362, 364–374. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Haas, H.L.; Selbach, O. Functions of neuronal adenosine receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2000, 362, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Ferré, S.; Sarasola, L.I.; Quiroz, C.; Ciruela, F. Presynaptic adenosine receptor heteromers as key modulators of glutamatergic and dopaminergic neurotransmission in the striatum. Neuropharmacology 2023, 223, 109329. [Google Scholar] [CrossRef] [PubMed]
- Rendón-Ochoa, E.A.; Padilla-Orozco, M.; Calderon, V.M.; Avilés-Rosas, V.H.; Hernández-González, O.; Hernández-Flores, T.; Perez-Ramirez, M.B.; Palomero-Rivero, M.; Galarraga, E.; Bargas, J. Dopamine D2 and Adenosine A2A Receptors Interaction on Ca2+ Current Modulation in a Rodent Model of Parkinsonism. ASN Neuro. 2022, 14, 17590914221102075. [Google Scholar] [CrossRef] [PubMed]
- Valle-León, M.; Callado, L.F.; Aso, E.; Cajiao-Manrique, M.M.; Sahlholm, K.; López-Cano, M.; Soler, C.; Altafaj, X.; Watanabe, M.; Ferré, S.; et al. Decreased striatal adenosine A2A-dopamine D2 receptor heteromerization in schizophrenia. Neuropsychopharmacology 2021, 46, 665–672. [Google Scholar] [CrossRef]
- Sánchez-Melgar, A.; Vultaggio-Poma, V.; Falzoni, S.; Fructuoso, C.; Albasanz, J.L.; Di Virgilio, F.; Martín, M. Mitochondrial Localization and Function of Adenosine Receptors. Int. J. Biol. Sci. 2025, 21, 1874–1893. [Google Scholar] [CrossRef]
- Merighi, S.; Gessi, S.; Borea, P.A. Adenosine Receptors: Structure, Distribution, and Signal Transduction. In The Adenosine Receptors; Borea, P.A., Varani, K., Gessi, S., Merighi, S., Vincenzi, F., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 33–57. [Google Scholar]
- Correia, A.S.; Vale, N. Exploring Oxidative Stress in Disease and Its Connection with Adenosine. Oxygen 2024, 4, 325–337. [Google Scholar] [CrossRef]
- Wu, L.; Meng, J.; Shen, Q.; Zhang, Y.; Pan, S.; Chen, Z.; Zhu, L.Q.; Lu, Y.; Huang, Y.; Zhang, G. Caffeine inhibits hypothalamic A1R to excite oxytocin neuron and ameliorate dietary obesity in mice. Nat. Commun. 2017, 8, 15904. [Google Scholar] [CrossRef]
- Hillaire-Buys, D.; Bertrand, G.; Gross, R.; Loubatières-Mariani, M.M. Evidence for an inhibitory A1 subtype adenosine receptor on pancreatic insulin-secreting cells. Eur. J. Pharmacol. 1987, 136, 109–112. [Google Scholar] [CrossRef]
- Johansson, S.M.; Salehi, A.; Sandström, M.E.; Westerblad, H.; Lundquist, I.; Carlsson, P.O.; Fredholm, B.B.; Katz, A. A1 receptor deficiency causes increased insulin and glucagon secretion in mice. Biochem. Pharmacol. 2007, 74, 1628–1635. [Google Scholar] [CrossRef]
- Ismail, N.A.; El Denshary, E.E.; Montague, W. Adenosine and the regulation of insulin secretion by isolated rat islets of Langerhans. Biochem. J. 1977, 164, 409–413. [Google Scholar] [CrossRef]
- Szkudelski, T.; Szkudelska, K. Regulatory role of adenosine in insulin secretion from pancreatic β-cells—Action via adenosine A1 receptor and beyond. J. Physiol. Biochem. 2015, 71, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Parandeh, F.; Fredholm, B.B.; Grapengiesser, E.; Hellman, B. Absence of adenosine A1 receptors unmasks pulses of insulin release and prolongs those of glucagon and somatostatin. Life Sci. 2009, 85, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Israeli, T.; Riahi, Y.; Saada, A.; Yefet, D.; Cerasi, E.; Tirosh, B.; Leibowitz, G. Opposing effects of intracellular versus extracellular adenine nucleotides on autophagy: Implications for β-cell function. J. Cell Sci. 2018, 131, jcs212969. [Google Scholar] [CrossRef]
- Sanni, O.; Terre’Blanche, G. Dual A(1) and A(2A) adenosine receptor antagonists, methoxy substituted 2-benzylidene-1-indanone, suppresses intestinal postprandial glucose and attenuates hyperglycaemia in fructose-streptozotocin diabetic rats. BMC Endocr. Disord. 2023, 23, 97. [Google Scholar] [CrossRef]
- Ohtani, M.; Oka, T.; Ohura, K. Possible involvement of A2A and A3 receptors in modulation of insulin secretion and β-cell survival in mouse pancreatic islets. Gen. Comp. Endocrinol. 2013, 187, 86–94. [Google Scholar] [CrossRef]
- Schulz, N.; Liu, K.C.; Charbord, J.; Mattsson, C.L.; Tao, L.; Tworus, D.; Andersson, O. Critical role for adenosine receptor A2a in β-cell proliferation. Mol. Metab. 2016, 5, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zhang, B.B. Glucagon and regulation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E671–E678. [Google Scholar] [CrossRef]
- Chapal, J.; Loubatières-Mariani, M.M.; Petit, P.; Roye, M. Evidence for an A2-subtype adenosine receptor on pancreatic glucagon secreting cells. Br. J. Pharmacol. 1985, 86, 565–569. [Google Scholar] [CrossRef]
- Liu, L.; El, K.; Dattaroy, D.; Barella, L.F.; Cui, Y.; Gray, S.M.; Guedikian, C.; Chen, M.; Weinstein, L.S.; Knuth, E.; et al. Intra-islet α-cell Gs signaling promotes glucagon release. Nat. Commun. 2024, 15, 5129. [Google Scholar] [CrossRef]
- Yang, G.K.; Fredholm, B.B.; Kieffer, T.J.; Kwok, Y.N. Improved blood glucose disposal and altered insulin secretion patterns in adenosine A1 receptor knockout mice. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E180–E190. [Google Scholar] [CrossRef]
- Yip, L.; Taylor, C.; Whiting, C.C.; Fathman, C.G. Diminished adenosine A1 receptor expression in pancreatic α-cells may contribute to the pathology of type 1 diabetes. Diabetes 2013, 62, 4208–4219. [Google Scholar] [CrossRef]
- John Hoffer, L.; Lowenstein, J.M. Effects of adenosine and adenosine analogues on glycogen metabolism in isolated rat hepatocytes. Biochem. Pharmacol. 1986, 35, 4529–4536. [Google Scholar] [CrossRef] [PubMed]
- González-Benı́tez, E.; Guinzberg, R.; Dı́az-Cruz, A.; Piña, E. Regulation of glycogen metabolism in hepatocytes through adenosine receptors. Role of Ca2+ and cAMP. Eur. J. Pharmacol. 2002, 437, 105–111. [Google Scholar] [CrossRef]
- Harada, H.; Asano, O.; Hoshino, Y.; Yoshikawa, S.; Matsukura, M.; Kabasawa, Y.; Niijima, J.; Kotake, Y.; Watanabe, N.; Kawata, T.; et al. 2-Alkynyl-8-aryl-9-methyladenines as novel adenosine receptor antagonists: Their synthesis and structure-activity relationships toward hepatic glucose production induced via agonism of the A2B receptor. J. Med. Chem. 2001, 44, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Figler, R.A.; Wang, G.; Srinivasan, S.; Jung, D.Y.; Zhang, Z.; Pankow, J.S.; Ravid, K.; Fredholm, B.; Hedrick, C.C.; Rich, S.S.; et al. Links between insulin resistance, adenosine A2B receptors, and inflammatory markers in mice and humans. Diabetes 2011, 60, 669–679. [Google Scholar] [CrossRef]
- Csóka, B.; Koscsó, B.; Töro, G.; Kókai, E.; Virág, L.; Németh, Z.H.; Pacher, P.; Bai, P.; Haskó, G. A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes 2014, 63, 850–866. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M.; Johnston-Cox, H.; Vezeridis, A.; Gavras, H.; Yang, D.; Zannis, V.; Ravid, K. A2b Adenosine Receptor Regulates Hyperlipidemia and Atherosclerosis. Circulation 2012, 125, 354–363. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Zhu, C.; Bucher, C.; Blazar, B.R.; Zhang, C.; Chen, J.F.; Linden, J.; Wu, C.; Huo, Y. Inactivation of the adenosine A2A receptor protects apolipoprotein E-deficient mice from atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1046–1052. [Google Scholar] [CrossRef]
- Johnston-Cox, H.; Koupenova, M.; Yang, D.; Corkey, B.; Gokce, N.; Farb, M.G.; LeBrasseur, N.; Ravid, K. The A2b Adenosine Receptor Modulates Glucose Homeostasis and Obesity. PLoS ONE 2012, 7, e40584. [Google Scholar] [CrossRef]
- Zhu, W.; Hong, Y.; Tong, Z.; He, X.; Li, Y.; Wang, H.; Gao, X.; Song, P.; Zhang, X.; Wu, X.; et al. Activation of hepatic adenosine A1 receptor ameliorates MASH via inhibiting SREBPs maturation. Cell Rep. Med. 2024, 5, 101477. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Jung, Y.-S.; Choi, D. Recent advance in brown adipose physiology and its therapeutic potential. Exp. Mol. Med. 2014, 46, e78. [Google Scholar] [CrossRef] [PubMed]
- Gnad, T.; Haufs-Brusberg, S.; von Kügelgen, I.; Scheele, C.; Kilić, A.; Glöde, A.; Hoffmann, L.S.; Reverte-Salisa, L.; Horn, P.; Mutlu, S.; et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 2014, 516, 395–399. [Google Scholar] [CrossRef]
- Ruan, C.-C.; Kong, L.-R.; Chen, X.-H.; Ma, Y.; Pan, X.-X.; Zhang, Z.-B.; Gao, P.-J. A2A Receptor Activation Attenuates Hypertensive Cardiac Remodeling via Promoting Brown Adipose Tissue-Derived FGF21. Cell Metab. 2018, 28, 476–489.e475. [Google Scholar] [CrossRef]
- Lahesmaa, M.; Oikonen, V.; Helin, S.; Luoto, P.; Din, M.U.; Pfeifer, A.; Nuutila, P.; Virtanen, K.A. Regulation of human brown adipose tissue by adenosine and A2A receptors—Studies with [15O]H2O and [11C]TMSX PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 743–750. [Google Scholar] [CrossRef]
- Kim, K.; Im, H.; Son, Y.; Kim, M.; Tripathi, S.K.; Jeong, L.S.; Lee, Y.H. Anti-obesity effects of the dual-active adenosine A2A/A3 receptor-ligand LJ-4378. Int. J. Obes. 2022, 46, 2128–2136. [Google Scholar] [CrossRef]
- Gnad, T.; Navarro, G.; Lahesmaa, M.; Reverte-Salisa, L.; Copperi, F.; Cordomi, A.; Naumann, J.; Hochhäuser, A.; Haufs-Brusberg, S.; Wenzel, D.; et al. Adenosine/A2B Receptor Signaling Ameliorates the Effects of Aging and Counteracts Obesity. Cell Metab. 2020, 32, 56–70.e57. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.M.; Lindgren, E.; Yang, J.N.; Herling, A.W.; Fredholm, B.B. Adenosine A1 receptors regulate lipolysis and lipogenesis in mouse adipose tissue-interactions with insulin. Eur. J. Pharmacol. 2008, 597, 92–101. [Google Scholar] [CrossRef]
- Vannucci, S.J.; Klim, C.M.; Martin, L.F.; LaNoue, K.F. A1-adenosine receptor-mediated inhibition of adipocyte adenylate cyclase and lipolysis in Zucker rats. Am. J. Physiol. 1989, 257, E871–E878. [Google Scholar] [CrossRef] [PubMed]
- Fatholahi, M.; Xiang, Y.; Wu, Y.; Li, Y.; Wu, L.; Dhalla, A.K.; Belardinelli, L.; Shryock, J.C. A Novel Partial Agonist of the A1 -Adenosine Receptor and Evidence of Receptor Homogeneity in Adipocytes. J. Pharmacol. Exp. Ther. 2006, 317, 676–684. [Google Scholar] [CrossRef]
- Granade, M.E.; Hargett, S.R.; Lank, D.S.; Lemke, M.C.; Luse, M.A.; Isakson, B.E.; Bochkis, I.M.; Linden, J.; Harris, T.E. Feeding desensitizes A1 adenosine receptors in adipose through FOXO1-mediated transcriptional regulation. Mol. Metab. 2022, 63, 101543. [Google Scholar] [CrossRef]
- Berkich, D.A.; Luthin, D.R.; Woodard, R.L.; Vannucci, S.J.; Linden, J.; LaNoue, K.F. Evidence for regulated coupling of A1 adenosine receptors by phosphorylation in Zucker rats. Am. J. Physiol. 1995, 268, E693–E704. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.M.; Fain, J.N.; Rivkees, S.A. A1 adenosine receptor activation increases adipocyte leptin secretion. Endocrinology 2000, 141, 1442–1445. [Google Scholar] [CrossRef] [PubMed]
- Faulhaber-Walter, R.; Jou, W.; Mizel, D.; Li, L.; Zhang, J.; Kim, S.M.; Huang, Y.; Chen, M.; Briggs, J.P.; Gavrilova, O.; et al. Impaired Glucose Tolerance in the Absence of Adenosine A1 Receptor Signaling. Diabetes 2011, 60, 2578–2587. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.A.; Bora, N.; Banerjee, D.; Arora, L.; Das, A.S.; Yadav, R.; Klotz, K.N.; Pal, D.; Jha, A.N.; Dasgupta, S. A novel small molecule A2A adenosine receptor agonist, indirubin-3′-monoxime, alleviates lipid-induced inflammation and insulin resistance in 3T3-L1 adipocytes. Biochem. J. 2019, 476, 2371–2391. [Google Scholar] [CrossRef]
- DeOliveira, C.C.; Paiva Caria, C.R.; Ferreira Gotardo, E.M.; Ribeiro, M.L.; Gambero, A. Role of A1 and A2A adenosine receptor agonists in adipose tissue inflammation induced by obesity in mice. Eur. J. Pharmacol. 2017, 799, 154–159. [Google Scholar] [CrossRef]
- Johnston-Cox, H.; Eisenstein, A.S.; Koupenova, M.; Carroll, S.; Ravid, K. The macrophage A2B adenosine receptor regulates tissue insulin sensitivity. PLoS ONE 2014, 9, e98775. [Google Scholar] [CrossRef]
- Sirikul, B.; Gower, B.A.; Hunter, G.R.; Larson-Meyer, D.E.; Newcomer, B.R. Relationship between insulin sensitivity and in vivo mitochondrial function in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E724–E728. [Google Scholar] [CrossRef]
- Lynge, J.; Hellsten, Y. Distribution of adenosine A1, A2A and A2B receptors in human skeletal muscle. Acta Physiol. Scand. 2000, 169, 283–290. [Google Scholar] [CrossRef]
- Haddad, M. Impact of Adenosine Analogue, Adenosine-5′-N-Ethyluronamide (NECA), on Insulin Signaling in Skeletal Muscle Cells. Biomed. Res. Int. 2021, 2021, 9979768. [Google Scholar] [CrossRef]
- Sacramento, J.F.; Martins, F.O.; Rodrigues, T.; Matafome, P.; Ribeiro, M.J.; Olea, E.; Conde, S.V. A2 Adenosine Receptors Mediate Whole-Body Insulin Sensitivity in a Prediabetes Animal Model: Primary Effects on Skeletal Muscle. Front. Endocrinol. 2020, 11, 262. [Google Scholar] [CrossRef]
- Bombassaro, B.; Araujo, E.P.; Velloso, L.A. The hypothalamus as the central regulator of energy balance and its impact on current and future obesity treatments. Arch. Endocrinol. Metab. 2024, 68, e240082. [Google Scholar] [CrossRef]
- Kumar, S.; Rai, S.; Hsieh, K.C.; McGinty, D.; Alam, M.N.; Szymusiak, R. Adenosine A2A receptors regulate the activity of sleep regulatory GABAergic neurons in the preoptic hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R31–R41. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qi, Y.; Yang, Y. Astrocytes control food intake by inhibiting AGRP neuron activity via adenosine A1 receptors. Cell Rep. 2015, 11, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Varin, C.; Rancillac, A.; Geoffroy, H.; Arthaud, S.; Fort, P.; Gallopin, T. Glucose Induces Slow-Wave Sleep by Exciting the Sleep-Promoting Neurons in the Ventrolateral Preoptic Nucleus: A New Link between Sleep and Metabolism. J. Neurosci. 2015, 35, 9900–9911. [Google Scholar] [CrossRef]
- Chen, C.; Lin, Y.; Cai, F.; Li, J.; Li, H.; Li, X. Adenosine Downregulates the Activities of Glutamatergic Neurons in the Paraventricular Hypothalamic Nucleus Required for Sleep. Front. Neurosci. 2022, 16, 907155. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Coughlin, M.; Cronstein, B.N.; Roy, R.M.; Desai, A.; Wessels, M.R. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J. Immunol. 2006, 177, 1956–1966. [Google Scholar] [CrossRef]
- Haskó, G.; Kuhel, D.G.; Chen, J.F.; Schwarzschild, M.A.; Deitch, E.A.; Mabley, J.G.; Marton, A.; Szabó, C. Adenosine inhibits IL-12 and TNF-α production via adenosine A2a receptor-dependent and independent mechanisms. Faseb. J. 2000, 14, 2065–2074. [Google Scholar] [CrossRef]
- Kreckler, L.M.; Wan, T.C.; Ge, Z.D.; Auchampach, J.A. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J. Pharmacol. Exp. Ther. 2006, 317, 172–180. [Google Scholar] [CrossRef]
- Sipka, S.; Kovács, I.; Szántó, S.; Szegedi, G.; Brugós, L.; Bruckner, G.; József Szentmiklósi, A. Adenosine inhibits the release of interleukin-1beta in activated human peripheral mononuclear cells. Cytokine 2005, 31, 258–263. [Google Scholar] [CrossRef]
- Hamano, R.; Takahashi, H.K.; Iwagaki, H.; Kanke, T.; Liu, K.; Yoshino, T.; Sendo, T.; Nishibori, M.; Tanaka, N. Stimulation of adenosine A2A receptor inhibits LPS-induced expression of intercellular adhesion molecule 1 and production of TNF-alpha in human peripheral blood mononuclear cells. Shock 2008, 29, 154–159. [Google Scholar] [CrossRef]
- Gebicke-Haerter, P.J.; Christoffel, F.; Timmer, J.; Northoff, H.; Berger, M.; Van Calker, D. BOTH ADENOSINE A1- AND A2-RECEPTORS ARE REQUIRED TO STIMULATE MICROGLIAL PROLIFERATION. Neurochem. Int. 1996, 29, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Si, Q.-s.; Nakamura, Y.; Schubert, P.; Rudolphi, K.; Kataoka, K. Adenosine and Propentofylline Inhibit the Proliferation of Cultured Microglial Cells. Exp. Neurol. 1996, 137, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, X.; Deng, P.; Wang, D.; Bai, X.; Li, Y.; Luo, C.; Belguise, K.; Wang, X.; Wei, X.; et al. Activation of adenosine A3 receptor reduces early brain injury by alleviating neuroinflammation after subarachnoid hemorrhage in elderly rats. Aging 2020, 13, 694–713. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Silva, J.; Aires, I.D.; Boia, R.; Ambrósio, A.F.; Santiago, A.R. Activation of Adenosine A3 Receptor Inhibits Microglia Reactivity Elicited by Elevated Pressure. Int. J. Mol. Sci. 2020, 21, 7218. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faraco, G.; Gaspar, J.M. The Role of Adenosine Signaling in Obesity-Driven Type 2 Diabetes: Revisiting Mechanisms and Implications for Metabolic Regulation. Diabetology 2025, 6, 43. https://doi.org/10.3390/diabetology6050043
Faraco G, Gaspar JM. The Role of Adenosine Signaling in Obesity-Driven Type 2 Diabetes: Revisiting Mechanisms and Implications for Metabolic Regulation. Diabetology. 2025; 6(5):43. https://doi.org/10.3390/diabetology6050043
Chicago/Turabian StyleFaraco, Giuseppe, and Joana M. Gaspar. 2025. "The Role of Adenosine Signaling in Obesity-Driven Type 2 Diabetes: Revisiting Mechanisms and Implications for Metabolic Regulation" Diabetology 6, no. 5: 43. https://doi.org/10.3390/diabetology6050043
APA StyleFaraco, G., & Gaspar, J. M. (2025). The Role of Adenosine Signaling in Obesity-Driven Type 2 Diabetes: Revisiting Mechanisms and Implications for Metabolic Regulation. Diabetology, 6(5), 43. https://doi.org/10.3390/diabetology6050043







