A Randomized Controlled Trial in a 14-Month Longitudinal Design to Analyze the Effects of a Peer Support Instant Messaging Service Intervention to Improve Diabetes Self-Management and Support
Abstract
1. Introduction
- H1: Peer support IMS intervention reduces HbA1c in patients with T2DM compared to standard therapy.
- H2: Peer support IMS intervention helps to maintain diabetes self-management behaviors in patients with T2DM compared to standard therapy.
- H3: Peer support IMS intervention improves the quality of life of patients with T2DM compared to standard therapy.
- H4: Peer support IMS intervention improves medication adherence in patients with T2DM compared to standard therapy.
- H5: Extraversion correlates positively with the benefits of peer support IMS intervention as measured by the frequency of IMS usage, quality of life, and HbA1c levels.
2. Materials and Methods
2.1. Study Design and Setting
2.2. Target Outcomes
- Social support was measured using the ‘Fragebogen zur Sozialen Unterstützung’ (F-SozU) [‘Questionnaire on social support’], [27]: the F-SozU operationalizes social support as perceived or anticipated support from the social environment. The short form consists of the following subscales: ‘emotional support’, ‘practical support’, ‘social integration’, ‘stress from the social network’. The F-SozU consists of14 items using a five-point Likert scale with the endpoints ‘1’ (does not apply) and ‘5’ (accurate).
- Self-efficacy was measured using the ‘General Self-Efficacy Scale’ (GSE) [28]: The GES consists of ten items designed on a four-point Likert scale with the endpoints ‘1’ (not at all true) and ‘4’ (completely true) and assesses optimistic self-beliefs to cope with several challenges in life.
- Depression was measured using the ‘Patient Health Questionnaire-9’ (PHQ-9) [29]: The PHQ-9 asks for all nine criteria of depression as defined in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) using a four-point Likert scale with the endpoints ‘0’ (not at all) and ‘3’ (nearly every day).
- Diabetes distress was measured using the ‘Diabetes Distress Scale’ (DDS) [30]: The DDS includes four dimensions of distress (‘emotional burden’, ‘regimen distress’, ‘interpersonal distress’, ‘physician distress’). The DDS consists of 17 items using a six-point Likert scale with the endpoints ‘1’ (not a problem) and ‘5’ (a very serious problem).
- Quality of life was measured using the ‘Short-Form Health Survey’ (SF-12) [31]: The SF-12 includes eight dimensions (‘physical functioning’, ‘role limitations due to physical problems’, ‘bodily pain’, ‘vitality’, ‘general health perceptions’, ‘social functioning’, ‘role limitations due to emotional problems’, ‘mental health’). The summary scores ‘Physical Summary Scale’ and ‘Psychological Summary Scale’ (0–100 scales) were calculated from the specified scales.
- Diabetes knowledge was measured using the ‘Diabetes Knowledge Test’ (DKT) [32] with forward and retranslation: The DKT consists of 20 statements about diabetes which have to be rated as ‘true’, ‘false’ or ‘I don’t know’. Based on the answers, a difficulty index (percentage of patients who scored correctly) was calculated.
- Medication adherence was measured using the ‘A14-scale’ [33]: The A14 consists of 14 items of non-adherent behaviors phrased in a non-threatening and non-judgemental way using a five-item Likert scale with the endpoints ‘4’ (never) to ‘0’ (very often).
- Dietary behavior and alcohol consumption during the past four weeks was measured based on a food frequency questionnaire using a nine-item Likert scale with the endpoints ‘0’ (never) to ‘9’ (more than four times a day).
- Smoking was measured using the questionnaire ‘Aktivrauchen—Kurzversion (Erwachsene)’ [‘Active smoking—short version (adults)’] [34]: The questionnaire assesses the current smoking behavior per week and that of the last six months. Participants indicated the number of cigarettes, cigarillos, cigars, or amount of tobacco (grams) used for pipes or hand-rolled cigarettes.
- Physical activity was measured using the ‘International Physical Activity Questionnaire Short Form’ (IPAQ-SF) [35,36]: The IPAQ-SF asks seven questions to assess ‘vigorous-intensity’ and ‘moderate-intensity’ physical activity as well as ‘walking’ and ‘sitting’. Participants indicate the time in minutes or hours for each activity level. Based on this information, three levels of physical activity (low, moderate, high) were calculated and expressed in the metabolic equivalent of task (MET) minutes per week.
- Diabetes self-management behaviors were measured using the ‘Summary of Diabetes Self-Care Activities German’ (SDSCA-G) [37]: The SDSCA-G focuses on the past seven days related to the diabetes self-care activities ‘nutrition’, ‘physical activities’, ‘blood glucose testing’, ‘footcare’, and ‘smoking’. The SDSCA-G consists of 11 items, and participants marked the number of days that the respective behavior was performed on an eight-point Likert scale with the endpoints ‘0’ (0 days) to ‘7’ (7 days). While the first ten items were calculated to a score and four sub scores (diet, exercise, blood glucose testing, foot care), the eleventh item focuses on smoking habits.
- Clinic and communication visits (health professional visits in the past six months, hospital stays in the past six months).
- Fasting blood glucose [mg/dL], total cholesterol [mg/dL], high-density lipoprotein (HDL), low-density lipoprotein (LDL) [mg/dL], and triglycerides [mg/dL] [mg/dL] were measured using Cobas 8000 (Roche Austria GmbH, Vienna, Austria).
- Blood pressure [mmHg] was measured using Boso Medicus uno OA.
- Body height [cm], body weight [kg], and body fat [%] were measured using Seca mBCA 555 (seca gmbh & co. kg, Hamburg, Germany). The device measures body weight with a scale, body height with ultrasound length measurement, and body composition by the voltage drop of the alternating current in one step. Body height and body weight were converted into Body Mass Index (kg/m2) and categorized according to the WHO classification [38]. Waist circumference [cm] to calculate the waist-to-height ratio and visceral body fat (Seca mBCA 555) was measured using an ergonomic, stepless, and extendable measuring tape.
2.3. Statistical Analyses
Missing Data
3. Results
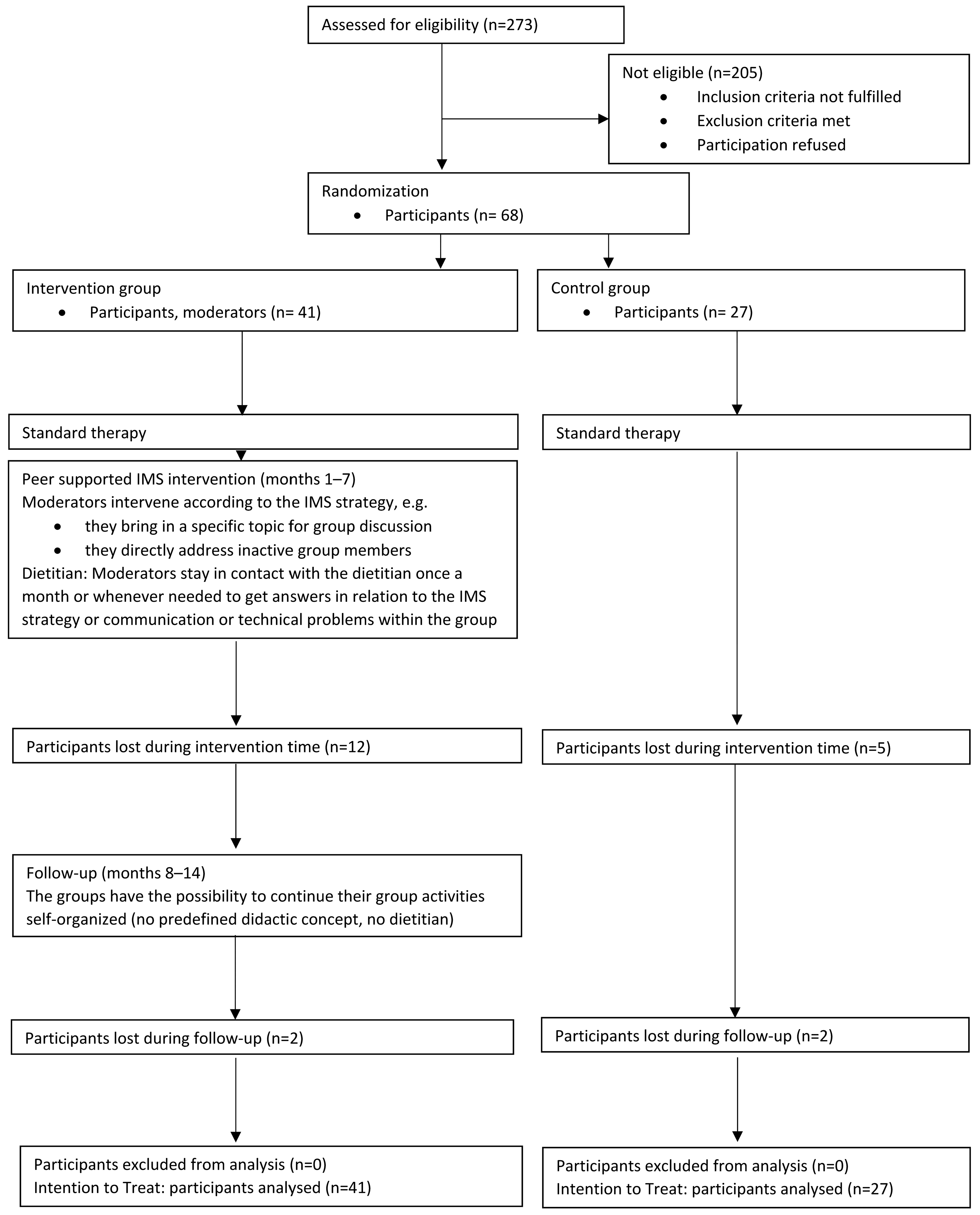
3.1. Dropout Analysis
3.2. DiabPeerS Participants
3.3. Verification of DiabPeerS Hypotheses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CG | control group |
| cm | centimeter |
| DDS | Diabetes Distress Scale |
| DKT | Diabetes Knowledge Test |
| DSM | diabetes self-management |
| DSME | diabetes self-management education |
| DSMES | diabetes self-management education and support |
| DSM-IV | Diagnostic and Statistical Manual of Mental Disorders |
| F-SozU | Fragebogen zur Sozialen Unterstützung |
| GSE | General Self-Efficacy Scale |
| HbA1c | glycated hemoglobin |
| IG | intervention group |
| IMS | instant messaging service |
| IPAQ-SF | International Physical Activity Questionnaire Short Form |
| kg | kilogram |
| mg/dL | milligram/deciliter |
| mmHg | millimeter of mercury |
| m2 | square meter |
| PHQ-9 | Patient Health Questionnaire-9 |
| RCT | randomized controlled trial |
| SDSCA-G | Summary of Diabetes Self-Care Activities German |
| SF-12 | Short-Form-Health Survey |
| T2DM | type 2 diabetes mellitus |
| USD | US Dollar |
References
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016; ISBN 978-92-4-156525-7. [Google Scholar]
- International Diabetes Federation. Clinical Guidelines Task Force; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Institute for Health Metrics and Evaluation (IHME). Global Burden of Disease 2021: Findings from the GBD 2021 Study; IHME: Seattle, WA, USA, 2024. [Google Scholar]
- Zhou, B.; Rayner, A.W.; Gregg, E.W.; Sheffer, K.E.; Carrillo-Larco, R.M.; Bennett, J.E.; Shaw, J.E.; Paciorek, C.J.; Singleton, R.K.; Pires, A.B.; et al. Worldwide Trends in Diabetes Prevalence and Treatment from 1990 to 2022: A Pooled Analysis of 1108 Population-Representative Studies with 141 Million Participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.D.; Ong, S.C.; Rafiq, A.; Kalam, M.N.; Sajjad, A.; Abdullah, M.; Malik, T.; Yaseen, F.; Babar, Z.-U.-D. A Systematic Review of the Economic Burden of Diabetes Mellitus: Contrasting Perspectives from High and Low Middle-Income Countries. J. Pharm. Policy Pract. 2024, 17, 2322107. [Google Scholar] [CrossRef]
- Mulcahy, K.; Maryniuk, M.; Peeples, M.; Peyrot, M.; Tomky, D.; Weaver, T.; Yarborough, P. Diabetes Self-Management Education Core Outcomes Measures. Diabetes Educ. 2003, 29, 768–770, 773–784, 787–788 passim. [Google Scholar] [CrossRef]
- Powers, M.A.; Bardsley, J.; Cypress, M.; Duker, P.; Funnell, M.M.; Fischl, A.H.; Maryniuk, M.D.; Siminerio, L.; Vivian, E. Diabetes Self-Management Education and Support in Type 2 Diabetes: A Joint Position Statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. Clin. Diabetes 2016, 34, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.A.; Bardsley, J.K.; Cypress, M.; Funnell, M.M.; Harms, D.; Hess-Fischl, A.; Hooks, B.; Isaacs, D.; Mandel, E.D.; Maryniuk, M.D.; et al. Diabetes Self-Management Education and Support in Adults With Type 2 Diabetes: A Consensus Report of the American Diabetes Association, the Association of Diabetes Care and Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association. J. Acad. Nutr. Diet. 2021, 121, 773–788.e9. [Google Scholar] [CrossRef]
- World Health Organization. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. Diabetes Res. Clin. Pract. 2011, 93, 299–309. [Google Scholar] [CrossRef]
- American Diabetes Association Foundations of Care. Education, Nutrition, Physical Activity, Smoking Cessation, Psychosocial Care, and Immunization. Diabetes Care 2014, 38, S20–S30. [Google Scholar] [CrossRef]
- Siegel, K.R.; Ali, M.K.; Zhou, X.; Ng, B.P.; Jawanda, S.; Proia, K.; Zhang, X.; Gregg, E.W.; Albright, A.L.; Zhang, P. Cost-Effectiveness of Interventions to Manage Diabetes: Has the Evidence Changed Since 2008? Diabetes Care 2020, 43, 1557–1592. [Google Scholar] [CrossRef]
- Ye, W.; Kuo, S.; Kieffer, E.C.; Piatt, G.; Sinco, B.; Palmisano, G.; Spencer, M.S.; Herman, W.H. Cost-Effectiveness of a Diabetes Self-Management Education and Support Intervention Led by Community Health Workers and Peer Leaders: Projections From the Racial and Ethnic Approaches to Community Health Detroit Trial. Diabetes Care 2021, 44, 1108–1115. [Google Scholar] [CrossRef]
- Qi, L.; Liu, Q.; Qi, X.; Wu, N.; Tang, W.; Xiong, H. Effectiveness of Peer Support for Improving Glycaemic Control in Patients with Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. BMC Public Health 2015, 15, 471. [Google Scholar] [CrossRef]
- Azmiardi, A.; Murti, B.; Febrinasari, R.P.; Tamtomo, D.G. The Effect of Peer Support in Diabetes Self-Management Education on Glycemic Control in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Epidemiol. Health 2021, 43, e2021090. [Google Scholar] [CrossRef]
- Dale, J.R.; Williams, S.M.; Bowyer, V. What Is the Effect of Peer Support on Diabetes Outcomes in Adults? A Systematic Review: A Systematic Review of Peer Support on Diabetes Outcomes in Adults. Diabet. Med. 2012, 29, 1361–1377. [Google Scholar] [CrossRef]
- STATISTIK AUSTRIA. Ergebnisse IKT-Einsatz in Haushalten 2024; STATISTIK AUSTRIA: Vienna, Austria, 2024. [Google Scholar]
- Gatlin, T.K.; Serafica, R.; Johnson, M. Systematic Review of Peer Education Intervention Programmes among Individuals with Type 2 Diabetes. J. Clin. Nurs. 2017, 26, 4212–4222. [Google Scholar] [CrossRef] [PubMed]
- Kitsiou, S.; Paré, G.; Jaana, M.; Gerber, B. Effectiveness of mHealth Interventions for Patients with Diabetes: An Overview of Systematic Reviews. PLoS ONE 2017, 12, e0173160. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, M.; Quade, M.; Kirch, W. Mobile Applications for Diabetics: A Systematic Review and Expert-Based Usability Evaluation Considering the Special Requirements of Diabetes Patients Age 50 Years or Older. J. Med. Internet Res. 2014, 16, e104. [Google Scholar] [CrossRef] [PubMed]
- Gabarron, E.; Årsand, E.; Wynn, R. Social Media Use in Interventions for Diabetes: Rapid Evidence-Based Review. J. Med. Internet Res. 2018, 20, e10303. [Google Scholar] [CrossRef]
- Österreichische Diabetes Gesellschaft. Diabetes Mellitus—Anleitungen Für-Die Praxis. Wien. Klin. Wochenschr. 2019, 131 (Suppl. S1), S1–S246.
- Höld, E.; Grüblbauer, J.; Wiesholzer, M.; Wewerka-Kreimel, D.; Stieger, S.; Kuschei, W.; Kisser, P.; Gützer, E.; Hemetek, U.; Ebner-Zarl, A.; et al. Improving Glycemic Control in Patients with Type 2 Diabetes Mellitus through a Peer Support Instant Messaging Service Intervention (DiabPeerS): Study Protocol for a Randomized Controlled Trial. Trials 2022, 23, 308. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Azami, G.; Soh, K.L.; Sazlina, S.G.; Salmiah, M.S.; Aazami, S.; Mozafari, M.; Taghinejad, H. Effect of a Nurse-Led Diabetes Self-Management Education Program on Glycosylated Hemoglobin among Adults with Type 2 Diabetes. J. Diabetes Res. 2018, 2018, 4930157. [Google Scholar] [CrossRef]
- Gagliardino, J.J.; Arrechea, V.; Assad, D.; Gagliardino, G.G.; González, L.; Lucero, S.; Rizzuti, L.; Zufriategui, Z.; Clark, C. Type 2 Diabetes Patients Educated by Other Patients Perform at Least as Well as Patients Trained by Professionals: Peer Diabetes Education. Diabetes Metab. Res. Rev. 2013, 29, 152–160. [Google Scholar] [CrossRef]
- Tshiananga, J.K.T.; Kocher, S.; Weber, C.; Erny-Albrecht, K.; Berndt, K.; Neeser, K. The Effect of Nurse-Led Diabetes Self-Management Education on Glycosylated Hemoglobin and Cardiovascular Risk Factors: A Meta-Analysis. Diabetes Educ. 2012, 38, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Fydrich, T.; Sommer, G.; Brähler, E. Fragebogen Zu Sozialen Unterstützung; Hogrefe: Göttingen, Germany, 2007. [Google Scholar]
- Schwarzer, R.; Jerusalem, M. Generalized Self-Efficacy Scale. In Measures in Health Psychology: A User’s Portfolio. Causal and Control Beliefs; NFER-NELSON: Winsor, UK, 1995; pp. 35–37. [Google Scholar]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a Brief Depression Severity Measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, W.H.; Fisher, L.; Earles, J.; Dudl, R.J.; Lees, J.; Mullan, J.; Jackson, R.A. Assessing Psychosocial Distress in Diabetes: Development of the Diabetes Distress Scale. Diabetes Care 2005, 28, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Morfeld, M.; Kirchberger, I.; Bullinger, M. SF-36 Fragebogen Zum Gesundheitszustand; 2., Ergänzte und Überarbeitete Auflage; Hogrefe: Göttingen, Germany, 2011. [Google Scholar]
- Collins, G.S.; Mughal, S.; Barnett, A.H.; Fitzgerald, J.; Lloyd, C.E. Modification and Validation of the Revised Diabetes Knowledge Scale. Diabet. Med. 2010, 38, 306–310. [Google Scholar] [CrossRef]
- Jank, S.; Bertsche, T.; Schellberg, D.; Herzog, W.; Haefeli, W.E. The A14-Scale: Development and Evaluation of a Questionnaire for Assessment of Adherence and Individual Barriers. Pharm. World Sci. 2009, 31, 426–431. [Google Scholar] [CrossRef]
- Latza, U.; Hoffmann, W.; Terschüren, C.; Chang-Claude, J.; Kreuzer, M.; Schaffrath Rosario, A.; Kropp, S.; Stang, A.; Ahrens, W.; Lampert, T. Erhebung, Quantifizierung und Analyse der Rauchexposition in Epidemiologischen Studien; Robert Koch-Institut: Berlin, Germany, 2005. [Google Scholar]
- IPAQ International Questionnaire Downloadable Questionnaires. Available online: https://sites.google.com/site/theipaq/questionnaire_links (accessed on 31 August 2018).
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A Systematic Review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef]
- Kamradt, M.; Bozorgmehr, K.; Krisam, J.; Freund, T.; Kiel, M.; Qreini, M.; Flum, E.; Berger, S.; Besier, W.; Szecsenyi, J.; et al. Assessing Self-Management in Patients with Diabetes Mellitus Type 2 in Germany: Validation of a German Version of the Summary of Diabetes Self-Care Activities Measure (SDSCA-G). Health Qual. Life Outcomes 2014, 12, 185. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; WHO Technical Report Series 894; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- United Nations Economic Commission for Europe. Conference of European Statisticians Recommendations for the 2020 Censuses of Population and Housing; United Nations Economic Commission for Europe: New York, NY, USA; Geneva, Switzerland, 2015. [Google Scholar]
- Danner, D.; Rammstedt, B.; Bluemke, M.; Treiber, L.; Berres, S.; Soto, C.; John, O. Die deutsche Version des Big Five Inventory 2 (BFI-2). In Zusammenstellung Sozialwissenschaftlicher Items und Skalen (ZIS); GESIS—Leibniz-Institut für Sozialwissenschaften: Köln, Germany, 2016. [Google Scholar] [CrossRef]
- Clodi, M.; Resl, M. Diabetes Mellitus—Anleitungen für die Praxis. Überarbeitete und erweiterte Fassung 2023. Wien. Klin. Wochenschr. 2023, 135 (Suppl. S1), S1–S330. [Google Scholar]
- Lönnberg, L.; Damberg, M.; Revenäs, Å. “It’s up to Me”: The Experience of Patients at High Risk of Cardiovascular Disease of Lifestyle Change. Scand. J. Prim. Health Care 2020, 38, 340–351. [Google Scholar] [CrossRef]
- Ambrož, M.; De Vries, S.T.; Buitenhuis, G.; Frost, J.; Denig, P. Willingness of People with Type 2 Diabetes to Engage in Healthy Eating, Physical Activity and Medication Taking. Primary Care Diabetes 2024, 18, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Hemetek, U.; Aubram, T.; Grüblbauer, J.; Höld, E. How to Facilitate Peer Support—Learnings from the Development of a Peer Support Program for People with T2DM via Instant Messaging Service to Improve Diabetes Self-Management. Front. Clin. Diabetes Healthc. 2024, 5, 1491865. [Google Scholar] [CrossRef] [PubMed]
- Nkhoma, D.E.; Soko, C.J.; Bowrin, P.; Manga, Y.B.; Greenfield, D.; Househ, M.; Li (Jack), Y.-C.; Iqbal, U. Digital Interventions Self-Management Education for Type 1 and 2 Diabetes: A Systematic Review and Meta-Analysis. Comput. Methods Programs Biomed. 2021, 210, 106370. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhao, X.; Wang, Y.; Xie, Q.; Cheng, L. Effectiveness of Smartphone Application–Based Self-Management Interventions in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Adv. Nurs. 2022, 78, 348–362. [Google Scholar] [CrossRef]
- Kerr, D.; Ahn, D.; Waki, K.; Wang, J.; Breznen, B.; Klonoff, D.C. Digital Interventions for Self-Management of Type 2 Diabetes Mellitus: Systematic Literature Review and Meta-Analysis. J. Med. Internet Res. 2024, 26, e55757. [Google Scholar] [CrossRef]
- Moschonis, G.; Siopis, G.; Jung, J.; Eweka, E.; Willems, R.; Kwasnicka, D.; Asare, B.Y.-A.; Kodithuwakku, V.; Verhaeghe, N.; Vedanthan, R.; et al. Effectiveness, Reach, Uptake, and Feasibility of Digital Health Interventions for Adults with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Lancet Digit. Health 2023, 5, e125–e143. [Google Scholar] [CrossRef]
- Ose, D.; Kamradt, M.; Kiel, M.; Freund, T.; Besier, W.; Mayer, M.; Krisam, J.; Wensing, M.; Salize, H.-J.; Szecsenyi, J. Care Management Intervention to Strengthen Self-Care of Multimorbid Patients with Type 2 Diabetes in a German Primary Care Network: A Randomized Controlled Trial. PLoS ONE 2019, 14, e0214056. [Google Scholar] [CrossRef]
- Pillay, J.; Armstrong, M.J.; Butalia, S.; Donovan, L.E.; Sigal, R.J.; Vandermeer, B.; Chordiya, P.; Dhakal, S.; Hartling, L.; Nuspl, M.; et al. Behavioral Programs for Type 2 Diabetes Mellitus. Ann. Intern. Med. 2015, 163, 848–860. [Google Scholar] [CrossRef]
- Alexandre, K.; Campbell, J.; Bugnon, M.; Henry, C.; Schaub, C.; Serex, M.; Elmers, J.; Desrichard, O.; Peytremann-Bridevaux, I. Factors Influencing Diabetes Self-Management in Adults: An Umbrella Review of Systematic Reviews. JBI Evid. Synth. 2021, 19, 1003–1118. [Google Scholar] [CrossRef]
- Leong, C.M.; Lee, T.-I.; Chien, Y.-M.; Kuo, L.-N.; Kuo, Y.-F.; Chen, H.-Y. Social Media–Delivered Patient Education to Enhance Self-Management and Attitudes of Patients with Type 2 Diabetes During the COVID-19 Pandemic: Randomized Controlled Trial. J. Med. Internet Res. 2022, 24, e31449. [Google Scholar] [CrossRef]
- Khiyali, Z.; Ghasemi, A.; Toghroli, R.; Ziapour, A.; Shahabi, N.; Dehghan, A.; Yari, A. The Effect of Peer Group on Self-Care Behaviors and Glycemic Index in Elders with Type II Diabetes. J. Educ. Health Promot. 2021, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.P.; Madalosso, M.M.; Bottino, L.G.; Monteiro, L.E.R.C.; Sparrenberger, K.; Schneiders, J.; Berlanda, G.; Blume, C.; Gossenheimer, A.N.; Telo, G.H.; et al. Optimization of Care for Adult Outpatients With Type 2 Diabetes Through the Diabetes Self-Management Multidisciplinary Program: A Randomized Clinical Trial. Can. J. Diabetes 2022, 46, 449–456.e3. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.M.; Sharaa, H.M.; Amer, F.G.M.; Shaban, M. Effect of Digital Based Nursing Intervention on Knowledge of Self-Care Behaviors and Self-Efficacy of Adult Clients with Diabetes. BMC Nurs. 2024, 23, 130. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chau, J.P.C.; Huo, L.; Li, X.; Wang, D.; Wu, H.; Zhang, Y. The Effects of a Nurse-Led Integrative Medicine-Based Structured Education Program on Self-Management Behaviors among Individuals with Newly Diagnosed Type 2 Diabetes: A Randomized Controlled Trial. BMC Nurs. 2022, 21, 217. [Google Scholar] [CrossRef]
- Jing, X.; Chen, J.; Dong, Y.; Han, D.; Zhao, H.; Wang, X.; Gao, F.; Li, C.; Cui, Z.; Liu, Y.; et al. Related Factors of Quality of Life of Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis. Health Qual. Life Outcomes 2018, 16, 189. [Google Scholar] [CrossRef]
- Aarthy, R.; Mikocka-Walus, A.; Pradeepa, R.; Anjana, R.M.; Mohan, V.; Aston-Mourney, K. Quality of Life and Diabetes in India: A Scoping Review. Indian J. Endocrinol. Metab. 2021, 25, 365–380. [Google Scholar] [CrossRef]
- Onu, D.U.; Ifeagwazi, C.M.; Prince, O.A. Social Support Buffers the Impacts of Diabetes Distress on Health-Related Quality of Life among Type 2 Diabetic Patients. J. Health Psychol. 2022, 27, 2305–2317. [Google Scholar] [CrossRef]
- Kretchy, I.A.; Koduah, A.; Ohene-Agyei, T.; Boima, V.; Appiah, B. The Association between Diabetes-Related Distress and Medication Adherence in Adult Patients with Type 2 Diabetes Mellitus: A Cross-Sectional Study. J. Diabetes Res. 2020, 2020, 4760624. [Google Scholar] [CrossRef]
- Balkhi, B.; Alwhaibi, M.; Alqahtani, N.; Alhawassi, T.; Alshammari, T.M.; Mahmoud, M.; Almetwazi, M.; Ata, S.; Kamal, K.M. Oral Antidiabetic Medication Adherence and Glycaemic Control among Patients with Type 2 Diabetes Mellitus: A Cross-Sectional Retrospective Study in a Tertiary Hospital in Saudi Arabia. BMJ Open 2019, 9, e029280. [Google Scholar] [CrossRef]
- Abdullah, N.F.; Khuan, L.; Theng, C.A.; Sowtali, S.N.; Juni, M.H. Effect of Patient Characteristics on Medication Adherence among Patients with Type 2 Diabetes Mellitus: A Cross-Sectional Survey. Contemp. Nurse 2019, 55, 27–37. [Google Scholar] [CrossRef]
- Dinkova, R.; Marinov, L.; Doneva, M.; Kamusheva, M. Medication Adherence among Patients with Diabetes Mellitus and Its Related Factors-A Real-World Pilot Study in Bulgaria. Medicina 2023, 59, 1205. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Goel, N.K.; Cheema, Y.S.; Garg, K. Medication Adherence and Its Predictors among Type 2 Diabetes Mellitus Patients: A Cross-Sectional Study. Indian. J. Community Med. 2023, 48, 781–785. [Google Scholar] [CrossRef]
- Chong, C.J.; Bakry, M.M.; Hatah, E.; Mohd Tahir, N.A.; Mustafa, N. Effects of Mobile Apps Intervention on Medication Adherence and Type 2 Diabetes Mellitus Control: A Systematic Review and Meta-Analysis. J. Telemed. Telecare 2023, 30, 1357633X231174933. [Google Scholar] [CrossRef] [PubMed]
- Enricho Nkhoma, D.; Jenya Soko, C.; Joseph Banda, K.; Greenfield, D.; Li, Y.-C.J.; Iqbal, U. Impact of DSMES App Interventions on Medication Adherence in Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis. BMJ Health Care Inform. 2021, 28, e100291. [Google Scholar] [CrossRef] [PubMed]
- Belete, A.M.; Gemeda, B.N.; Akalu, T.Y.; Aynalem, Y.A.; Shiferaw, W.S. What Is the Effect of Mobile Phone Text Message Reminders on Medication Adherence among Adult Type 2 Diabetes Mellitus Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. BMC Endocr. Disord. 2023, 23, 18. [Google Scholar] [CrossRef]
- Shrivastava, T.P.; Goswami, S.; Gupta, R.; Goyal, R.K. Mobile App Interventions to Improve Medication Adherence Among Type 2 Diabetes Mellitus Patients: A Systematic Review of Clinical Trials. J. Diabetes Sci. Technol. 2023, 17, 458–466. [Google Scholar] [CrossRef]
- Hakami, A.M.; Almutairi, B.; Alanazi, A.S.; Alzahrani, M.A. Effect of Mobile Apps on Medication Adherence of Type 2 Diabetes Mellitus: A Systematic Review of Recent Studies. Cureus 2024, 16, e51791. [Google Scholar] [CrossRef]
- Dudzik, J.M.; Senkus, K.E.; Evert, A.B.; Raynor, H.A.; Rozga, M.; Handu, D.; Moloney, L.M. The Effectiveness of Medical Nutrition Therapy Provided by a Dietitian in Adults with Prediabetes: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2023, 118, 892–910. [Google Scholar] [CrossRef]
- Razaz, J.M.; Rahmani, J.; Varkaneh, H.K.; Thompson, J.; Clark, C.; Abdulazeem, H.M. The Health Effects of Medical Nutrition Therapy by Dietitians in Patients with Diabetes: A Systematic Review and Meta-Analysis: Nutrition Therapy and Diabetes. Prim. Care Diabetes 2019, 13, 399–408. [Google Scholar] [CrossRef]
- Gianotti, L.; Belcastro, S.; D’Agnano, S.; Tassone, F. The Stress Axis in Obesity and Diabetes Mellitus: An Update. Endocrines 2021, 2, 334–347. [Google Scholar] [CrossRef]
- Sharma, K.; Akre, S.; Chakole, S.; Wanjari, M.B. Stress-Induced Diabetes: A Review. Cureus 2022, 14, e29142. [Google Scholar] [CrossRef] [PubMed]
- Preiser, J.-C.; Ichai, C.; Orban, J.-C.; Groeneveld, A.B.J. Metabolic Response to the Stress of Critical Illness. Br. J. Anaesth. 2014, 113, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Stefanaki, C.; Pervanidou, P.; Boschiero, D.; Chrousos, G.P. Chronic Stress and Body Composition Disorders: Implications for Health and Disease. Hormones 2018, 17, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Sunena; Mishra, D.N. Stress Etiology of Type 2 Diabetes. Curr. Diabetes Rev. 2022, 18, e240222201413. [Google Scholar] [CrossRef]
- Wong, H.; Singh, J.; Go, R.M.; Ahluwalia, N.; Guerrero-Go, M.A. The Effects of Mental Stress on Non-Insulin-Dependent Diabetes: Determining the Relationship Between Catecholamine and Adrenergic Signals from Stress, Anxiety, and Depression on the Physiological Changes in the Pancreatic Hormone Secretion. Cureus 2019, 11, e5474. [Google Scholar] [CrossRef]
- Rog, J.; Nowak, K.; Wingralek, Z. The Relationship between Psychological Stress and Anthropometric, Biological Outcomes: A Systematic Review. Medicina 2024, 60, 1253. [Google Scholar] [CrossRef]


| IG | CG | |
|---|---|---|
| M (SD) | M (SD) | |
| Age (years) | 62.00 (8.54) | 61.11 (8.85) |
| p = 0.816 | ||
| HbA1c (%) | 7.33 (0.98) | 7.16 (0.94) |
| p = 0.543 | ||
| n (%) | n (%) | |
| Gender | ||
| Male | 27 (65.9) | 20 (74.1) |
| Female | 14 (34.1) | 7 (25.9) |
| Diverse | 0 (0.0) | 0 (0.0) |
| p = 0.473 | ||
| BMI according to [38] | ||
| Underweight | 0 (0.0) | 0 (0.0) |
| Normal weight | 4 (14.8) | 2 (10.0) |
| Overweight | 7 (25.9) | 6 (30.0) |
| Obesity | 14 (51.9) | 12 (60.0) |
| Missing * | 2 (7.4) | 0 (0.0) |
| p = 0.543 | ||
| Family status | ||
| Single | 3 (7.3) | 2 (7.4) |
| Married/partnership | 33 (80.5) | 19 (70.4) |
| Separated | 1 (2.4) | 0 (0.0) |
| Divorced | 1 (2.4) | 3 (11.1) |
| Widowed | 1 (2.4) | 2 (7.4) |
| Other/missing | 1 (2.4) | 1 (3.7) |
| p = 0.422 | ||
| Education | ||
| No formal education | 0 (0.0) | 1 (3.7) |
| Secondary school | 4 (9.8) | 2 (7.4) |
| Apprenticeship | 14 (34.1) | 9 (33.3) |
| Master craftsman qualification | 4 (9.8) | 2 (7.4) |
| A-level | 11 (26.8) | 5 (18.5) |
| University degree | 3 (7.3) | 6 (20.2) |
| Other/missing | 5 (12.2) | 2 (7.4) |
| p = 0.563 | ||
| Occupation | ||
| Employed | 15 (36.6) | 10 (37.0) |
| Self-employed | 3 (7.3) | 2 (7.4) |
| Unemployed | 1 (2.4) | 1 (3.7) |
| Retired | 20 (48.8) | 14 (51.9) |
| Other/missing | 2 (4.8) | 0 (0.0) |
| p = 0.943 | ||
| Net household income | ||
| >1000 € | 2 (4.9) | 2 (7.4) |
| 1000–2000 € | 11 (26.8) | 7 (25.9) |
| 2000–3000 € | 11 (26.8) | 8 (29.6) |
| 3000–4000 € | 6 (14.6) | 5 (18.5) |
| <4000 € | 8 (19.5) | 5 (18.5) |
| Missing | 3 (7.3) | 0 (0.0) |
| p = 0.990 | ||
| Immigration background † | ||
| Yes | 0 (0.0) | 1 (3.7) |
| No | 33 (80.5) | 22 (81.5) |
| Missing | 8 (19.5) | 4 (14.8) |
| p = 0.411 | ||
| T0 | T1 | T2 | T3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | M (SD) | N | M (SD) | N | M (SD) | N | M (SD) | ||
| HbA1c (H1) | IG | 41 | 7.33 (0.98) | 31 | 7.05 (0.59) | 27 | 7.04 (0.67) | 27 | 7.06 (0.77) |
| CG | 25 | 7.16 (0.94) | 24 | 7.21 (1.08) | 22 | 6.79 (0.81) | 19 | 7.22 (1.86) | |
| SDSCA-G: Diet (H2) | IG | 40 | 3.72 (1.47) | 33 | 4.25 (1.35) | 28 | 3.96 (1.50) | 27 | 3.74 (1.40) |
| CG | 27 | 4.08 (1.58) | 23 | 4.21 (1.21) | 22 | 4.17 (1.32) | 20 | 4.54 (1.57) | |
| SDSCA-G: Exercise (H2) | IG | 41 | 3.22 (1.82) | 33 | 3.08 (1.95) | 29 | 3.31 (1.94) | 27 | 3.09 (1.92) |
| CG | 27 | 3.78 (1.87) | 23 | 3.85 (1.77) | 22 | 4.18 (1.83) | 20 | 3.30 (1.68) | |
| SDSCA-G: BST (H2) | IG | 39 | 3.49 (2.91) | 32 | 4.31 (2.75) | 29 | 3.53 (2.87) | 26 | 3.90 (2.74) |
| CG | 26 | 2.90 (2.76) | 23 | 3.04 (3.00) | 21 | 3.45 (2.78) | 20 | 3.45 (2.91) | |
| SDSCA-G: Footcare (H2) | IG | 41 | 1.66 (1.99) | 33 | 2.00 (1.86) | 29 | 1.98 (1.81) | 27 | 2.35 (2.05) |
| CG | 27 | 2.37 (2.34) | 23 | 2.37 (2.34) | 22 | 2.59 (2.38) | 20 | 2.83 (2.56) | |
| SDSCA-G: Overall (H2) | IG | 38 | 3.09 (1.27) | 32 | 3.57 (1.13) | 28 | 3.34 (1.36) | 26 | 3.30 (1.08) |
| CG | 26 | 3.41 (1.37) | 23 | 3.57 (1.32) | 21 | 3.75 (1.45) | 20 | 3.73 (1.32) | |
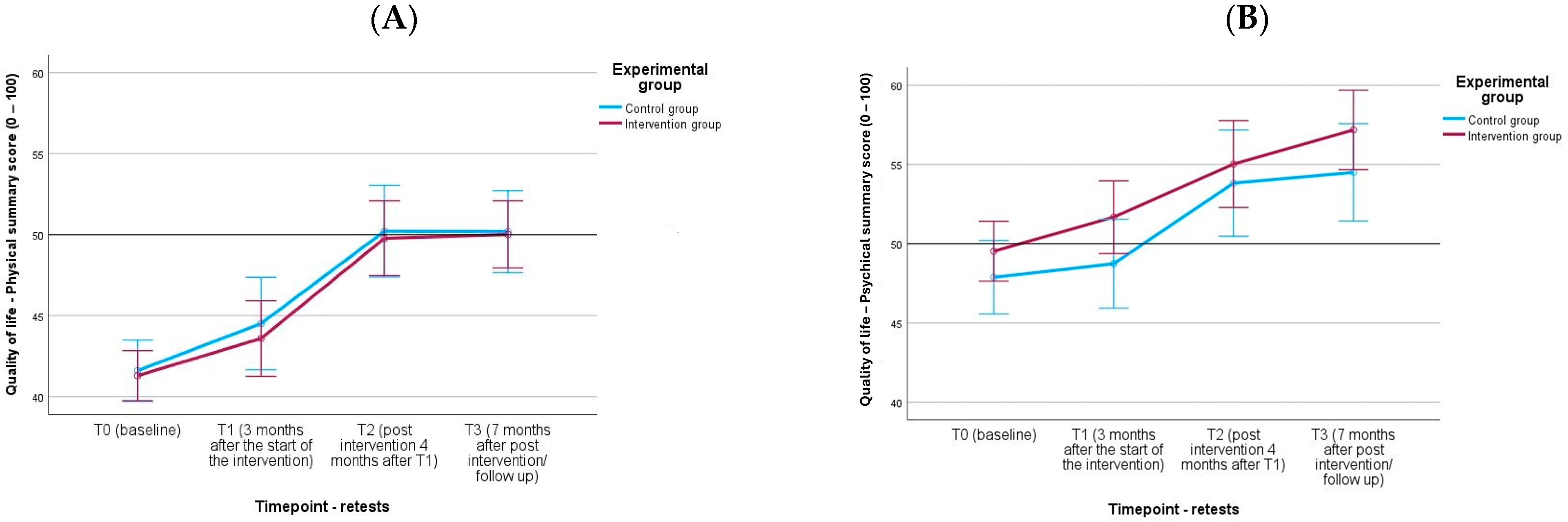
| SF-12: QoL—KSK (H3) | IG | 41 | 41.41 (5.36) | 41 | 43.71 (7.76) | 39 | 49.78 (7.72) | 41 | 50.33 (6.57) |
| CG | 27 | 41.70 (5.08) | 27 | 44.20 (6.88) | 26 | 50.21 (6.36) | 27 | 50.43 (6.30) | |
| SF-12: QoL—PSK (H3) | IG | 41 | 49.88 (5.66) | 41 | 51.97 (6.57) | 39 | 55.03 (7.70) | 41 | 57.36 (6.75) |
| CG | 27 | 47.33 (6.97) | 27 | 48.26 (8.21) | 26 | 53.83 (9.71) | 27 | 54.74 (8.99) | |
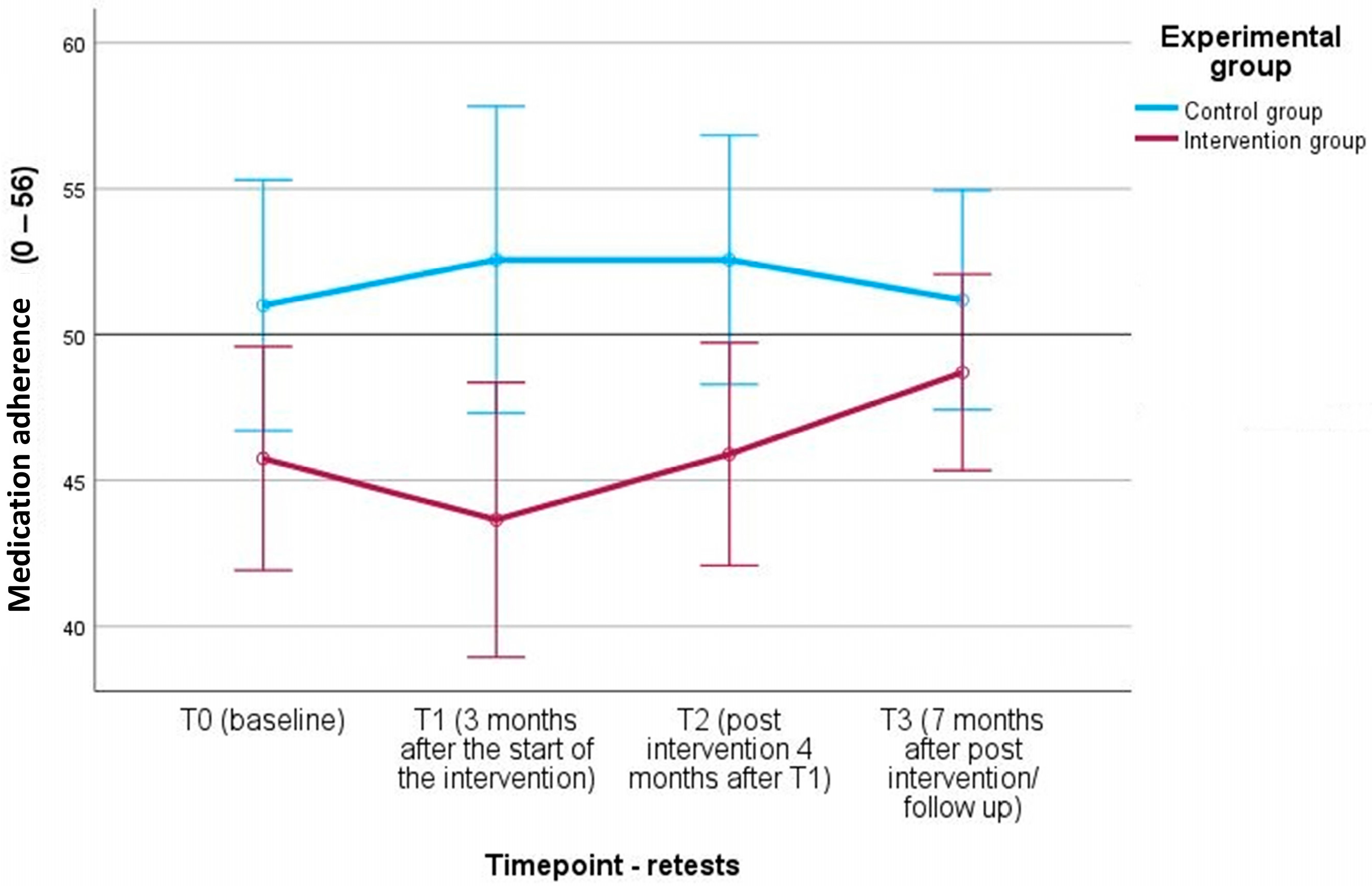
| A14: Medication ad. (H4) | IG | 36 | 45.81 (9.59) | 31 | 45.26 (12.03) | 28 | 46.71 (10.11) | 26 | 49.12 (6.68) |
| CG | 26 | 49.27 (7.99) | 22 | 51.41 (5.04) | 21 | 51.95 (5.04) | 19 | 50.84 (8.04) |
| Group/Between | Pre-Post/Within | Interaction | |
|---|---|---|---|
| Hypothesis 1 | |||
| HbA1c | F(1.38) = 0.06, ηp2 = 0.002 | F(3.114) = 2.08, ηp2 = 0.052 | F(3.114) =1.40, ηp2 = 0.036 |
| Hypothesis 2 | |||
| SDSCA-G: Diet | F(1.40) = 0.78, ηp2 = 0.019 | F(3.120) = 0.86, ηp2 = 0.021 | F(3.120) = 3.87 **, ηp2 = 0.088 |
| SDSCA-G: Exercise | F(1.42) = 1.11, ηp2 = 0.026 | F(3.126) = 3.25 *, ηp2 = 0.072 | F(3.126) = 1.52, ηp2 = 0.035 |
| SDSCA-G: Blood sugar | F(1.39) < 0.01, ηp2 < 0.001 | F(3.117) = 0.44, ηp2 = 0.011 | F(3.117) = 1.98, ηp2 = 0.048 |
| SDSCA-G: Footcare | F(1.42) = 2.95, ηp2 = 0.066 | F(3.126) = 0.64, ηp2 = 0.015 | F(3.126) = 0.50, ηp2 = 0.012 |
| SDSCA-G: Overall | F(1.37) = 2.87, ηp2 = 0.072 | F(3.111) = 0.93, ηp2 = 0.024 | F(3.111) = 1.54, ηp2 = 0.040 |
| Hypothesis 3 | |||
| SF-12: QoL—KSK | F(1.63) = 0.16, ηp2 = 0.002 | F(3.189) = 41.75 ***, ηp2 = 0.399 | F(3.189) = 0.06, ηp2 = 0.001 |
| SF-12: QoL—PSK | F(1.63) = 2.31, ηp2 = 0.035 | F(3.189) = 21.48 ***, ηp2 = 0.254 | F(3.189) = 0.32, ηp2 = 0.005 |
| Hypothesis 4 | |||
| A-14: Medication ad. | F(1.34) = 7.46 **, ηp2 = 0.180 | F(3.102) = 0.53, ηp2 = 0.015 | F(3.102) = 1.36, ηp2 = 0.038 |
| IG | CG | |||
|---|---|---|---|---|
| M (SD) | M (SD) | t | Cohen d | |
| HbA1c [%] (T0) | 7.33 (0.98) | 7.16 (0.94) | 0.674 | 0.966 |
| HbA1c [%] (T1) | 7.05 (0.60) | 7.21 (1.08) | −0.704 | 0.841 |
| HbA1c [%] (T2) | 7.04 (0.67) | 6.80 (0.81) | 1.162 | 0.737 |
| HbA1c [%] (T3) | 7.06 (0.77) | 7.22 (1.86) | −0.408 | 1.325 |
| SDSCA-G: Diet (T0) | 3.72 (1.47) | 4.08 (1.58) | −0.104 | 0.024 |
| SDSCA-G: Diet (T1) | 4.25 (1.21) | 4.21 (1.21) | −1.425 | 0.034 |
| SDSCA-G: Diet (T2) | 3.96 (1.50) | 4.17 (1.32) | −1.425 | −0.151 |
| SDSCA-G: Diet (T3) | 3.74 (1.46) | 4.54 (1.57) | −3.285 * | −0.542 |
| SDSCA-G: Exercise (T0) | 3.80 (1.80) | 2.87 (1.54) | 1.413 | −0.303 |
| SDSCA-G: Exercise (T1) | 3.14 (2.00) | 3.76 (1.71) | −1.216 | −0.411 |
| SDSCA-G: Exercise (T2) | 3.31 (1.93) | 4.18 (1.84) | −1.629 | −0.461 |
| SDSCA-G: Exercise (T3) | 3.08 (1.91) | 3.31 (1.70) | −0.436 | −0.114 |
| SDSCA-G: Blood sugar (T0) | 3.96 (2.95) | 3.00 (3.07) | 0.815 | 0.205 |
| SDSCA-G: Blood sugar (T1) | 4.02 (2.94) | 3.46 (2.88) | 0.702 | 0.444 |
| SDSCA-G: Blood sugar (T2) | 3.53 (3.01) | 3.45 (2.55) | 0.101 | 0.029 |
| SDSCA-G: Blood sugar (T3) | 3.75 (2.87) | 3.65 (2.76) | 0.119 | 0.161 |
| SDSCA-G: Footcare (T0) | 1.93 (2.17) | 1.75 (2.10) | 0.221 | −0.334 |
| SDSCA-G: Footcare (T1) | 1.92 (1.92) | 2.65 (2.20) | 0.221 | −0.262 |
| SDSCA-G: Footcare (T2) | 1.97 (1.81) | 2.61 (2.37) | −1.318 | −0.294 |
| SDSCA-G: Footcare (T3) | 2.30 (2.08) | 2.88 (2.48) | −0.890 | −0.208 |
| SF-12: Quality of life—KSK (T0) | 41.41 (5.36) | 41.70 (5.08) | −0.222 | −0.055 |
| SF-12: Quality of life—KSK (T1) | 43.71 (7.76) | 44.20 (6.88) | −0.266 | −0.066 |
| SF-12: Quality of life—KSK (T2) | 49.78 (7.72) | 50.21 (6.36) | −0.237 | −0.060 |
| SF-12: Quality of life—KSK (T3) | 50.33 (6.57) | 50.43 (6.30) | −0.059 | −0.015 |
| SF-12: Quality of life—PSK (T0) | 49.88 (5.66) | 47.33 (6.97) | 1.657 | 0.411 |
| SF-12: Quality of life—PSK (T1) | 51.97 (6.57) | 48.26 (8.21) | 2.062 | 0.511 |
| SF-12: Quality of life—PSK (T2) | 55.03 (7.70) | 53.83 (9.72) | 0.554 | 0.140 |
| SF-12: Quality of life—PSK (T3) | 57.36 (6.75) | 54.74 (9.00) | 1.372 | 0.340 |
| A-14: Medication adherence (T0) | 45.81 (9.60) | 49.27 (8.00) | −1.503 | −0.387 |
| A-14: Medication adherence (T1) | 45.26 (12.02) | 51.41 (5.04) | −2.258 | −0.629 |
| A-14: Medication adherence (T2) | 46.71 (10.11) | 51.95 (5.04) | −2.177 | −0.628 |
| A-14: Medication adherence (T3) | 49.12 (6.68) | 50.84 (8.04) | −0.785 | −0.237 |
| BFI: Conscientiousness | 3.872 (0.56) | 3.73 (0.62) | 0.616 | 0.585 |
| BFI: Neuroticism | 2.24 (0.47) | 2.31 (0.87) | −0.270 | 0.670 |
| BFI: Agreeableness | 3.82 (0.51) | 3.87 (0.38) | −0.269 | 0.460 |
| BFI: Open-mindedness | 3.50 (0.66) | 3.77 (0.58) | −1.033 | 0.632 |
| BFI: Extraversion | 3.60 (0.66) | 3.30 (0.500) | 1.220 | 0.600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Höld, E.; Hemetek, U.; Tremmel, K.; Aubram, T.; Grüblbauer, J.; Wiesholzer, M.; Schwanda, M.; Stieger, S. A Randomized Controlled Trial in a 14-Month Longitudinal Design to Analyze the Effects of a Peer Support Instant Messaging Service Intervention to Improve Diabetes Self-Management and Support. Diabetology 2025, 6, 44. https://doi.org/10.3390/diabetology6050044
Höld E, Hemetek U, Tremmel K, Aubram T, Grüblbauer J, Wiesholzer M, Schwanda M, Stieger S. A Randomized Controlled Trial in a 14-Month Longitudinal Design to Analyze the Effects of a Peer Support Instant Messaging Service Intervention to Improve Diabetes Self-Management and Support. Diabetology. 2025; 6(5):44. https://doi.org/10.3390/diabetology6050044
Chicago/Turabian StyleHöld, Elisabeth, Ursula Hemetek, Katharina Tremmel, Tatjana Aubram, Johanna Grüblbauer, Martin Wiesholzer, Manuel Schwanda, and Stefan Stieger. 2025. "A Randomized Controlled Trial in a 14-Month Longitudinal Design to Analyze the Effects of a Peer Support Instant Messaging Service Intervention to Improve Diabetes Self-Management and Support" Diabetology 6, no. 5: 44. https://doi.org/10.3390/diabetology6050044
APA StyleHöld, E., Hemetek, U., Tremmel, K., Aubram, T., Grüblbauer, J., Wiesholzer, M., Schwanda, M., & Stieger, S. (2025). A Randomized Controlled Trial in a 14-Month Longitudinal Design to Analyze the Effects of a Peer Support Instant Messaging Service Intervention to Improve Diabetes Self-Management and Support. Diabetology, 6(5), 44. https://doi.org/10.3390/diabetology6050044







