Abstract
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by inattention, impulsivity and/or hyperactivity. In recent years, metabolic alterations, primarily obesity, insulin resistance, and diabetes, have emerged as frequent comorbidities in individuals with ADHD, suggesting a bidirectional relationship between neurodevelopmental and metabolic dysfunctions. Emerging evidence indicates that dysregulation of dopaminergic signaling, disturbances in the hypothalamic-pituitary-adrenal (HPA) axis, and chronic low-grade inflammation are central to both ADHD symptomatology and metabolic impairments. For instance, alterations in dopamine-related genes (e.g., DRD4, DAT1) not only affect cognitive and behavioral functions but also play a role in appetite regulation and glucose homeostasis. Epidemiological studies further demonstrate that individuals with ADHD exhibit poorer glycemic control and a higher prevalence of both type 1 and type 2 diabetes, while early-life metabolic challenges such as maternal diabetes may predispose offspring to ADHD. This review aims to comprehensively synthesize the epidemiological, genetic, and pathogenetic evidence linking ADHD to metabolic alterations. We discuss key pathophysiological pathways—including dopaminergic dysregulation, HPA axis disturbances, inflammation, and oxidative stress—and evaluate their contributions to the co-occurrence of ADHD and metabolic disorders. In addition, we explore the clinical implications and integrated treatment approaches that encompass lifestyle modifications, pharmacological therapies, and multidisciplinary care. Finally, we outline future research directions to develop personalized and holistic interventions.
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental condition that is frequently comorbid with other psychiatric disorders and creates a substantial burden for the individual, their family, and the community [1]. ADHD is characterized by core symptoms of age-inappropriate inattention, impulsivity, and/or hyperactivity, with its clinical manifestations evolving across the lifespan. While inattention often persists from childhood into adulthood, hyperactivity in adults might instead present as an inability to relax or internal restlessness, and impulsivity may manifest as impatience, inappropriate risk-taking or emotional lability [2,3,4,5].
The global prevalence of ADHD in childhood is estimated at approximately 5%, based on meta-regression analyses [6]. Interestingly, the prevalence in adults appears higher than would be expected by the persistence rates from children, suggesting the emergence of new diagnoses during adulthood. This trend is likely influenced by evolving diagnostic criteria, as defined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), as well as increased awareness of the disorder, partly driven by social media [7]. Despite this change, ADHD remains an underdiagnosed and undertreated condition in adults, often resulting in significant impairment [8]. Beyond its clinical manifestations, ADHD is increasingly understood as a disorder of disrupted neurotransmitter systems, particularly involving dopamine pathways in the prefrontal cortex. These abnormalities affect executive functions such as attention, behavioral inhibition, and emotional regulation, which are central to ADHD pathophysiology [1]. Emerging evidence also suggests that neuroinflammation and oxidative stress may contribute to these neurochemical imbalances, linking ADHD to broader systemic alterations, including metabolic dysfunction [1,9].
The association between ADHD and obesity has been documented in the literature, with various proposed mechanisms linking the two conditions such as abnormal eating patterns, decreased physical activity and sleep disruption. Preliminary evidence has also revealed possible common genetic underpinnings [9]. Obesity itself can be considered as a real pandemic as its global prevalence nearly tripled between 1975 and 2016, affecting both high-income and low-to-middle-income countries [10]. Its prevalence has also increased in the last decade in pediatric populations. In particular, one in five children or adolescents have experienced excess weight [11]. Since 2000, childhood obesity has continued to rise, particularly in economically disadvantaged countries [12]. This widespread phenomenon has contributed to the increasing prevalence of metabolic disorders, including non-alcoholic fatty liver disease and type 2 diabetes (T2D) [13,14].
Throughout history, the clash of different pandemics has led to unexpected and often deleterious effects. A recent example is the interplay between obesity and COVID-19, where their overlap resulted in severe clinical consequences for affected individuals [15]. Given the rising global prevalence of both ADHD and obesity, it is necessary to investigate their interconnection and the potential implications of their convergence. This review aims to synthesize the current evidence about ADHD and metabolism, their interrelationships starting from the pathophysiological mechanisms and common risk factors, and then moving toward management and treatment concerns. Among the metabolic impairments, we will specifically focus on obesity and diabetes.
2. Pathophysiological Mechanisms Linking ADHD Neurobiological Factors and Metabolism
ADHD is a highly heritable disorder, but not all of its risk is genetic. Estimates suggest that environmental factors may account for between 10% and 40% of the variance associated with ADHD [16]. Although the etiology is still uncertain, hypo-efficient dopamine systems, and the subsequent neurochemical imbalances, have been linked to ADHD onset and its features, such as sustained attention deficits, overactivity and impulsiveness [17]. Reduced postsynaptic activation in the prefrontal and striatal regions has been observed in several MRI studies [18,19]. In the striatum, dopamine activates postsynaptic neurons but ADHD patients experience a delayed dopamine signal, rather than the immediate anticipatory dopamine signal, that normal subjects experience [20]. Furthermore, a number of other neuroendocrine imbalances may be present in ADHD: a higher cumulative diurnal cortisol level has been found, with increased morning and afternoon cortisol, after controlling for symptoms of anxiety, depression and conduct disorder [21], with a reduced bedtime salivary cortisol [22], reflecting a different circadian cortisol profile. Leptin levels may be normal or increased in ADHD [23,24], while ghrelin and neuropeptide Y remain normal [24,25]. Based on these data, individuals with ADHD may also exhibit altered energetic metabolism. The interlinks between ADHD behavioral patterns and metabolism could be bidirectional. Recently, mice fed a high-fat diet were observed to have developed behavioral deficits resembling ADHD phenotypes, such as decreased wakefulness, increased REM (rapid eye movement) sleep with fragmented patterns, and impaired visuospatial memory, suggesting that dietary patterns can affect the dopaminergic system [26].
Emerging evidence underscores a shared genetic architecture underlying ADHD and metabolic dysfunction, including obesity and type 2 diabetes [27]. Genome-wide association studies have revealed that multiple genetic loci implicated in ADHD also overlap with those associated with metabolic disorders, reflecting common biological pathways [28,29] (Figure 1).
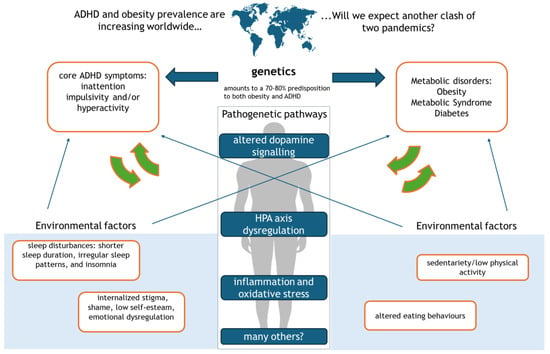
Figure 1.
The bidirectional link between ADHD and obesity.
This figure illustrates the complex interplay between ADHD, obesity, and diabetes, driven by genetic, environmental, and neurobiological factors. Altered dopamine signaling contributes to sleep disorders, which in turn lead to dysregulated eating behaviors. HPA axis dysfunction exacerbates ADHD core symptoms—inattention, impulsivity, and hyperactivity—fostering sedentary behavior and obesity. Chronic inflammation and oxidative stress further reinforce emotional dysregulation, internalized stigma, and metabolic disturbances, ultimately increasing the risk of type 2 diabetes.
2.1. Dopaminergic System and Its Role in ADHD and Metabolism
Polymorphisms in dopamine-related genes, such as DRD4 and DRD5 (dopamine receptor genes) and DAT1 (dopamine transporter gene), are well-established contributors to ADHD pathophysiology [30,31]. These genes regulate dopaminergic signaling, which is crucial for attention, reward processing, and impulse control [32,33]. However, their effects extend beyond the central nervous system to metabolic regulation. Dysregulation in dopaminergic pathways can impair reward sensitivity, leading to altered eating behaviors such as hyperphagia [34,35]. This disruption in reward systems may explain the higher prevalence of obesity observed in individuals with ADHD [35]. Additionally, dopamine plays a role in modulating insulin secretion and glucose homeostasis, linking these polymorphisms to an increased risk of insulin resistance [36].
2.2. HPA Axis Dysregulation and Genetic Variants
The hypothalamic–pituitary–adrenal (HPA) axis, a key regulator of stress responses and energy balance, is frequently disrupted in ADHD [37]. Genetic variations in NR3C1, which encodes the glucocorticoid receptor, have been associated with dysregulated cortisol secretion in individuals with ADHD [38]. These alterations may contribute to heightened stress reactivity, promoting abdominal fat deposition and insulin resistance through prolonged exposure to elevated cortisol levels [39]. Dysfunctional HPA axis activity also influences neurodevelopment and behavior, further intertwining ADHD symptoms with metabolic derangements [40].
2.3. Genetic Links to Inflammatory Pathways
Chronic low-grade inflammation is a hallmark of metabolic disorders and has been increasingly recognized in ADHD [41,42]. Polymorphisms in pro-inflammatory cytokine genes, such as IL-6 and TNF-α, have demonstrated dual associations with ADHD and metabolic dysfunction [43,44]. Elevated levels of these cytokines are observed in both conditions and may exacerbate neuroinflammation, impair neuroplasticity, and contribute to the systemic inflammation observed in obesity and type 2 diabetes [45,46].
2.4. Epigenetic Modifications and Environmental Interactions
Epigenetic mechanisms provide a dynamic link between genetic predisposition and environmental exposures, influencing both ADHD and metabolic dysfunction [47,48]. Prenatal stress, maternal obesity, poor maternal nutrition, and exposure to environmental toxins (e.g., tobacco smoke, heavy metals) are known risk factors for ADHD [49,50]. These exposures can lead to epigenetic changes, such as DNA methylation or histone modification, in genes regulating neurodevelopment and metabolic processes [51,52]. For example, maternal stress during pregnancy has been associated with altered methylation of genes involved in HPA axis regulation and energy homeostasis, potentially predisposing offspring to ADHD and obesity later in life [53].
2.5. Shared Polygenic Risk Scores and Heritability
Studies using polygenic risk scores (PRS) suggest that ADHD and metabolic disorders share common genetic determinants, contributing to their co-occurrence [54]. Twin studies further support this, with heritability estimates for ADHD and obesity ranging between 70–80%, indicating significant genetic contributions to both conditions [55,56]. Interestingly, the co-heritability of ADHD and increased body mass index (BMI) has also been observed, suggesting a partially overlapping genetic etiology [57].
3. Inflammation and Oxidative Stress as Neurobiological Bridges Between ADHD and Metabolic Dysfunction
Chronic inflammation and oxidative stress are key mechanisms linking ADHD and metabolic dysfunction, creating a complex interplay of neuroimmune and metabolic pathways that exacerbate both conditions [58,59,60]. These shared pathophysiological processes highlight the profound connections between systemic inflammation, oxidative damage, and their cumulative impact on neurodevelopment and metabolism [58,59]. Notably, oxidative stress directly affects neurotransmitter systems by altering the synthesis, release, and reuptake of key monoamines such as dopamine and serotonin, neurotransmitters that are central to ADHD pathophysiology and are also involved in appetite, mood regulation, and energy homeostasis [46,59]. This interference further contributes to the overlapping cognitive, behavioral, and metabolic dysfunctions observed in ADHD and related disorders.
ADHD has been consistently associated with elevated levels of pro-inflammatory cytokines, including interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP), which are similarly upregulated in metabolic disorders such as obesity and type 2 diabetes [60,61]. This inflammatory milieu is not only a hallmark of systemic immune dysregulation but also a driver of neurodevelopmental impairments and metabolic disturbances [46]. IL-6, for instance, influences synaptic plasticity and neurotransmitter signaling, critical for cognitive and behavioral regulation in ADHD, while also playing a role in hepatic glucose production and lipid metabolism, thereby contributing to insulin resistance and obesity [62,63,64]. Similarly, TNF-α exacerbates insulin resistance by impairing insulin receptor signaling, while simultaneously promoting neuroinflammatory processes that worsen core ADHD symptoms like attention deficits and impulsivity [65,66,67]. Elevated CRP, a marker of systemic inflammation, bridges these disorders by linking them to vascular dysfunction and heightened cardiometabolic risk [68,69].
Inflammation further disrupts the HPA axis, a key regulator of stress responses and energy homeostasis [70]. Chronic inflammation-induced dysregulation of cortisol secretion amplifies the pathophysiology of both ADHD and metabolic disorders [71,72]. Altered cortisol rhythms in ADHD impair attention and emotional regulation, while excess cortisol in metabolic disorders promotes visceral fat accumulation, insulin resistance, and dyslipidemia [71,72,73]. Furthermore, inflammatory signals disrupt the regulation of dopamine and serotonin pathways, indirectly influencing neurotransmitter homeostasis and exacerbating core ADHD symptoms. These disturbances also contribute to metabolic dysfunction by impairing mechanisms involved in appetite regulation, glucose metabolism, and mood [59,74].
Oxidative stress adds another layer to this interplay, characterized by an imbalance between reactive oxygen species (ROS) production and antioxidant defenses [46]. Excessive ROS levels cause oxidative damage to lipids, proteins, and DNA, particularly in dopamine-rich brain regions such as the prefrontal cortex, impairing synaptic function and contributing to cognitive and behavioral symptoms of ADHD [46]. In metabolic disorders, oxidative stress impairs insulin signaling pathways and promotes pancreatic beta-cell dysfunction, accelerating the progression of insulin resistance and hyperglycemia [75]. Mitochondrial dysfunction emerges as a central mechanism in this context, as mitochondria, being primary energy producers and sources of ROS, amplify oxidative damage and reduce ATP availability [76]. In ADHD, this dysfunction limits neuronal energy resources, affecting processes like attention and memory, while in metabolic disorders, it hampers fatty acid oxidation and glucose metabolism, exacerbating insulin resistance and promoting weight gain [77].
The bidirectional relationship between inflammation and oxidative stress creates a self-perpetuating loop, where inflammation drives ROS production and oxidative stress exacerbates inflammatory responses through pathways like nuclear factor-kappa B (NF-κB) activation [46]. This synergistic interaction not only deepens the neurodevelopmental and metabolic impairments seen in ADHD and metabolic dysfunction but also influences behaviors such as appetite regulation and energy expenditure, leading to overeating, obesity, and poor glycemic control, which in turn exacerbate ADHD symptoms [78,79].
Addressing these shared mechanisms offers promising avenues for therapeutic interventions. Anti-inflammatory strategies, including selective cytokine inhibitors and anti-inflammatory diets rich in omega-3 fatty acids and polyphenols, hold potential to mitigate systemic and neuroinflammation [80]. Similarly, antioxidant therapies, whether through nutrient-rich diets or pharmacological agents targeting mitochondrial function, such as coenzyme Q10 and N-acetylcysteine, may reduce oxidative damage and restore cellular energy balance [81,82]. An integrated approach that combines pharmacological treatments with lifestyle modifications and dietary interventions may provide synergistic benefits, as we will discuss in the next paragraphs [75].
5. Clinical Implications and Treatment Considerations
In the era of precision medicine and multiple drug choices, comorbidities play a crucial role in shaping treatment strategies, guiding clinicians toward therapies that address both neurological and metabolic concerns. We have previously discussed that the interconnections between ADHD and metabolism are primarily epidemiological and, at times, clinical, but the underlying biochemical mechanisms linking obesity and diabetes to ADHD remain largely unexplored. Preclinical research on the intersection of ADHD-related neuronal circuit dysfunction and metabolic parameters is still in its early stages. However, some longitudinal studies suggest that hyperglycemia may play a causal role in the onset of ADHD, although the exact pathophysiological mechanisms and additional etiological factors remain unclear [142]. One key piece of evidence supporting this connection is the neurological impact of diabetic ketoacidosis (DKA), an acute complication arising from severe insulin deficiency, leading to hyperglycemia and ketone body production. DKA has been associated with neurological complications as morphological and functional brain changes, particularly affecting the frontal white matter [143]. We have discussed how reduced postsynaptic activation in prefrontal region is a possible finding in ADHD. This suggests a potential link between metabolic dysregulation and cognitive function. However, while the impact of metabolic disorders on ADHD risk has been explored to some extent, the reverse relationship—how ADHD may contribute to metabolic disease—remains insufficiently studied. Longitudinal research on this subject is still lacking, making it difficult to establish management recommendations for ADHD patients with metabolic comorbidities. Nonetheless, existing therapeutic strategies for both conditions offer valuable insights to help guide clinical decision-making.
ADHD has been shown to worsen glycemic and metabolic outcomes in children and adolescents. Given this, a more intensive and closely monitored treatment approach is warranted to prevent acute complications such as DKA, which is particularly common in adolescents and in individuals with psychiatric comorbidities [144]. Continuous glucose monitoring (CGM) should be preferred over traditional self-monitoring of blood glucose, as CGM provides real-time data and can help to prevent glycemic fluctuations [145].
In adults, treatment priorities should focus on reducing both acute and chronic complications [146]. Cardiovascular risk is a major concern, as it increases with age and is closely linked to cumulative LDL cholesterol exposure [147,148,149]. A recent meta-analysis confirmed that ADHD itself is associated with an increased risk of cardiovascular diseases, regardless of the presence of metabolic comorbidities [150]. Therefore, a comprehensive cardiovascular risk management in ADHD patients, incorporating lipid control, blood pressure management, and lifestyle modifications, is encouraged. Given the interplay between psychiatric and metabolic factors, a multidisciplinary approach is recommended. For example, therapeutic choices would benefit from this shared approach. Some general considerations are provided that could serve as a common ground among the clinicians:
5.1. Lifestyle Modifications and Nutritional Approaches
Physical activity should be strongly encouraged in ADHD patients, as it not only improves focus and executive functioning but also reduces obesity risk [151]. Notably, moderate to vigorous activity has been associated with a lower incidence of sleep disorders in men but not in women, highlighting the importance of gender-specific considerations in ADHD management [152]. Dietary interventions may also play a role. An omega-3 enriched diet should be encouraged, as these nutrients not only confer cardiovascular benefits [153] but may also reduce impulsive behavior in ADHD patients [154] (Table 1).
5.2. Pharmacological Considerations
While methylphenidate remains the main therapeutic strategy for ADHD treatment [155], combining it with psychosocial interventions—such as home-based behavioral therapy, school-based programs, and summer treatment programs—has been shown to improve functional outcomes beyond pharmacotherapy alone, including academic performance, parent–child relationships, and social skills [156]. However, the metabolic effects of these interventions remain unclear and warrant further study.
In managing diabetes, therapeutic agents with pleiotropic effects may offer promise for patients with comorbid ADHD. While insulin remains the standard treatment for type 1 diabetes, the role of adjunctive therapies in this population is still debated [157]. For T2D, recent advancements in treatment—particularly sodium–glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide-1 receptor agonists (GLP-1 RAs)—offer beneficial metabolic effects beyond glycemic control [158,159,160,161]. For instance, GLP-1 receptor agonists have shown promise in combating obesity while potentially enhancing cognitive function [162,163]. Further, given the role of gut peptides in the brain’s reward system, GLP1-RA may have a positive influence [164]. Further research is needed to clarify the impact of these drugs on the central nervous system.

Table 1.
Summary of pathophysiological mechanisms and therapeutic implications in ADHD and metabolic dysfunction.
Table 1.
Summary of pathophysiological mechanisms and therapeutic implications in ADHD and metabolic dysfunction.
| Mechanism | Clinical Effect | Associated Metabolic Outcomes | Therapeutic Implications | References |
|---|---|---|---|---|
| Dopaminergic Dysregulation | Altered dopamine signaling affects attention, impulsivity, and reward sensitivity. | Impaired appetite regulation, altered insulin secretion, increased obesity risk. | Dopamine modulators, nutritional interventions (e.g., omega-3 supplementation). | [17,20,36] |
| HPA Axis Dysregulation | Dysregulated cortisol secretion and increased stress reactivity. | Visceral fat accumulation, insulin resistance, and heightened diabetes risk. | Stress management strategies, HPA-targeted therapies, lifestyle modifications. | [21,22,23] |
| Inflammation and Oxidative Stress | Chronic low-grade inflammation and increased ROS contribute to neuroinflammation. | Insulin resistance, dyslipidemia, obesity, and type 2 diabetes. | Anti-inflammatory diets, antioxidant therapies, and interventions targeting ROS production. | [125,126,145] |
| Genetic and Epigenetic Factors | Shared genetic loci and epigenetic modifications create a common predisposition for both ADHD and metabolic dysfunction. | Predisposition to metabolic syndrome, obesity, and diabetes. | Personalized medicine approaches, early screening, and targeted lifestyle modifications. | [122,139,140] |
| Integrated Clinical Approaches | Comorbid ADHD and metabolic disorders require holistic, multidisciplinary management. | Improved glycemic control, weight management, and overall cognitive function. | Combined pharmacological treatments (e.g., CGM, SGLT2 inhibitors, GLP-1 RAs), behavioral therapies, and lifestyle programs. | [147,152,159] |
HPA: Hypothalamic–pituitary–adrenal; ROS: Reactive oxygen species; ADHD: Attention deficit hyperactivity disorder; CGM: Continuous glucose monitoring; SGLT2: Sodium–glucose cotransporter-2; GLP-1 RA: Glucagon-like peptide-1 receptor agonist.
Similarly, insulin-sensitizing agents like metformin may offer adjunctive benefits in ADHD treatment due to their neuroprotective properties and positive effects on metabolism [165]. Metformin, widely used for treating insulin resistance and T2D, has demonstrated potential benefits in managing weight gain associated with psychotropic medications, particularly in children treated with mixed serotonin and dopamine receptor antagonists [166]. Given its favorable safety profile, metformin may be a valuable option for young, obese, insulin-resistant ADHD patients, although specific data on its effects in this population are currently lacking. Interactions of these drugs with methylphenidate and other psychiatric medications have not yet been fully investigated.
5.3. Bariatric Surgery
For severely obese ADHD patients, bariatric surgery remains a viable treatment option, demonstrating comparable efficacy to its outcomes in non-ADHD individuals. Studies suggest that surgical interventions may be superior to pharmacotherapy in achieving sustained weight loss and metabolic improvements [167,168]. However, the long-term effects of bariatric surgery on ADHD symptoms and cognitive function require further exploration. Given the complex interplay between ADHD and metabolic disorders, treatment strategies must adopt a holistic, patient-centered approach, integrating psychiatric and metabolic care to optimize outcomes [169,170,171].
6. Future Directions and Research Needs
Despite growing evidence linking ADHD to metabolic dysfunction, several critical knowledge gaps remain that must be addressed to develop targeted interventions mitigating both ADHD symptoms and metabolic complications. A more comprehensive understanding of the interplay between ADHD and metabolism is essential, and current research must evolve beyond predominantly cross-sectional designs. Longitudinal studies tracking metabolic health from childhood through adulthood in individuals with ADHD are needed to establish causal relationships, identify early predictive markers, and elucidate disease trajectories. Additionally, standardized metabolic biomarkers for assessing risk remain elusive. However, emerging candidates such as adiponectin, leptin, and inflammatory markers like CRP may offer insight into early metabolic alterations in ADHD [24,172,173]. Future investigations should focus on identifying specific metabolic signatures that could enhance diagnostic precision and inform treatment monitoring.
The relationship between ADHD and metabolic disorders is further complicated by shared genetic and epigenetic mechanisms [174]. Variants in dopamine signaling pathways, appetite regulation genes such as FTO and MC4R, and circadian rhythm regulators suggest a biological overlap that warrants closer examination [175]. Understanding how these genetic predispositions interact with environmental factors like diet and physical activity will be key to unraveling their collective impact on metabolic health. Moreover, emerging research on the gut–brain axis points to the composition of gut microbiota as a potential mediator of both neurodevelopmental and metabolic outcomes [176]. Investigating microbiome-targeted therapies, including probiotics and specific dietary interventions, could reveal new strategies for modulating ADHD symptoms while improving metabolic profiles. In parallel, the long-term metabolic effects of ADHD medications require careful scrutiny; while stimulant medications often lead to appetite suppression and potential weight loss, some non-stimulant treatments have been associated with increased risks of insulin resistance and obesity. Addressing these challenges calls for innovative therapeutic strategies that integrate neurodevelopmental and metabolic perspectives. Novel pharmacological approaches are being explored, including medications that modulate both dopamine and metabolic pathways. The development of multi-target drugs that address both executive dysfunction and metabolic abnormalities represents another promising avenue. Beyond pharmacotherapy, non-pharmacological interventions that promote neuroplasticity and healthy lifestyles are critical. Cognitive training combined with exercise regimens could simultaneously boost executive function and improve insulin sensitivity and cardiovascular health [177,178]. The link between sleep duration, obesity, and type 2 diabetes suggests that interventions targeting sleep hygiene may help mitigate metabolic risks in individuals with ADHD [179,180]. In addition, implementing school-based programs focused on early metabolic health could help prevent obesity and type 2 diabetes in high-risk populations.
The metabolic aspects of ADHD also carry significant public health implications, particularly as the prevalence of both ADHD and obesity continues to rise [181]. Incorporating routine metabolic screening into ADHD clinical guidelines, especially for children and adolescents at heightened risk, is imperative for early detection and prevention. The establishment of interdisciplinary care models that bring together pediatricians, psychiatrists, endocrinologists, and nutritionists will be crucial for delivering comprehensive management of ADHD and its metabolic comorbidities. Furthermore, public health initiatives must work to raise awareness about the interconnected nature of ADHD and metabolic dysfunction, ensuring broader access to integrative treatment strategies and preventive measures [182,183]. Finally, given the influence of socioeconomic and environmental factors on both conditions, future research should prioritize the examination of health disparities and the development of targeted interventions for high-risk populations [184,185].
This review offers a comprehensive synthesis of current knowledge on the interplay between ADHD and metabolic dysfunction. However, several limitations should be acknowledged. As a narrative review, it is inherently subject to selection and publication bias and lacks the methodological rigor of a systematic review. The heterogeneity of the studies included, in terms of design, diagnostic criteria, and population characteristics, limits the ability to draw definitive conclusions. Moreover, given the rapid pace of research in both neurodevelopmental and metabolic fields, some emerging findings may not have been captured. These limitations underscore the need for continued research and periodic reassessment of the evolving evidence base.
7. Conclusions
There is a robust, bidirectional link between ADHD and metabolic disorders, driven by shared mechanisms such as dopaminergic dysregulation, HPA axis disruption, and chronic inflammation. These overlapping pathways suggest that therapeutic strategies targeting both neurodevelopmental and metabolic dysfunctions may yield significant clinical benefits. However, further research is necessary to clarify the genetic, epigenetic, and environmental factors at play, and to refine personalized treatment approaches that integrate metabolic and psychiatric care. Addressing these challenges is critical for reducing the long-term burden of these comorbid conditions.
Author Contributions
Conceptualization, U.C., I.M., A.C.; writing—original draft preparation, U.C., I.M., A.C.; writing—review and editing, U.C., I.M., A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The authors have reviewed literature data and have reported results coming from studies approved by local ethics committees.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gallo, E.F.; Posner, J. Moving towards causality in attention-deficit hyperactivity disorder: Overview of neural and genetic mechanisms. Lancet Psychiatry 2016, 3, 555–567. [Google Scholar] [CrossRef]
- Biederman, J. Pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: Findings from a longitudinal follow-up of youths with and without ADHD. J. Clin. Psychiatry 2003, 64, 3–8. [Google Scholar] [PubMed]
- Franke, B.; Michelini, G.; Asherson, P.; Banaschewski, T.; Bilbow, A.; Buitelaar, J.K.; Cormand, B.; Faraone, S.V.; Ginsberg, Y.; Haavik, J.; et al. Live fast, die young? A review on the developmental trajectories of ADHD across the lifespan. Eur. Neuropsychopharmacol. 2018, 28, 1059–1088. [Google Scholar] [CrossRef] [PubMed]
- Turgay, A.; Goodman, D.W.; Asherson, P.; Lasser, R.A.; Babcock, T.F.; Pucci, M.L.; Barkley, R.; ADHD Transition Phase Model Working Group. Lifespan persistence of ADHD: The life transition model and its application. J. Clin. Psychiatry 2012, 73, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Hechtman, L.T. Hyperactive Children Grown Up: ADHD in Children, Adolescents, and Adults, 2nd ed.; Guilford Press: New York, NY, USA, 1993. [Google Scholar]
- Polanczyk, G.; de Lima, M.S.; Horta, B.L.; Biederman, J.; Rohde, L.A. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am. J. Psychiatry 2007, 164, 942–948. [Google Scholar] [CrossRef]
- Abdelnour, E.; Jansen, M.O.; Gold, J.A. ADHD Diagnostic Trends: Increased Recognition or Overdiagnosis? Mo. Med. 2022, 119, 467–473. [Google Scholar]
- Ginsberg, Y.; Quintero, J.; Anand, E.; Casillas, M.; Upadhyaya, H.P. Underdiagnosis of attention-deficit/hyperactivity disorder in adult patients: A review of the literature. Prim. Care Companion CNS Disord. 2014, 16, 13r01600. [Google Scholar] [CrossRef]
- Cortese, S.; Tessari, L. Attention-Deficit/Hyperactivity Disorder (ADHD) and Obesity: Update 2016. Curr. Psychiatry Rep. 2017, 19, 4. [Google Scholar] [CrossRef]
- The Lancet Gastroenterology Hepatology. Obesity: Another ongoing pandemic. Lancet Gastroenterol. Hepatol. 2021, 6, 411. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Ni, Y.; Yi, C.; Fang, Y.; Ning, Q.; Shen, B.; Zhang, K.; Liu, Y.; Yang, L.; et al. Global Prevalence of Overweight and Obesity in Children and Adolescents: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2024, 178, 800–813. [Google Scholar] [CrossRef]
- Jenssen, B.P.; Kelly, M.K.; Powell, M.; Bouchelle, Z.; Mayne, S.L.; Fiks, A.G. COVID-19 and Changes in Child Obesity. Pediatrics 2021, 147, 147. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.B. COVID-19, Childhood Obesity, and NAFLD: Colliding Pandemics. Lancet Gastroenterol. Hepatol. 2022, 7, 499–501. [Google Scholar] [CrossRef]
- Caturano, A.; Galiero, R.; Loffredo, G.; Vetrano, E.; Medicamento, G.; Acierno, C.; Rinaldi, L.; Marrone, A.; Salvatore, T.; Monda, M.; et al. Effects of a Combination of Empagliflozin Plus Metformin vs. Metformin Monotherapy on NAFLD Progression in Type 2 Diabetes: The IMAGIN Pilot Study. Biomedicines 2023, 11, 322. [Google Scholar] [CrossRef]
- Mundi, M.S.; Patel, J.J.; Mohamed Elfadil, O.; Patel, J.; Patel, I.; Nanda, S.; Hurt, R.T. When Pandemics Collide: The Interplay of Obesity and COVID-19. Curr. Gastroenterol. Rep. 2021, 23, 26. [Google Scholar] [CrossRef] [PubMed]
- Sciberras, E.; Mulraney, M.; Silva, D.; Coghill, D. Prenatal Risk Factors and the Etiology of ADHD—Review of Existing Evidence. Curr. Psychiatry Rep. 2017, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Sagvolden, T.; Sergeant, J.A. Attention Deficit/Hyperactivity Disorder—From Brain Dysfunctions to Behaviour. Behav. Brain Res. 1998, 94, 1–10. [Google Scholar] [PubMed]
- Casey, B.J.; Nigg, J.T.; Durston, S. New Potential Leads in the Biology and Treatment of Attention Deficit-Hyperactivity Disorder. Curr. Opin. Neurol. 2007, 20, 119–124. [Google Scholar] [CrossRef]
- Scheres, A.; Dijkstra, M.; Ainslie, E.; Balkan, J.; Reynolds, B.; Sonuga-Barke, E.; Castellanos, F.X. Temporal and Probabilistic Discounting of Rewards in Children and Adolescents: Effects of Age and ADHD Symptoms. Neuropsychologia 2006, 44, 2092–2103. [Google Scholar] [CrossRef]
- Tripp, G.; Wickens, J.R. Neurobiology of ADHD. Neuropharmacology 2009, 57, 579–589. [Google Scholar] [CrossRef]
- Berens, A.; LeMoult, J.; Kircanski, K.; Gotlib, I.H. ADHD Symptoms and Diurnal Cortisol in Adolescents: The Importance of Comorbidities. Psychoneuroendocrinology 2023, 148, 105990. [Google Scholar] [CrossRef]
- Chang, J.P.; Mondelli, V.; Satyanarayanan, S.K.; Chiang, Y.J.; Chen, H.T.; Su, K.P.; Pariante, C.M. Cortisol, Inflammatory Biomarkers, and Neurotrophins in Children and Adolescents with Attention Deficit Hyperactivity Disorder (ADHD) in Taiwan. Brain Behav. Immun. 2020, 88, 105–113. [Google Scholar] [CrossRef]
- Petropoulos, A.; Anesiadou, S.; Michou, M.; Lymperatou, A.; Roma, E.; Chrousos, G.; Pervanidou, P. Functional Gastrointestinal Symptoms in Children with Autism and ADHD: Profiles of Hair and Salivary Cortisol, Serum Leptin Concentrations and Externalizing/Internalizing Problems. Nutrients 2024, 16, 1538. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.W.; Huang, K.L.; Ba, Y.M.; Tsai, S.J.; Chen, M.H. Role of Appetite Hormone Dysregulation in Symptomology and Executive Function in Adolescents with Attention Deficit Hyperactivity Disorder. Int. J. Neuropsychopharmacol. 2023, 26, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Özcan, Ö.; Arslan, M.; Güngör, S.; Yüksel, T.; Selimoğlu, M.A. Plasma Leptin, Adiponectin, Neuropeptide Y Levels in Drug Naive Children with ADHD. J. Atten. Disord. 2018, 22, 896–900. [Google Scholar] [CrossRef]
- Kang, J.; Park, M.; Oh, C.M.; Kim, T. High-Fat Diet-Induced Dopaminergic Dysregulation Induces REM Sleep Fragmentation and ADHD-Like Behaviors. Psychiatry Res. 2023, 327, 115412. [Google Scholar] [CrossRef]
- Du Rietz, E.; Xie, T.; Wang, R.; Cheesman, R.; Garcia-Argibay, M.; Dong, Z.; Zhang, J.; Niebuur, J.; Vos, M.; Snieder, H.; et al. The Contribution of Attention-Deficit/Hyperactivity Disorder Polygenic Load to Metabolic and Cardiovascular Health Outcomes: A Large-Scale Population and Sibling Study. Transl. Psychiatry 2024, 14, 470. [Google Scholar] [CrossRef] [PubMed]
- Demontis, D.; Walters, G.B.; Athanasiadis, G.; Walters, R.; Therrien, K.; Nielsen, T.T.; Farajzadeh, L.; Voloudakis, G.; Bendl, J.; Zeng, B.; et al. Genome-Wide Analyses of ADHD Identify 27 Risk Loci, Refine the Genetic Architecture and Implicate Several Cognitive Domains. Nat. Genet. 2023, 55, 198–208. [Google Scholar] [CrossRef]
- Faraone, S.V.; Larsson, H. Genetics of Attention Deficit Hyperactivity Disorder. Mol. Psychiatry 2019, 24, 562–575. [Google Scholar] [CrossRef]
- Ptácek, R.; Kuzelová, H.; Stefano, G.B. Dopamine D4 Receptor Gene DRD4 and Its Association with Psychiatric Disorders. Med. Sci. Monit. 2011, 17, RA215–RA220. [Google Scholar] [CrossRef]
- Tunbridge, E.M.; Narajos, M.; Harrison, C.H.; Beresford, C.; Cipriani, A.; Harrison, P.J. Which Dopamine Polymorphisms Are Functional? Systematic Review and Meta-Analysis of COMT, DAT, DBH, DDC, DRD1-5, MAOA, MAOB, TH, VMAT1, and VMAT2. Biol. Psychiatry 2019, 86, 608–620. [Google Scholar] [CrossRef]
- Tahir, E.; Yazgan, Y.; Cirakoglu, B.; Ozbay, F.; Waldman, I.; Asherson, P.J. Association and Linkage of DRD4 and DRD5 with Attention Deficit Hyperactivity Disorder (ADHD) in a Sample of Turkish Children. Mol. Psychiatry 2000, 5, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, J.; Huang, Z.; Fan, X.; Wang, X.; Chen, X.; Guo, H.; Liu, H.; Li, S.; Yu, S.; et al. Dopaminergic System and Neurons: Role in Multiple Neurological Diseases. Neuropharmacology 2024, 260, 110133. [Google Scholar] [CrossRef] [PubMed]
- Dresp-Langley, B. From Reward to Anhedonia—Dopamine Function in the Global Mental Health Context. Biomedicines 2023, 11, 2469. [Google Scholar] [CrossRef] [PubMed]
- Tafet, G.E.; Ortiz Alonso, T. Psychoneurobiology of Dopaminergic Pathways and the Reward System. In Psychiatry and Neuroscience Update—Vol. V.; Gargiulo, P.Á., Mesones-Arroyo, H.L., Eds.; Springer: Cham, Switzerland, 2024; pp. 321–329. [Google Scholar] [CrossRef]
- Lisco, G.; De Tullio, A.; Iovino, M.; Disoteo, O.; Guastamacchia, E.; Giagulli, V.A.; Triggiani, V. Dopamine in the Regulation of Glucose Homeostasis, Pathogenesis of Type 2 Diabetes, and Chronic Conditions of Impaired Dopamine Activity/Metabolism: Implication for Pathophysiological and Therapeutic Purposes. Biomedicines 2023, 11, 2993. [Google Scholar] [CrossRef]
- Dunlavey, C.J. Introduction to the Hypothalamic-Pituitary-Adrenal Axis: Healthy and Dysregulated Stress Responses, Developmental Stress and Neurodegeneration. J. Undergrad. Neurosci. Educ. 2018, 16, R59–R60. [Google Scholar]
- Plieger, T.; Felten, A.; Splittgerber, H.; Duke, É.; Reuter, M. The Role of Genetic Variation in the Glucocorticoid Receptor (NR3C1) and Mineralocorticoid Receptor (NR3C2) in the Association between Cortisol Response and Cognition under Acute Stress. Psychoneuroendocrinology 2018, 87, 173–180. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Y.H.; Chen, H.; Liu, Y.Y.; Wang, Y.X. The Function of Hypothalamus-Pituitary-Adrenal Axis in Children with ADHD. Brain Res. 2011, 1368, 159–162. [Google Scholar] [CrossRef]
- Lasselin, J.; Capuron, L. Chronic Low-Grade Inflammation in Metabolic Disorders: Relevance for Behavioral Symptoms. Neuroimmunomodulation 2014, 21, 95–101. [Google Scholar] [CrossRef]
- Cifuentes, M.; Verdejo, H.E.; Castro, P.F.; Corvalan, A.H.; Ferreccio, C.; Quest, A.F.G.; Kogan, M.J.; Lavandero, S. Low-Grade Chronic Inflammation: A Shared Mechanism for Chronic Diseases. Physiology 2025, 40, 4–25. [Google Scholar] [CrossRef]
- Oda, K.; Tanaka, N.; Arai, T.; Araki, J.; Song, Y.; Zhang, L.; Kuchiba, A.; Hosoi, T.; Shirasawa, T.; Muramatsu, M.; et al. Polymorphisms in Pro- and Anti-Inflammatory Cytokine Genes and Susceptibility to Atherosclerosis: A Pathological Study of 1503 Consecutive Autopsy Cases. Hum. Mol. Genet. 2007, 16, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The Interplay between Cytokines, Inflammation, and Antioxidants: Mechanistic Insights and Therapeutic Potentials of Various Antioxidants and Anti-Cytokine Compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; Rocco, M.; Tagliaferri, G.; Piacevole, A.; Nilo, D.; Di Lorenzo, G.; Iadicicco, I.; Donnarumma, M.; Galiero, R.; Acierno, C.; et al. Oxidative Stress and Cardiovascular Complications in Type 2 Diabetes: From Pathophysiology to Lifestyle Modifications. Antioxidants 2025, 14, 72. [Google Scholar] [CrossRef]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative Stress and Inflammation in the Pathogenesis of Neurological Disorders: Mechanisms and Implications. Acta Pharm. Sin. B 2024, 15, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Klibaner-Schiff, E.; Simonin, E.M.; Akdis, C.A.; Cheong, A.; Johnson, M.M.; Karagas, M.R.; Kirsh, S.; Kline, O.; Mazumdar, M.; Oken, E.; et al. Environmental Exposures Influence Multigenerational Epigenetic Transmission. Clin. Epigenetics 2024, 16, 145. [Google Scholar] [CrossRef]
- Cecil, C.A.M.; Nigg, J.T. Epigenetics and ADHD: Reflections on Current Knowledge, Research Priorities and Translational Potential. Mol. Diagn. Ther. 2022, 26, 581–606. [Google Scholar] [CrossRef]
- Padula, A.M.; Monk, C.; Brennan, P.A.; Borders, A.; Barrett, E.S.; McEvoy, C.T.; Foss, S.; Desai, P.; Alshawabkeh, A.; Wurth, R.; et al. A Review of Maternal Prenatal Exposures to Environmental Chemicals and Psychosocial Stressors-Implications for Research on Perinatal Outcomes in the ECHO Program. J. Perinatol. 2020, 40, 10–24. [Google Scholar] [CrossRef]
- Musillo, C.; Berry, A.; Cirulli, F. Prenatal Psychological or Metabolic Stress Increases the Risk for Psychiatric Disorders: The “Funnel Effect” Model. Neurosci. Biobehav. Rev. 2022, 136, 104624. [Google Scholar] [CrossRef]
- Ho, S.M.; Johnson, A.; Tarapore, P.; Janakiram, V.; Zhang, X.; Leung, Y.K. Environmental Epigenetics and Its Implication on Disease Risk and Health Outcomes. ILAR J. 2012, 53, 289–305. [Google Scholar] [CrossRef]
- Tiffon, C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3425. [Google Scholar] [CrossRef]
- Kotsakis Ruehlmann, A.; Sammallahti, S.; Cortés Hidalgo, A.P.; Bakulski, K.M.; Binder, E.B.; Campbell, M.L.; Caramaschi, D.; Cecil, C.A.M.; Colicino, E.; Cruceanu, C.; et al. Epigenome-Wide Meta-Analysis of Prenatal Maternal Stressful Life Events and Newborn DNA Methylation. Mol. Psychiatry 2023, 28, 5090–5100. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Baroud, E.; DiSalvo, M.; Faraone, S.V.; Biederman, J. Examining the Impact of ADHD Polygenic Risk Scores on ADHD and Associated Outcomes: A Systematic Review and Meta-Analysis. J. Psychiatr. Res. 2022, 155, 49–67. [Google Scholar] [CrossRef]
- Brikell, I.; Kuja-Halkola, R.; Larsson, H. Heritability of Attention-Deficit Hyperactivity Disorder in Adults. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2015, 168, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Larsson, H.; Chang, Z.; D’Onofrio, B.M.; Lichtenstein, P. The Heritability of Clinically Diagnosed Attention Deficit Hyperactivity Disorder Across the Lifespan. Psychol. Med. 2014, 44, 2223–2229. [Google Scholar] [CrossRef]
- Karhunen, V.; Bond, T.A.; Zuber, V.; Hurtig, T.; Moilanen, I.; Järvelin, M.R.; Evangelou, M.; Rodriguez, A. The Link between Attention Deficit Hyperactivity Disorder (ADHD) Symptoms and Obesity-Related Traits: Genetic and Prenatal Explanations. Transl. Psychiatry 2021, 11, 455. [Google Scholar] [CrossRef]
- Gambini, J.; Stromsnes, K. Oxidative Stress and Inflammation: From Mechanisms to Therapeutic Approaches. Biomedicines 2022, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Corona, J.C. Role of Oxidative Stress and Neuroinflammation in Attention-Deficit/Hyperactivity Disorder. Antioxidants 2020, 9, 1039. [Google Scholar] [CrossRef]
- Schnorr, I.; Siegl, A.; Luckhardt, S.; Wenz, S.; Friedrichsen, H.; El Jomaa, H.; Steinmann, A.; Kilencz, T.; Arteaga-Henríquez, G.; Ramos-Sayalero, C.; et al. Inflammatory biotype of ADHD is linked to chronic stress: A data-driven analysis of the inflammatory proteome. Transl. Psychiatry. 2024, 14, 37. [Google Scholar] [CrossRef]
- Misiak, B.; Wójta-Kempa, M.; Samochowiec, J.; Schiweck, C.; Aichholzer, M.; Reif, A.; Samochowiec, A.; Stańczykiewicz, B. Peripheral blood inflammatory markers in patients with attention deficit/hyperactivity disorder (ADHD): A systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2022, 118, 110581. [Google Scholar] [CrossRef]
- Gruol, D.L. IL-6 regulation of synaptic function in the CNS. Neuropharmacology. 2015, 96, 42–54. [Google Scholar] [CrossRef]
- Stampanoni Bassi, M.; Iezzi, E.; Mori, F.; Simonelli, I.; Gilio, L.; Buttari, F.; Sica, F.; De Paolis, N.; Mandolesi, G.; Musella, A.; et al. Interleukin-6 Disrupts Synaptic Plasticity and Impairs Tissue Damage Compensation in Multiple Sclerosis. Neurorehabil Neural Repair. 2019, 33, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; Galiero, R.; Vetrano, E.; Sardu, C.; Rinaldi, L.; Russo, V.; Monda, M.; Marfella, R.; Sasso, F.C. Insulin–Heart Axis: Bridging Physiology to Insulin Resistance. Int. J. Mol. Sci. 2024, 25, 8369. [Google Scholar] [CrossRef] [PubMed]
- Castelli, V.; Kacem, H.; Brandolini, L.; Giorgio, C.; Scenna, M.S.; Allegretti, M.; Cimini, A.; d’Angelo, M. TNFα-CXCR1/2 partners in crime in insulin resistance conditions. Cell Death Discov. 2024, 10, 486. [Google Scholar] [CrossRef]
- Caturano, A.; Vetrano, E.; Galiero, R.; Sardu, C.; Rinaldi, L.; Russo, V.; Monda, M.; Marfella, R.; Sasso, F.C. Advances in the Insulin–Heart Axis: Current Therapies and Future Directions. Int. J. Mol. Sci. 2024, 25, 10173. [Google Scholar] [CrossRef]
- Olmos, G.; Lladó, J. Tumor necrosis factor alpha: A link between neuroinflammation and excitotoxicity. Mediat. Inflamm. 2014, 2014, 861231. [Google Scholar] [CrossRef]
- Rolver, M.G.; Emanuelsson, F.; Nordestgaard, B.G.; Benn, M. Contributions of elevated CRP, hyperglycaemia, and type 2 diabetes to cardiovascular risk in the general population: Observational and Mendelian randomization studies. Cardiovasc. Diabetol. 2024, 23, 165. [Google Scholar] [CrossRef] [PubMed]
- Amezcua-Castillo, E.; González-Pacheco, H.; Sáenz-San Martín, A.; Méndez-Ocampo, P.; Gutierrez-Moctezuma, I.; Massó, F.; Sierra-Lara, D.; Springall, R.; Rodríguez, E.; Arias-Mendoza, A.; et al. C-Reactive Protein: The Quintessential Marker of Systemic Inflammation in Coronary Artery Disease—Advancing toward Precision Medicine. Biomedicines 2023, 11, 2444. [Google Scholar] [CrossRef]
- Chortis, V.; Breault, D.T. Inflammation-induced adrenal dysfunction. Nat. Rev. Endocrinol. 2023, 19, 622–623. [Google Scholar] [CrossRef]
- Knezevic, E.; Nenic, K.; Milanovic, V.; Knezevic, N.N. The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders. Cells 2023, 12, 2726. [Google Scholar] [CrossRef]
- de Guia, R.M. Stress, glucocorticoid signaling pathway, and metabolic disorders. Diabetes Metab. Syndr. 2020, 14, 1273–1280. [Google Scholar] [CrossRef]
- Corominas, M.; Ramos-Quiroga, J.A.; Ferrer, M.; Sáez-Francàs, N.; Palomar, G.; Bosch, R.; Casas, M. Cortisol responses in children and adults with attention deficit hyperactivity disorder (ADHD): A possible marker of inhibition deficits. Atten. Defic. Hyperact. Disord. 2012, 4, 63–75. [Google Scholar] [CrossRef]
- Ma, P.; Ou, Y. Correlation between the dopaminergic system and inflammation disease: A review. Mol. Biol. Rep. 2023, 50, 7043–7053. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Öğütlü, H.; Kaşak, M.; Tutku Tabur, S. Mitochondrial Dysfunction in Attention Deficit Hyperactivity Disorder. Eurasian J. Med. 2022, 54, 187–195. [Google Scholar] [CrossRef]
- Joseph, N.; Zhang-James, Y.; Perl, A.; Faraone, S.V. Oxidative Stress and ADHD: A Meta-Analysis. J. Atten. Disord. 2015, 19, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Leffa, D.T.; Torres, I.L.S.; Rohde, L.A. A Review on the Role of Inflammation in Attention-Deficit/Hyperactivity Disorder. Neuroimmunomodulation 2018, 25, 328–333. [Google Scholar] [CrossRef]
- Kim, M.E.; Lee, J.S. Mechanisms and Emerging Regulators of Neuroinflammation: Exploring New Therapeutic Strategies for Neurological Disorders. Curr. Issues Mol. Biol. 2025, 47, 8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yin, J.; Chen, J.; Ma, X.; Wu, M.; Liu, G.; Yao, K.; Tan, B.; Yin, Y. Mitochondria-Targeted Antioxidants: A Step towards Disease Treatment. Oxid. Med. Cell Longev. 2020, 2020, 8837893. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Del-Ponte, B.; Quinte, G.C.; Cruz, S.; Grellert, M.; Santos, I.S. Dietary Patterns and Attention Deficit/Hyperactivity Disorder (ADHD): A Systematic Review and Meta-Analysis. J. Affect. Disord. 2019, 252, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Mambrini, S.P.; Menichetti, F.; Ravella, S.; Pellizzari, M.; De Amicis, R.; Foppiani, A.; Battezzati, A.; Bertoli, S.; Leone, A. Ultra-Processed Food Consumption and Incidence of Obesity and Cardiometabolic Risk Factors in Adults: A Systematic Review of Prospective Studies. Nutrients 2023, 15, 2583. [Google Scholar] [CrossRef]
- Ríos-Hernández, A.; Alda, J.A.; Farran-Codina, A.; Ferreira-García, E.; Izquierdo-Pulido, M. The Mediterranean Diet and ADHD in Children and Adolescents. Pediatrics 2017, 139, e20162027. [Google Scholar] [CrossRef] [PubMed]
- Granero, R.; Pardo-Garrido, A.; Carpio-Toro, I.L.; Ramírez-Coronel, A.A.; Martínez-Suárez, P.C.; Reivan-Ortiz, G.G. The Role of Iron and Zinc in the Treatment of ADHD among Children and Adolescents: A Systematic Review of Randomized Clinical Trials. Nutrients 2021, 13, 4059. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, J.Y.; Lee, J.; Jeong, G.H.; Lee, E.; Lee, S.; Lee, K.H.; Kronbichler, A.; Stubbs, B.; Solmi, M.; et al. Environmental Risk Factors, Protective Factors, and Peripheral Biomarkers for ADHD: An Umbrella Review. Lancet Psychiatry 2020, 7, 955–970. [Google Scholar] [CrossRef] [PubMed]
- Arango, C.; Dragioti, E.; Solmi, M.; Cortese, S.; Domschke, K.; Murray, R.M.; Jones, P.B.; Uher, R.; Carvalho, A.F.; Reichenberg, A.; et al. Risk and Protective Factors for Mental Disorders Beyond Genetics: An Evidence-Based Atlas. World Psychiatry 2021, 20, 417–436. [Google Scholar] [CrossRef]
- Suchert, V.; Pedersen, A.; Hanewinkel, R.; Isensee, B. Relationship between Attention-Deficit/Hyperactivity Disorder and Sedentary Behavior in Adolescence: A Cross-Sectional Study. Atten. Defic. Hyperact. Disord. 2017, 9, 213–218. [Google Scholar] [CrossRef]
- Khalife, N.; Kantomaa, M.; Glover, V.; Tammelin, T.; Laitinen, J.; Ebeling, H.; Hurtig, T.; Jarvelin, M.R.; Rodriguez, A. Childhood Attention-Deficit/Hyperactivity Disorder Symptoms Are Risk Factors for Obesity and Physical Inactivity in Adolescence. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 425–436. [Google Scholar] [CrossRef]
- Banerjee, T.D.; Middleton, F.; Faraone, S.V. Environmental Risk Factors for Attention-Deficit Hyperactivity Disorder. Acta Paediatr. 2007, 96, 1269–1274. [Google Scholar] [CrossRef]
- Lagerström, M.; Johnsson, P.; Orrenius, B.; Järvholm, K.; Olbers, T.; Engström, M. Internalized Shame in Treatment-Seeking Adults with Obesity Class II-III and Its Association with Quality of Life, Body Image, and Self-Esteem. Obes. Facts 2025, 5, 1–12. [Google Scholar] [CrossRef]
- Visser, M.J.; Peters, R.M.H.; Luman, M. Unmet Needs of Children and Young Adults with ADHD: Insights from Key Stakeholders on Priorities for Stigma Reduction. J. Atten. Disord. 2025, 29, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Pyszkowska, A.; Nowacki, A.; Celban, J. The Daydream Spectrum: The Role of Emotional Dysregulation, Internalized Stigma and Self-Esteem in Maladaptive Daydreaming Among Adults with ADHD, ASD, and Double Diagnosis. J. Atten. Disord. 2025, 29, 53–69. [Google Scholar] [CrossRef]
- Anthony, D.C.; Probert, F.; Gorlova, A.; Hebert, J.; Radford-Smith, D.; Nefedova, Z.; Umriukhin, A.; Nedorubov, A.; Cespuglio, R.; Shulgin, B.; et al. Impact of Serotonin Transporter Absence on Brain Insulin Receptor Expression, Plasma Metabolome Changes, and ADHD-like Behavior in Mice Fed a Western Diet. Biomolecules 2024, 14, 884. [Google Scholar] [CrossRef]
- Hur, S.; Oh, B.; Kim, H.; Kwon, O. Associations of Diet Quality and Sleep Quality with Obesity. Nutrients 2021, 13, 3181. [Google Scholar] [CrossRef]
- Elagizi, A.; Kachur, S.; Carbone, S.; Lavie, C.J.; Blair, S.N. A Review of Obesity, Physical Activity, and Cardiovascular Disease. Curr. Obes. Rep. 2020, 9, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Moreira-Maia, C.R.; St Fleur, D.; Morcillo-Peñalver, C.; Rohde, L.A.; Faraone, S.V. Association Between ADHD and Obesity: A Systematic Review and Meta-Analysis. Am. J. Psychiatry 2016, 173, 34–43. [Google Scholar] [CrossRef]
- Colomer, C.; Berenguer, C.; Roselló, B.; Baixauli, I.; Miranda, A. The Impact of Inattention, Hyperactivity/Impulsivity Symptoms, and Executive Functions on Learning Behaviors of Children with ADHD. Front. Psychol. 2017, 8, 540. [Google Scholar] [CrossRef] [PubMed]
- Aster, H.C.; Waltmann, M.; Busch, A.; Romanos, M.; Gamer, M.; van Noort, B.M.; Beck, A.; Kappel, V.; Deserno, L. Impaired Flexible Reward Learning in ADHD Patients Is Associated with Blunted Reinforcement Sensitivity and Neural Signals in Ventral Striatum and Parietal Cortex. Neuroimage Clin. 2024, 42, 103588. [Google Scholar] [CrossRef]
- Killeen, P.R.; Russell, V.A.; Sergeant, J.A. A Behavioral Neuroenergetics Theory of ADHD. Neurosci. Biobehav. Rev. 2013, 37, 625–657. [Google Scholar] [CrossRef]
- Gonzalez, J.T.; Batterham, A.M.; Atkinson, G.; Thompson, D. Perspective: Is the Response of Human Energy Expenditure to Increased Physical Activity Additive or Constrained? Adv. Nutr. 2023, 14, 406–419. [Google Scholar] [CrossRef]
- Miklós, M.; Futó, J.; Komáromy, D.; Balázs, J. Executive Function and Attention Performance in Children with ADHD: Effects of Medication and Comparison with Typically Developing Children. Int. J. Environ. Res. Public Health 2019, 16, 3822. [Google Scholar] [CrossRef]
- Devi, K.A.; Singh, S.K. The Hazards of Excessive Screen Time: Impacts on Physical Health, Mental Health, and Overall Well-Being. J. Educ. Health Promot. 2023, 12, 413. [Google Scholar] [CrossRef] [PubMed]
- Kurnik Mesarič, K.; Pajek, J.; Logar Zakrajšek, B.; Bogataj, Š.; Kodrič, J. Cognitive Behavioral Therapy for Lifestyle Changes in Patients with Obesity and Type 2 Diabetes: A Systematic Review and Meta-Analysis. Sci. Rep. 2023, 13, 12793. [Google Scholar] [CrossRef]
- Palakodeti, S.S.; Sarangi, A.; Mehta, T.R. The ADHD Conundrum—A Review of Non-pharmacological Approach to Management. Curr. Dev. Disord. Rep. 2024, 12, 6. [Google Scholar] [CrossRef]
- Cortese, S.; Vincenzi, B. Obesity and ADHD: Clinical and Neurobiological Implications. Curr. Top. Behav. Neurosci. 2012, 9, 199–218. [Google Scholar] [CrossRef]
- Bulik, C.M.; Coleman, J.R.I.; Hardaway, J.A.; Breithaupt, L.; Watson, H.J.; Bryant, C.D.; Breen, G. Genetics and Neurobiology of Eating Disorders. Nat. Neurosci. 2022, 25, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Le Thuc, O.; Stobbe, K.; Cansell, C.; Nahon, J.L.; Blondeau, N.; Rovère, C. Hypothalamic Inflammation and Energy Balance Disruptions: Spotlight on Chemokines. Front. Endocrinol. 2017, 8, 197. [Google Scholar] [CrossRef]
- da Silva, B.S.; Grevet, E.H.; Silva, L.C.F.; Ramos, J.K.N.; Rovaris, D.L.; Bau, C.H.D. An Overview on Neurobiology and Therapeutics of Attention-Deficit/Hyperactivity Disorder. Discov. Ment. Health 2023, 3, 2. [Google Scholar] [CrossRef]
- Bellato, A.; Arora, I.; Hollis, C.; Groom, M.J. Is Autonomic Nervous System Function Atypical in Attention Deficit Hyperactivity Disorder (ADHD)? A Systematic Review of the Evidence. Neurosci. Biobehav. Rev. 2020, 108, 182–206. [Google Scholar] [CrossRef]
- Geiss, L.; Stemmler, M.; Beck, B.; Hillemacher, T.; Widder, M.; Hösl, K.M. Dysregulation of the Autonomic Nervous System in Adult Attention Deficit Hyperactivity Disorder: A Systematic Review. Cogn. Neuropsychiatry 2023, 28, 285–306. [Google Scholar] [CrossRef]
- Arnsten, A.F. Stimulants: Therapeutic Actions in ADHD. Neuropsychopharmacology 2006, 31, 2376–2383. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V. The Pharmacology of Amphetamine and Methylphenidate: Relevance to the Neurobiology of Attention-Deficit/Hyperactivity Disorder and Other Psychiatric Comorbidities. Neurosci. Biobehav. Rev. 2018, 87, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Reas, D.L.; Grilo, C.M. Pharmacological Treatment of Binge Eating Disorder: Update Review and Synthesis. Expert Opin. Pharmacother. 2015, 16, 1463–1478. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.D.; Viswanath, O.; Urits, I.; Boyer, A.G.; et al. Selective Serotonin Reuptake Inhibitors and Adverse Effects: A Narrative Review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef]
- Wynchank, D.; Bijlenga, D.; Lamers, F.; Kooij, J.J.S.; Bron, T.I.; Beekman, A.T.F.; Penninx, B.W.J.H. The Association Between Metabolic Syndrome, Obesity-Related Outcomes, and ADHD in Adults with Comorbid Affective Disorders. J. Atten. Disord. 2018, 22, 460–471. [Google Scholar] [CrossRef]
- Di Girolamo, G.; Bracco, I.F.; Portigliatti Pomeri, A.; Puglisi, S.; Oliva, F. Prevalence of Metabolic Syndrome and Insulin Resistance in a Sample of Adult ADHD Outpatients. Front. Psychiatry 2022, 13, 891479. [Google Scholar] [CrossRef]
- Xiang, A.H.; Wang, X.; Martinez, M.P.; Getahun, D.; Page, K.A.; Buchanan, T.A.; Feldman, K. Maternal Gestational Diabetes Mellitus, Type 1 Diabetes, and Type 2 Diabetes During Pregnancy and Risk of ADHD in Offspring. Diabetes Care 2018, 41, 2502–2508. [Google Scholar] [CrossRef]
- Guo, D.; Ju, R.; Zhou, Q.; Mao, J.; Tao, H.; Jing, H.; Zhu, C.; Dai, J. Association of Maternal Diabetes with Attention Deficit/Hyperactivity Disorder (ADHD) in Offspring: A Meta-Analysis and Review. Diabetes Res. Clin. Pract. 2020, 165, 108269. [Google Scholar] [CrossRef]
- Feig, D.S.; Artani, A.; Asaf, A.; Li, P.; Booth, G.L.; Shah, B.R. Long-Term Neurobehavioral and Metabolic Outcomes in Offspring of Mothers with Diabetes During Pregnancy: A Large, Population-Based Cohort Study in Ontario, Canada. Diabetes Care 2024, 47, 1568–1575. [Google Scholar] [CrossRef]
- Liu, S.; Kuja-Halkola, R.; Larsson, H.; Lichtenstein, P.; Ludvigsson, J.F.; Svensson, A.M.; Gudbjörnsdottir, S.; Tideman, M.; Serlachius, E.; Butwicka, A. Poor Glycaemic Control is Associated with Increased Risk of Neurodevelopmental Disorders in Childhood-Onset Type 1 Diabetes: A Population-Based Cohort Study. Diabetologia 2021, 64, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.N.; Lei, X.; Xiao, C.Y.; Li, Y.M.; Lei, X.Y. Association Between Type 1 Diabetes and Neurodevelopmental Disorders in Children and Adolescents: A Systematic Review and Meta-Analysis. Front. Psychiatry 2022, 13, 982696. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Lin, C.L.; Hsu, W.H.; Lin, C.C.; Fu, Y.C. Association of Attention Deficit Hyperactivity Disorder with Recurrent Hypoglycemia in Type 1 Diabetes Mellitus. Pediatr. Diabetes 2019, 20, 189–196. [Google Scholar] [CrossRef]
- Koren, D. Growth and Development in Type 1 Diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 57–64. [Google Scholar] [CrossRef]
- Zare Dehnavi, A.; Elmitwalli, I.; Alsharif, H.O.H.; Shervin Razavi, A.; Gumpel, T.A.; Smith, A.; Weinstock, R.S.; Faraone, S.V.; Zhang-James, Y. Effects of ADHD and ADHD Treatment on Glycemic Management in Type 1 Diabetes: A Systematic Review and Meta-Analysis of Observational Studies. Diabetes Res. Clin. Pract. 2024, 209, 111566. [Google Scholar] [CrossRef]
- Jendle, J.; Agvall, B.; Galozy, A.; Adolfsson, P. Patterns and Predictors Associated with Long-Term Glycemic Control in Pediatric and Young Adult Patients with Type 1 Diabetes. J. Diabetes Sci. Technol. 2023, 17, 1243–1251. [Google Scholar] [CrossRef]
- Vinker-Shuster, M.; Golan-Cohen, A.; Merhasin, I.; Merzon, E. Attention-Deficit Hyperactivity Disorder in Pediatric Patients with Type 1 Diabetes Mellitus: Clinical Outcomes and Diabetes Control. J. Dev. Behav. Pediatr. 2019, 40, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Yazar, A.; Akın, F.; Akça, Ö.F.; Eklioğlu, B.S.; Türe, E.; Coşkun, F.; Atabek, M.E. The Effect of Attention Deficit/Hyperactivity Disorder and Other Psychiatric Disorders on the Treatment of Pediatric Diabetes Mellitus. Pediatr. Diabetes 2019, 20, 345–352. [Google Scholar] [CrossRef]
- Merzon, E.; Grossman, J.; Vinker, S.; Merhasin, I.; Levit, S.; Golan-Cohen, A. Factors Associated with Withdrawal from Insulin Pump Therapy: A Large-Population-Based Study. Diabetes Metab. Res. Rev. 2020, 36, e3288. [Google Scholar] [CrossRef]
- Dehnavi, A.Z.; Zhang-James, Y.; Draytsel, D.; Carguello, B.; Faraone, S.V.; Weinstock, R.S. Association of ADHD Symptoms with Type 2 Diabetes and Cardiovascular Comorbidities in Adults Receiving Outpatient Diabetes Care. J. Clin. Transl. Endocrinol. 2023, 32, 100318. [Google Scholar] [CrossRef]
- Garcia-Argibay, M.; Li, L.; Du Rietz, E.; Zhang, L.; Yao, H.; Jendle, J.; Ramos-Quiroga, J.A.; Ribasés, M.; Chang, Z.; Brikell, I.; et al. The Association between Type 2 Diabetes and Attention-Deficit/Hyperactivity Disorder: A Systematic Review, Meta-Analysis, and Population-Based Sibling Study. Neurosci. Biobehav. Rev. 2023, 147, 105076. [Google Scholar] [CrossRef]
- Liu, N.; Tan, J.S.; Liu, L.; Li, H.; Wang, Y.; Yang, Y.; Qian, Q. Roles of Obesity in Mediating the Causal Effect of Attention-Deficit/Hyperactivity Disorder on Diabetes. Epidemiol. Psychiatr. Sci. 2023, 32, e32. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, N.; Takatsuka, N.; Shimizu, H. Sleep Disturbance and Onset of Type 2 Diabetes. Diabetes Care 2004, 27, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Charach, G.; Karniel, E.; Grosskopf, I.; Rabinovich, A.; Charach, L. Methylphenidate Has Mild Hyperglycemic and Hypokalemia Effects and Increases Leukocyte and Neutrophil Counts. Medicine 2020, 99, e20931. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.; Chandhoke, V.; Cao, H.; Zhang, F. Shared Genetics and Bidirectional Causal Relationships Between Type 2 Diabetes and Attention-Deficit/Hyperactivity Disorder. Gen. Psychiatr. 2023, 36, e100996. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Prasad, R.B.; Groop, L. Subtypes of Type 2 Diabetes Determined From Clinical Parameters. Diabetes 2020, 69, 2086–2093. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Jayyousi, A.; DeFronzo, R.A.; Asaad, N.; Al-Suwaidi, J. Insulin Resistance the Link between T2DM and CVD: Basic Mechanisms and Clinical Implications. Curr. Vasc. Pharmacol. 2019, 17, 153–163. [Google Scholar] [CrossRef]
- Thota, P.; Perez-Lopez, F.R.; Benites-Zapata, V.A.; Pasupuleti, V.; Hernandez, A.V. Obesity-related Insulin Resistance in Adolescents: A Systematic Review and Meta-Analysis of Observational Studies. Gynecol. Endocrinol. 2017, 33, 179–184. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tobin, J.D.; Andres, R. Glucose Clamp Technique: A Method for Quantifying Insulin Secretion and Resistance. Am. J. Physiol. 1979, 237, E214–E223. [Google Scholar] [CrossRef]
- Ai, Y.; Zhao, J.; Liu, H.; Li, J.; Zhu, T. The Relationship Between Diabetes Mellitus and Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis. Front. Pediatr. 2022, 10, 936813. [Google Scholar] [CrossRef]
- Cameron, F.J.; Scratch, S.E.; Nadebaum, C.; Northam, E.A.; Koves, I.; Jennings, J.; Finney, K.; Neil, J.J.; Wellard, R.M.; Mackay, M.; et al. Neurological Consequences of Diabetic Ketoacidosis at Initial Presentation of Type 1 Diabetes in a Prospective Cohort Study of Children. Diabetes Care 2014, 37, 1554–1562. [Google Scholar] [CrossRef]
- Ehrmann, D.; Kulzer, B.; Roos, T.; Haak, T.; Al-Khatib, M.; Hermanns, N. Risk Factors and Prevention Strategies for Diabetic Ketoacidosis in People with Established Type 1 Diabetes. Lancet Diabetes Endocrinol. 2020, 8, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Teo, E.; Hassan, N.; Tam, W.; Koh, S. Effectiveness of Continuous Glucose Monitoring in Maintaining Glycaemic Control Among People with Type 1 Diabetes Mellitus: A Systematic Review of Randomised Controlled Trials and Meta-Analysis. Diabetologia 2022, 65, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Nevola, R.; Alfano, M.; Pafundi, P.C.; Brin, C.; Gragnano, F.; Calabrò, P.; Adinolfi, L.E.; Rinaldi, L.; Sasso, F.C.; Caturano, A. Cardiorenal Impact of SGLT-2 Inhibitors: A Conceptual Revolution in The Management of Type 2 Diabetes, Heart Failure and Chronic Kidney Disease. Rev. Cardiovasc. Med. 2022, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Domanski, M.J.; Tian, X.; Wu, C.O.; Reis, J.P.; Dey, A.K.; Gu, Y.; Zhao, L.; Bae, S.; Liu, K.; Hasan, A.A.; et al. Time Course of LDL Cholesterol Exposure and Cardiovascular Disease Event Risk. J. Am. Coll. Cardiol. 2020, 76, 1507–1516. [Google Scholar] [CrossRef]
- Mormone, A.; Tortorella, G.; Esposito, F.; Caturano, A.; Marrone, A.; Cozzolino, D.; Galiero, R.; Marfella, R.; Sasso, F.C.; Rinaldi, L. Advances in Pharmacological Approaches for Managing Hypercholesterolemia: A Comprehensive Overview of Novel Treatments. Biomedicines 2024, 12, 432. [Google Scholar] [CrossRef]
- Russo, V.; Napolitano, N.; Ascrizzi, A.; Leonardi, S.; Pisacane, F.; Di Micco, P.; Imbalzano, E.; Sasso, F.C.; D’Andrea, A.; Caturano, A.; et al. The Lipid-Lowering Efficacy of a Nutraceutical Combination Including Leucoselect Phytosome, Red Yeast Rice, Policosanol and Folic Acid in Dyslipidaemia Patients: Real-World Insights. Pharmaceuticals 2024, 17, 447. [Google Scholar] [CrossRef]
- Li, L.; Yao, H.; Zhang, L.; Garcia-Argibay, M.; Du Rietz, E.; Brikell, I.; Solmi, M.; Cortese, S.; Ramos-Quiroga, J.A.; Ribasés, M.; et al. Attention-deficit/hyperactivity disorder is associated with increased risk of cardiovascular diseases: A systematic review and meta-analysis. J. Child Psychol. Psychiatry Adv. 2023, 3, e12158. [Google Scholar] [CrossRef]
- García-Hermoso, A.; Ezzatvar, Y.; Izquierdo, M.; López-Gil, J.F. Can an Active Lifestyle Reduce the Risk of Obesity in Adulthood among Adolescents with Attention-Deficit/Hyperactivity Disorder Symptoms? An Ambispective Cohort Study. Psychiatry Res. 2024, 334, 115770. [Google Scholar] [CrossRef]
- Zhu, F.; Liu, B.; Kuang, D.; Zhu, X.; Bi, X.; Song, Y.; Quan, T.; Yang, Y.; Ren, Y. The Association between Physical Activity and Sleep in Adult ADHD Patients with Stimulant Medication Use. Front. Psychiatry 2023, 14, 1236636. [Google Scholar] [CrossRef]
- Mason, R.P.; Jacob, R.F.; Shrivastava, S.; Sherratt, S.C.R.; Chattopadhyay, A. Eicosapentaenoic Acid Reduces Membrane Fluidity, Inhibits Cholesterol Domain Formation, and Normalizes Bilayer Width in Atherosclerotic-Like Model Membranes. Biochim. Biophys. Acta 2016, 1858, 2614–2623. [Google Scholar] [CrossRef] [PubMed]
- San Mauro Martin, I.; Sanz Rojo, S.; González Cosano, L.; Conty de la Campa, R.; Garicano Vilar, E.; Blumenfeld Olivares, J.A. Impulsiveness in Children with Attention-Deficit/Hyperactivity Disorder after an 8-Week Intervention with the Mediterranean Diet and/or Omega-3 Fatty Acids: A Randomised Clinical Trial. Neurologia 2022, 37, 513–523. [Google Scholar] [CrossRef]
- Kimko, H.C.; Cross, J.T.; Abernethy, D.R. Pharmacokinetics and Clinical Effectiveness of Methylphenidate. Clin. Pharmacokinet. 1999, 37, 457–470. [Google Scholar] [CrossRef]
- The MTA Cooperative Group. A 14-Month Randomized Clinical Trial of Treatment Strategies for Attention-Deficit/Hyperactivity Disorder. Arch. Gen. Psychiatry 1999, 56, 1073–1086. [Google Scholar] [CrossRef]
- Edwards, K.; Li, X.; Lingvay, I. Clinical and Safety Outcomes with GLP-1 Receptor Agonists and SGLT2 Inhibitors in Type 1 Diabetes: A Real-World Study. J. Clin. Endocrinol. Metab. 2023, 108, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Gallwitz, B. The Cardiovascular Benefits Associated with the Use of Sodium-Glucose Cotransporter 2 Inhibitors—Real-World Data. Eur. Endocrinol. 2018, 14, 17–23. [Google Scholar] [CrossRef]
- Fadini, G.P.; Longato, E.; Morieri, M.L.; Del Prato, S.; Avogaro, A.; Solini, A.; DARWIN-Renal Study Investigators. Long-term Benefits of Dapagliflozin on Renal Outcomes of Type 2 Diabetes under Routine Care: A Comparative Effectiveness Study on Propensity Score Matched Cohorts at Low Renal Risk. Lancet Reg. Health Eur. 2024, 38, 100847. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Husain, M.; Lehrke, M.; Verma, S.; Sattar, N. GLP-1 Receptor Agonists for the Reduction of Atherosclerotic Cardiovascular Risk in Patients with Type 2 Diabetes. Circulation 2022, 146, 1882–1894. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Li, L.; Yang, Y.X.; Cao, Y.; Yu, X.; Samuel, R.; Ali, B.; Desiderio, R.; Cholankeril, G.; et al. GLP-1 Receptor Agonists and Risk for Cirrhosis and Related Complications in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease. JAMA Intern. Med. 2024, 184, 1314–1323. [Google Scholar] [CrossRef]
- Popoviciu, M.-S.; Păduraru, L.; Yahya, G.; Metwally, K.; Cavalu, S. Emerging Role of GLP-1 Agonists in Obesity: A Comprehensive Review of Randomised Controlled Trials. Int. J. Mol. Sci. 2023, 24, 10449. [Google Scholar] [CrossRef]
- Monney, M.; Jornayvaz, F.R.; Gariani, K. GLP-1 receptor agonists effect on cognitive function in patients with and without type 2 diabetes. Diabetes Metab. 2023, 49, 101470. [Google Scholar] [CrossRef] [PubMed]
- Karaivazoglou, K.; Aggeletopoulou, I.; Triantos, C. The Contribution of the Brain-Gut Axis to the Human Reward System. Biomedicines 2024, 12, 1861. [Google Scholar] [CrossRef]
- Tabatabaei Malazy, O.; Bandarian, F.; Qorbani, M.; Mohseni, S.; Mirsadeghi, S.; Peimani, M.; Larijani, B. The effect of metformin on cognitive function: A systematic review and meta-analysis. J. Psychopharmacol. 2022, 36, 666–679. [Google Scholar] [CrossRef]
- Levy-Shraga, Y.; Madi, L.R.; Shalev, M.; Mazor-Aronovitch, K.; Schwartz-Lifshitz, M.; Gothelf, D. Effectiveness of Metformin for Weight Reduction in Children and Adolescents Treated with Mixed Dopamine and Serotonin Receptor Antagonists: A Naturalistic Cohort Study. J. Child Adolesc. Psychopharmacol. 2021, 31, 376–380. [Google Scholar] [CrossRef]
- Dickinson, K.; Parmar, P.; Reyes, A.B.; Hale, E.W. Bariatric Surgery Is Highly Effective and Underutilized in Patients with ADHD: A 5-Year Retrospective Cohort Study. Obes. Surg. 2024, 34, 2066–2072. [Google Scholar] [CrossRef]
- Mocanu, V.; Tavakoli, I.; MacDonald, A.; Dang, J.T.; Switzer, N.; Birch, D.W.; Karmali, S. The Impact of ADHD on Outcomes Following Bariatric Surgery: A Systematic Review and Meta-Analysis. Obes. Surg. 2019, 29, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Galiero, R.; Caturano, A.; Vetrano, E.; Monda, M.; Marfella, R.; Sardu, C.; Salvatore, T.; Rinaldi, L.; Sasso, F.C. Precision Medicine in Type 2 Diabetes Mellitus: Utility and Limitations. Diabetes Metab. Syndr. Obes. Targets Ther. 2023, 16, 3669–3689. [Google Scholar] [CrossRef] [PubMed]
- Dhieb, D.; Bastaki, K. Pharmaco-Multiomics: A New Frontier in Precision Psychiatry. Int. J. Mol. Sci. 2025, 26, 1082. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; di Filippo, L.; Facciorusso, A.; Adler, R.A.; Binkley, N.; Bollerslev, J.; Bouillon, R.; Casanueva, F.F.; Cavestro, G.M.; Chakhtoura, M.; et al. Vitamin D Status and Supplementation Before and After Bariatric Surgery: Recommendations Based on a Systematic Review and Meta-Analysis. Rev. Endocr. Metab. Disord. 2023, 24, 1011–1029. [Google Scholar] [CrossRef]
- Luo, Y.; Blakey, R.; Gkatzionis, A.; Stergiakouli, E.; Dardani, C. Investigating the Relationship between Attention-Deficit Hyperactivity Disorder (ADHD) and C-Reactive Protein (CRP): Observational, Polygenic Risk Score, and Mendelian Randomization Analyses. Psychol. Med. 2025, 55, e103. [Google Scholar] [CrossRef]
- Mavroconstanti, T.; Halmøy, A.; Haavik, J. Decreased Serum Levels of Adiponectin in Adult Attention Deficit Hyperactivity Disorder. Psychiatry Res. 2014, 216, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Predescu, E.; Vaidean, T.; Rapciuc, A.-M.; Sipos, R. Metabolomic Markers in Attention-Deficit/Hyperactivity Disorder (ADHD) among Children and Adolescents—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 4385. [Google Scholar] [CrossRef]
- Dark, C.; Homman-Ludiye, J.; Bryson-Richardson, R.J. The role of ADHD associated genes in neurodevelopment. Dev. Biol. 2018, 438, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Checa-Ros, A.; Jeréz-Calero, A.; Molina-Carballo, A.; Campoy, C.; Muñoz-Hoyos, A. Current Evidence on the Role of the Gut Microbiome in ADHD Pathophysiology and Therapeutic Implications. Nutrients 2021, 13, 249. [Google Scholar] [CrossRef]
- Ludyga, S.; Held, S.; Rappelt, L.; Donath, L.; Klatt, S. A network meta-analysis comparing the effects of exercise and cognitive training on executive function in young and middle-aged adults. Eur. J. Sport Sci. 2023, 23, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Magkos, F.; Conte, C.; Mittendorfer, B.; Patterson, B.W.; Okunade, A.L.; Klein, S. Validation of a Novel Index to Assess Insulin Resistance of Adipose Tissue Lipolytic Activity in Obese Subjects. J. Lipid Res. 2012, 53, 321–324. [Google Scholar] [CrossRef]
- Becker, S.P. ADHD and sleep: Recent advances and future directions. Curr. Opin. Psychol. 2020, 34, 50–56. [Google Scholar] [CrossRef]
- Cortese, S. The Association between ADHD and Obesity: Intriguing, Progressively More Investigated, but Still Puzzling. Brain Sci. 2019, 9, 256. [Google Scholar] [CrossRef]
- Antza, C.; Kostopoulos, G.; Mostafa, S.; Nirantharakumar, K.; Tahrani, A. The Links Between Sleep Duration, Obesity, and Type 2 Diabetes Mellitus. J. Endocrinol. 2021, 252, 125–141. [Google Scholar] [CrossRef]
- Khorram-Manesh, A.; Dulebenets, M.A.; Goniewicz, K. Implementing Public Health Strategies—The Need for Educational Initiatives: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 5888. [Google Scholar] [CrossRef]
- Caron, R.M.; Noel, K.; Reed, R.N.; Sibel, J.; Smith, H.J. Health Promotion, Health Protection, and Disease Prevention: Challenges and Opportunities in a Dynamic Landscape. AJPM Focus. 2023, 3, 100167. [Google Scholar] [CrossRef] [PubMed]
- Riley, W.J. Health Disparities: Gaps in Access, Quality and Affordability of Medical Care. Trans. Am. Clin. Climatol. Assoc. 2012, 123, 167–172, discussion 172–174. [Google Scholar] [PubMed]
- Agurs-Collins, T.; Persky, S.; Paskett, E.D.; Barkin, S.L.; Meissner, H.I.; Nansel, T.R.; Arteaga, S.S.; Zhang, X.; Das, R.; Farhat, T. Designing and Assessing Multilevel Interventions to Improve Minority Health and Reduce Health Disparities. Am. J. Public Health 2019, 109, S86–S93. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).