From Classic to Contemporary, Evolving Therapies in Diabetic Kidney Disease: The Point of View of the Nephrologist and the Diabetologist
Abstract
1. Introduction
2. Role and Importance of Kidney Biopsy for the Diagnosis of DKD
3. Metformin-Associated Lactic Acidosis (MALA)
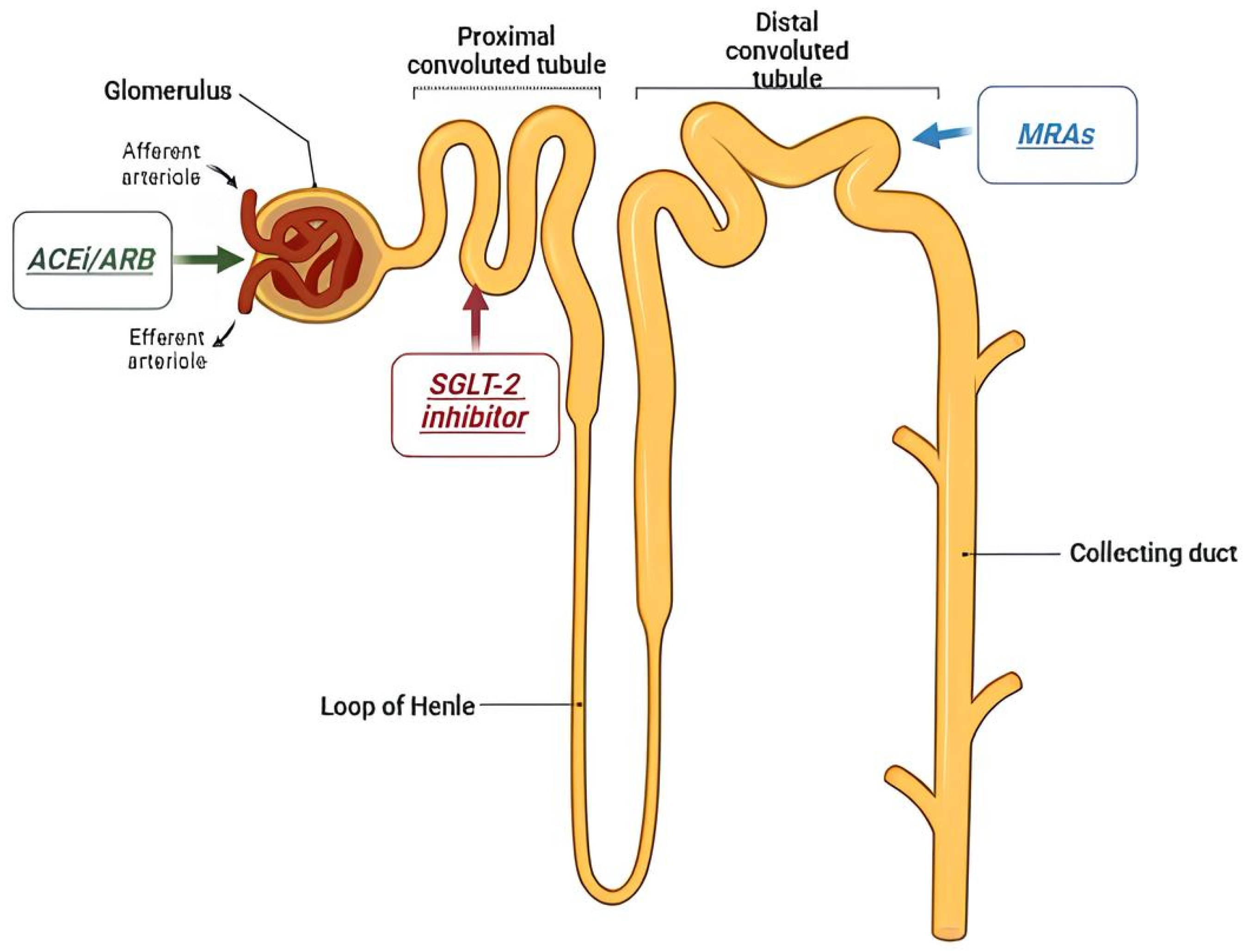
4. Contemporary Therapies
4.1. Angiotensin-Converting Enzyme Inhibitor and Angiotensin II Receptor Blocker
| Trial | Treatment | Population | Follow Up | Results |
|---|---|---|---|---|
| RENAAL (2001) [33] | Losartan |
| 3.4 years | Significant reduction in the progression of diabetic nephropathy, slowing the onset of ESKD and doubling serum creatinine. It did not have a significant impact on overall mortality or cardiovascular events, however. |
| INDT (2005) [33] | Irbestartan |
| 2.6 years | Effectiveness in slowing the progression of diabetic nephropathy in patients with type 2 diabetes and hypertension, independent of the effect on blood pressure. |
| INNOVATION (2007) [34] | Telmisartan |
| 2 years | Efficacy in delaying the worsening of diabetic kidney disease in patients with type 2 diabetes who had microalbuminuria; inhibition of the progression from microalbuminuria to overt proteinuria by approximately 60% compared with placebo. |
| IRMA-2 (2001) [35] | Irbesartan |
| 2 years | Inhibition of the progression from microalbuminuria to overt proteinuria by approximately 70%. |
| MARVAL (2002) [36] | Valsartan |
| 6 months | The comparison between an ARB with a calcium channel blocker showed that only ARB was effective in lowering microalbuminuria, indicating that RAS inhibitors have an inhibitory effect on nephropathy and a blood pressure-lowering ability. |
| BENEDICT (2004) [37] | Tradolapril |
| 3.6 years | ARBs reduced the incidence of microalbuminuria among patients who did not present with microalbuminuria. |
| ROADMAP (2011) [38] | Olmesartan |
| 5 years | ARBs reduced the incidence of microalbuminuria among patients who did not present with microalbuminuria. |
4.2. SGLT2 Inhibitors
| Trial | Treatment | Population | Follow Up | Results |
|---|---|---|---|---|
| EMPA-REG OUTCOME (2015) [50] | Empaglifozin |
| 3.1 years | Significant reduction in cardiovascular events in type 2 diabetes mellitus patients with pre-existing cardiovascular disease and in progression of nephropathy |
| CANVAS (2017) [48] | Canaglifozin |
| 4.3 years | Reduction in cardiovascular death, nonfatal myocardial infarction (MI), or nonfatal stroke by 14% compared with placebo 33% reduction in hospitalization for heart failure 40% reduction in the risk of a composite renal outcome |
| CREDENCE (2019) [55] | Canaglifozin |
| 2.6 years | Significant reduction in the risk of ESKD or cardiovascular death (30%) compared with placebo |
| DAPA-CKD (2020) [56] | Dapaglifozin |
| 2.4 years | Significant reduction in the risk of ESKD, ≥50% sustained decline in eGFR or cardiovascular death |
| EMPA-KIDNEY (2023) [45] | Empaglifozin |
| 2 years | Reduction in risk of CKD progression or cardiovascular death by 28% with empagliflozin compared with placebo Reduction in the risk of all-cause hospitalization by 14% |
4.3. Glucagon-like Peptide-1 Receptor Agonists
4.4. Mineralocorticoids Receptor Antagonists (MRAs)
4.5. SGLT-1 Inhibitors
4.6. Endothelin Receptor Antagonists
5. Special Populations
5.1. Kidney Transplant Recipients
5.2. Pharmacologic Management
- -
- Insulin therapy: Insulin remains the cornerstone of diabetes management in kidney transplant recipients, especially in those with significant hyperglycemia or advanced stages of PTDM. Given the altered pharmacokinetics of insulin in renal failure, dosing adjustments are necessary to avoid hypoglycemia, especially in patients with impaired kidney function [107].
- -
- Oral hypoglycemic agents: The use of oral agents is considered in patients with mild PTDM and preserved renal function. Metformin is generally avoided in patients with significant renal dysfunction due to the risk of lactic acidosis. Sulfonylureas and thiazolidinediones can be used cautiously, but their use is often limited by their side effects, including weight gain and fluid retention, which can exacerbate cardiovascular risks [110].
- -
- Glucagon-like Peptide-1 (GLP-1) Receptor Agonists: They are increasingly being used in kidney transplant recipients, especially in those with mild to moderate PTDM, especially for the benefit of weight loss [77].
- -
- SGLT2 inhibitors: Their positive cardiovascular and kidney effects make them an attractive option for PTDM in kidney transplant recipients [111,112,113]. A multicenter study involving 750 KTRs recently reported that SGLT2 inhibitors significantly reduced the risk of major adverse cardiovascular events (MACEs), including myocardial infarction and cardiovascular mortality, without increasing the incidence of urinary tract infections (UTIs). Furthermore, a systematic review of 18 studies reported that SGLT2 inhibitors led to modest reductions in HbA1c and body weight, without a significant impact on eGFR or systolic blood pressure [114]. Despite these benefits, concerns remain regarding the potential risk of UTIs, in immunosuppressed patients, and the long-term effects on graft function [114].
6. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.-W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef]
- Cameron, J.S. The discovery of diabetic nephropathy: From small print to centre stage. J. Nephrol. 2006, 19 (Suppl. 10), S75–S87. [Google Scholar] [PubMed]
- Pugliese, G.; Penno, G.; Natali, A.; Barutta, F.; Di Paolo, S.; Reboldi, G.; Gesualdo, L.; De Nicola, L. Diabetic kidney disease: New clinical and therapeutic issues. Joint position statement of the Italian Diabetes Society and the Italian Society of Nephrology on “The natural history of diabetic kidney disease and treatment of hyperglycemia in patients with type 2 diabetes and impaired renal function”. J. Nephrol. 2020, 33, 9–35. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Tervaert, T.W.C.; Mooyaart, A.L.; Amann, K.; Cohen, A.H.; Cook, H.T.; Drachenberg, C.B.; Ferrario, F.; Fogo, A.B.; Haas, M.; de Heer, E.; et al. Pathologic classification of diabetic nephropathy. J. Am. Soc. Nephrol. 2010, 21, 556–563. [Google Scholar] [CrossRef]
- Doshi, S.M.; Friedman, A.N. Diagnosis and Management of Type 2 Diabetic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 1366–1373. [Google Scholar] [CrossRef]
- Deng, Y.; Li, N.; Wu, Y.; Wang, M.; Yang, S.; Zheng, Y.; Deng, X.; Xiang, D.; Zhu, Y.; Xu, P.; et al. Global, Regional, and National Burden of Diabetes-Related Chronic Kidney Disease from 1990 to 2019. Front. Endocrinol. 2021, 12, 672350. [Google Scholar] [CrossRef]
- Gesualdo, L.; Fiorentino, M.; Conserva, F.; Pontrelli, P. Should we enlarge the indication for kidney biopsy in patients with diabetes? The pro part. Clin. Kidney J. 2024, 17, sfad266. [Google Scholar] [CrossRef]
- Yamazaki, T.; Mimura, I.; Tanaka, T.; Nangaku, M. Treatment of Diabetic Kidney Disease: Current and Future. Diabetes Metab. J. 2021, 45, 11–26. [Google Scholar] [CrossRef]
- Reddy, M.A.; Zhang, E.; Natarajan, R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia 2015, 58, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Elendu, C.; Okah, M.M.J.; Fiemotongha, K.D.J.M.; Adeyemo, B.I.M.; Bassey, B.N.M.; Omeludike, E.K.M.; Obidigbo, B.M. Comprehensive advancements in the prevention and treatment of diabetic nephropathy: A narrative review. Medicine 2023, 102, e35397. [Google Scholar] [CrossRef]
- Limonte, C.P.; Kretzler, M.; Pennathur, S.; Pop-Busui, R.; de Boer, I.H. Present and future directions in diabetic kidney disease. J. Diabetes Complicat. 2022, 36, 108357. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Neeland, I.J.; Tuttle, K.R.; Chow, S.L.; Mathew, R.O.; Khan, S.S.; Coresh, J.; Baker-Smith, C.M.; Carnethon, M.R.; Després, J.-P.; et al. A Synopsis of the Evidence for the Science and Clinical Management of Cardiovascular-Kidney-Metabolic (CKM) Syndrome: A Scientific Statement from the American Heart Association. Circulation 2023, 148, 1636–1664. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory from the American Heart Association. Circulation 2023, 148, 1606–1635. [Google Scholar] [CrossRef] [PubMed]
- Mutruc, V.; Bologa, C.; Șorodoc, V.; Ceasovschih, A.; Morărașu, B.C.; Șorodoc, L.; Catar, O.E.; Lionte, C. Cardiovascular–Kidney–Metabolic Syndrome: A New Paradigm in Clinical Medicine or Going Back to Basics? J. Clin. Med. 2025, 14, 2833. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-K.; Kao, J.T.-W.; Wong, C.-S.; Liao, C.-T.; Lo, W.-C.; Chien, K.-L.; Wen, C.-P.; Wu, M.-S.; Wu, M.-Y. Cardiovascular–kidney–metabolic syndrome and all-cause and cardiovascular mortality: A retrospective cohort study. PLoS Med. 2025, 22, e1004629. [Google Scholar] [CrossRef]
- Ortiz, A. Should we enlarge the indication for kidney biopsy in diabetics? The con part. Clin. Kidney J. 2024, 17, sfad267. [Google Scholar] [CrossRef]
- Penno, G.; Solini, A.; Bonora, E.; Fondelli, C.; Orsi, E.; Zerbini, G.; Trevisan, R.; Vedovato, M.; Gruden, G.; Cavalot, F.; et al. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J. Hypertens. 2011, 29, 1802–1809. [Google Scholar] [CrossRef]
- Sulkin, T.V.; Bosman, D.; Krentz, A.J. Contraindications to metformin therapy in patients with NIDDM. Diabetes Care 1997, 20, 925–928. [Google Scholar] [CrossRef]
- Hung, S.-C.; Chang, Y.-K.; Liu, J.-S.; Kuo, K.-L.; Chen, Y.-H.; Hsu, C.-C.; Tarng, D.-C. Metformin use and mortality in patients with advanced chronic kidney disease: National, retrospective, observational, cohort study. Lancet Diabetes Endocrinol. 2015, 3, 605–614. [Google Scholar] [CrossRef]
- Lalau, J.D. Lactic acidosis induced by metformin: Incidence, management and prevention. Drug Saf. 2010, 33, 727–740. [Google Scholar] [CrossRef]
- Seidowsky, A.; Nseir, S.; Houdret, N.; Fourrier, F. Metformin-associated lactic acidosis: A prognostic and therapeutic study. Crit. Care Med. 2009, 37, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- Greco, P.; Regolisti, G.; Maggiore, U.; Ferioli, E.; Fani, F.; Locatelli, C.; Parenti, E.; Maccari, C.; Gandolfini, I.; Fiaccadori, E. Sustained low-efficiency dialysis for metformin-associated lactic acidosis in patients with acute kidney injury. J. Nephrol. 2019, 32, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Thammavaranucupt, K.; Phonyangnok, B.; Parapiboon, W.; Wongluechai, L.; Pichitporn, W.; Sumrittivanicha, J.; Sungkanuparph, S.; Nongnuch, A.; Jayanama, K. Metformin-associated lactic acidosis and factors associated with 30-day mortality. PLoS ONE 2022, 17, e0273678. [Google Scholar] [CrossRef] [PubMed]
- Rivera, D.; Onisko, N.; Cao, J.D.; Koyfman, A.; Long, B. High risk and low prevalence diseases: Metformin toxicities. Am. J. Emerg. Med. 2023, 72, 107–112. [Google Scholar] [CrossRef]
- Lewis, E.J.; Hunsicker, L.G.; Bain, R.P.; Rohde, R.D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 1993, 329, 1456–1462. [Google Scholar] [CrossRef]
- Ali, M.K.; Bullard, K.M.; Saaddine, J.B.; Cowie, C.C.; Imperatore, G.; Gregg, E.W. Achievement of goals in U.S. diabetes care, 1999-2010. N. Engl. J. Med. 2013, 368, 1613–1624. [Google Scholar] [CrossRef]
- Jacobsen, P.; Andersen, S.; Rossing, K.; Jensen, B.R.; Parving, H.-H. Dual blockade of the renin-angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathy. Kidney Int. 2003, 63, 1874–1880. [Google Scholar] [CrossRef]
- Scheen, A.J. Renin-angiotensin system inhibition prevents type 2 diabetes mellitus. Part 2. Overview of physiological and biochemical mechanisms. Diabetes Metab. 2004, 30, 498–505. [Google Scholar] [CrossRef]
- Mann, J.F.; Schmieder, R.E.; McQueen, M.; Dyal, L.; Schumacher, H.; Pogue, J.; Wang, X.; Maggioni, A.; Budaj, A.; Chaithiraphan, S.; et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet 2008, 372, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.F.; Emanuele, N.; Zhang, J.H.; Brophy, M.; Conner, T.A.; Duckworth, W.; Leehey, D.J.; McCullough, P.A.; O’Connor, T.; Palevsky, P.M.; et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N. Engl. J. Med. 2013, 369, 1892–1903. [Google Scholar] [CrossRef] [PubMed]
- Lambers Heerspink, H.J.; Weldegiorgis, M.; Inker, L.A.; Gansevoort, R.; Parving, H.-H.; Dwyer, J.P.; Mondal, H.; Coresh, J.; Greene, T.; Levey, A.S.; et al. Estimated GFR decline as a surrogate end point for kidney failure: A post hoc analysis from the Reduction of End Points in Non-Insulin-Dependent Diabetes with the Angiotensin II Antagonist Losartan (RENAAL) study and Irbesartan Diabetic Nephropathy Trial (IDNT). Am. J. Kidney Dis. 2014, 63, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Makino, H.; Haneda, M.; Babazono, T.; Moriya, T.; Ito, S.; Iwamoto, Y.; Kawamori, R.; Takeuchi, M.; Katayama, S. The telmisartan renoprotective study from incipient nephropathy to overt nephropathy–rationale, study design, treatment plan and baseline characteristics of the incipient to overt: Angiotensin II receptor blocker, telmisartan, Investigation on Type 2 Diabetic Nephropathy (INNOVATION) Study. J. Int. Med. Res. 2005, 33, 677–686. [Google Scholar] [CrossRef]
- Hellemons, M.E.; Persson, F.; Bakker, S.J.; Rossing, P.; Parving, H.-H.; De Zeeuw, D.; Heerspink, H.J.L. Initial angiotensin receptor blockade-induced decrease in albuminuria is associated with long-term renal outcome in type 2 diabetic patients with microalbuminuria: A post hoc analysis of the IRMA-2 trial. Diabetes Care 2011, 34, 2078–2083. [Google Scholar] [CrossRef]
- Viberti, G.; Wheeldon, N.M.; MicroAlbuminuria Reduction with VALsartan (MARVAL) Study Investigators. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: A blood pressure-independent effect. Circulation 2002, 106, 672–678. [Google Scholar] [CrossRef]
- BENEDICT Group. The BErgamo NEphrologic DIabetes Complications Trial (BENEDICT): Design and baseline characteristics. Control. Clin. Trials 2003, 24, 442–461. [Google Scholar] [CrossRef]
- Grassi, G. The ROADMAP trial: Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. Expert Opin. Pharmacother. 2011, 12, 2421–2424. [Google Scholar] [CrossRef]
- Gohda, T.; Murakoshi, M. Sodium-Glucose Cotransporter-2 Inhibitors—Miracle Drugs for the Treatment of Chronic Kidney Disease Irrespective of the Diabetes Status: Lessons from the Dedicated Kidney Disease-Focused CREDENCE and DAPA-CKD Trials. Int. J. Mol. Sci. 2022, 23, 13749. [Google Scholar] [CrossRef]
- Upadhyay, A. SGLT2 Inhibitors and Kidney Protection: Mechanisms beyond Tubuloglomerular Feedback. Kidney360 2024, 5, 771–782. [Google Scholar] [CrossRef]
- Gregg, L.P.; Richardson, P.A.; Nambi, V.; Petersen, L.A.; Matheny, M.E.; Virani, S.S.; Navaneethan, S.D. Sodium-Glucose Cotransporter-2 Inhibitor and Glucagon-Like Peptide-1 Receptor Agonist Discontinuation in Patients with CKD. J. Am. Soc. Nephrol. 2024, 36, 87–98. [Google Scholar] [CrossRef]
- Russo, E.; Zanetti, V.; Macciò, L.; Benizzelli, G.; Carbone, F.; La Porta, E.; Esposito, P.; Verzola, D.; Garibotto, G.; Viazzi, F. SGLT2 inhibition to target kidney aging. Clin. Kidney J. 2024, 17, sfae133. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Reeves, W.B.; Awad, A.S. Pathophysiology of diabetic kidney disease: Impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 2021, 17, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Günes-Altan, M.; Bosch, A.; Striepe, K.; Bramlage, P.; Schiffer, M.; Schmieder, R.E.; Kannenkeril, D. Is GFR decline induced by SGLT2 inhibitor of clinical importance? Cardiovasc. Diabetol. 2024, 23, 184. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fernandez, B.; Sarafidis, P.; Soler, M.J.; Ortiz, A. EMPA-KIDNEY: Expanding the range of kidney protection by SGLT2 inhibitors. Clin. Kidney J. 2023, 16, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, Q.; Sun, Z.; Liang, G.; Yan, F.; Niu, Y. Pretransplant Diabetes Mellitus and Kidney Transplant Outcomes: A Systematic Review and Meta-Analysis. Transplant. Proc. 2024, 56, 2149–2157. [Google Scholar] [CrossRef]
- Kluger, A.Y.; Tecson, K.M.; Barbin, C.M.; Lee, A.Y.; Lerma, E.V.; Rosol, Z.P.; Rangaswami, J.; Lepor, N.E.; Cobble, M.E.; McCullough, P.A. Cardiorenal Outcomes in the CANVAS, DECLARE-TIMI 58, and EMPA-REG OUTCOME Trials: A Systematic Review. Rev. Cardiovasc. Med. 2018, 19, 41–49. [Google Scholar] [CrossRef]
- Perkovic, V.; de Zeeuw, D.; Mahaffey, K.W.; Fulcher, G.; Erondu, N.; Shaw, W.; Barrett, T.D.; Weidner-Wells, M.; Deng, H.; Matthews, D.R.; et al. Canagliflozin and renal outcomes in type 2 diabetes: Results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018, 6, 691–704. [Google Scholar] [CrossRef]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef]
- Cherney, D.Z.I.; Zinman, B.; Inzucchi, S.E.; Koitka-Weber, A.; Mattheus, M.; von Eynatten, M.; Wanner, C. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: An exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 610–621. [Google Scholar] [CrossRef]
- Rådholm, K.; Figtree, G.; Perkovic, V.; Solomon, S.D.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Barrett, T.D.; Shaw, W.; Desai, M.; et al. Canagliflozin and Heart Failure in Type 2 Diabetes Mellitus: Results from the CANVAS Program. Circulation 2018, 138, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Kaplinsky, E. DAPA-HF trial: Dapagliflozin evolves from a glucose-lowering agent to a therapy for heart failure. Drugs Context 2020, 9, 2019-11-3. [Google Scholar] [CrossRef] [PubMed]
- Somagutta, M.R.; Agadi, K.; Hange, N.; Jain, M.S.; Batti, E.; Emuze, B.O.; Amos-Arowoshegbe, E.O.; Popescu, S.; Hanan, S.; Kumar, V.R.; et al. Euglycemic Diabetic Ketoacidosis and Sodium-Glucose Cotransporter-2 Inhibitors: A Focused Review of Pathophysiology, Risk Factors, and Triggers. Cureus 2021, 13, e13665. [Google Scholar] [CrossRef] [PubMed]
- AlKindi, F.; Boobes, Y.; Shalwani, F.; Ansari, J.; Almazrouei, R. Sodium-Glucose Cotransporter 2 Inhibitor (SGLT2i) Associated Diabetic Ketoacidosis in Oncology Patients: A Case Series and Literature Review. Cureus 2024, 16, e53816. [Google Scholar] [CrossRef]
- Heerspink, H.J.; Oshima, M.; Zhang, H.; Li, J.; Agarwal, R.; Capuano, G.; Charytan, D.M.; Craig, J.; de Zeeuw, D.; Di Tanna, G.L.; et al. Canagliflozin and Kidney-Related Adverse Events in Type 2 Diabetes and CKD: Findings from the Randomized CREDENCE Trial. Am. J. Kidney Dis. 2022, 79, 244–256.e1. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Stefansson, B.V.; Batiushin, M.; Bilchenko, O.; Cherney, D.Z.I.; Chertow, G.M.; Douthat, W.; Dwyer, J.P.; Escudero, E.; Pecoits-Filho, R.; et al. TThe dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial: Baseline characteristics. Nephrol. Dial. Transplant. 2020, 35, 1700–1711. [Google Scholar] [CrossRef]
- Mohamed, A.J.; AlSaffar, A.H.; Mohamed, A.A.; Khamis, M.H.; Khalaf, A.A.; AlAradi, H.J.; Abuhamaid, A.I.; Sanad, A.H.; Abbas, H.L.; Abdulla, A.M.; et al. Effect of GLP-1 Receptor Agonists on Renal Functions and Diabetic Nephropathy in Type 2 Diabetes Mellitus (T2DM) Patients: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e71739. [Google Scholar] [CrossRef]
- Michos, E.D.; Tuttle, K.R. GLP-1 receptor agonists in diabetic kidney disease. Clin. J. Am. Soc. Nephrol. 2021, 16, 1578–1580. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- McGuire, D.K.; Marx, N.; Mulvagh, S.L.; Deanfield, J.E.; Inzucchi, S.E.; Pop-Busui, R.; Mann, J.F.; Emerson, S.S.; Poulter, N.R.; Engelmann, M.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in High-Risk Type 2 Diabetes. N. Engl. J. Med. 2025, 392, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- Alfaris, N.; Waldrop, S.; Johnson, V.; Boaventura, B.; Kendrick, K.; Stanford, F.C. GLP-1 single, dual, and triple receptor agonists for treating type 2 diabetes and obesity: A narrative review. EClinicalMedicine 2024, 75, 102782. [Google Scholar] [CrossRef] [PubMed]
- Rossing, P.; Baeres, F.M.M.; Bakris, G.; Bosch-Traberg, H.; Gislum, M.; Gough, S.C.L.; Idorn, T.; Lawson, J.; Mahaffey, K.W.; Mann, J.F.E.; et al. The rationale, design and baseline data of FLOW, a kidney outcomes trial with once-weekly semaglutide in people with type 2 diabetes and chronic kidney disease. Nephrol. Dial. Transplant. 2023, 38, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liang, X.; Sun, N.; Zhang, D. Influence of glucagon-like peptide-1 receptor agonists on renal parameters: A meta-analysis of randomized controlled trials. BMC Endocr. Disord. 2025, 25, 124. [Google Scholar] [CrossRef]
- Kim, S.; An, J.N.; Song, Y.R.; Kim, S.G.; Lee, H.S.; Cho, A.; Kim, J.-K. Effect of once-weekly dulaglutide on renal function in patients with chronic kidney disease. PLoS ONE 2022, 17, e0273004. [Google Scholar] [CrossRef]
- Botros, F.T.; Gerstein, H.C.; Malik, R.; Nicolay, C.; Hoover, A.; Turfanda, I.; Colhoun, H.M.; Shaw, J.E. Dulaglutide and Kidney Function–Related Outcomes in Type 2 Diabetes: A REWIND Post Hoc Analysis. Diabetes Care 2023, 46, 1524–1530. [Google Scholar] [CrossRef]
- Mahaffey, K.W.; Tuttle, K.R.; Arici, M.; Baeres, F.M.M.; Bakris, G.; Charytan, D.M.; Cherney, D.Z.I.; Chernin, G.; Correa-Rotter, R.; Gumprecht, J.; et al. Cardiovascular outcomes with semaglutide by severity of chronic kidney disease in type 2 diabetes: The FLOW trial. Eur. Heart J. 2024, 46, 1096–1108. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Kaplan, L.M.; Frías, J.P.; Wu, Q.; Du, Y.; Gurbuz, S.; Coskun, T.; Haupt, A.; Milicevic, Z.; Hartman, M.L. Triple–Hormone-Receptor Agonist Retatrutide for Obesity—A Phase 2 Trial. N. Engl. J. Med. 2023, 389, 514–526. [Google Scholar] [CrossRef]
- Heerspink, H.L.; Lu, Z.; DU, Y.; Duffin, K.L.; Coskun, T.; Haupt, A.; Hartman, M.L. 754-P: Effect of Retatrutide on Kidney Parameters in People with Type 2 Diabetes and/or Obesity—A Post-Hoc Analysis of Two Phase 2 Trials. Diabetes 2024, 73 (Suppl. 1), 754-P. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Sattar, N.; Pavo, I.; Haupt, A.; Duffin, K.L.; Yang, Z.; Wiese, R.J.; Tuttle, K.R.; Cherney, D.Z.I. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: Post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022, 10, 774–785. [Google Scholar] [CrossRef]
- Chu, L.; Bradley, R.M.; Auerbach, P.; Abitbol, A. Real-world impact of adding a glucagon-like peptide-1 receptor agonist compared with basal insulin on metabolic targets in adults living with type 2 diabetes and chronic kidney disease already treated with a sodium-glucose co-transporter-2 inhibitor: The Impact GLP-1 CKD study. Diabetes Obes. Metab. 2024, 26, 4674–4683. [Google Scholar] [CrossRef]
- Colhoun, H.M.; Lingvay, I.; Brown, P.M.; Deanfield, J.; Brown-Frandsen, K.; Kahn, S.E.; Plutzky, J.; Node, K.; Parkhomenko, A.; Rydén, L.; et al. Long-term kidney outcomes of semaglutide in obesity and cardiovascular disease in the SELECT trial. Nat. Med. 2024, 30, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Praga, M.; Morales, E. Obesity-related renal damage: Changing diet to avoid progression. Kidney Int. 2010, 78, 633–635. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.N.; Yu, Z.; Juliar, B.E.; Nguyen, J.T.; Strother, M.; Quinney, S.K.; Li, L.; Inman, M.; Gomez, G.; Shihabi, Z.; et al. Independent influence of dietary protein on markers of kidney function and disease in obesity. Kidney Int. 2010, 78, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Ussher, J.R.; Drucker, D.J. Glucagon-like peptide 1 receptor agonists: Cardiovascular benefits and mechanisms of action. Nat. Rev. Cardiol. 2023, 20, 463–474. [Google Scholar] [CrossRef]
- de Boer, I.H.; Caramori, M.L.; Chan, J.C.; Heerspink, H.J.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; Sadusky, T.; et al. GLP-1 receptor agonists and other incretin mimetics for diabetes and chronic kidney disease—A KDIGO commentary. Kidney Int. 2025, 107, 767–771. [Google Scholar] [CrossRef]
- Shaman, A.M.; Bain, S.C.; Bakris, G.L.; Buse, J.B.; Idorn, T.; Mahaffey, K.W.; Mann, J.F.E.; Nauck, M.A.; Rasmussen, S.; Rossing, P.; et al. Effect of the glucagon-likePeptide-1 receptor agonists Semaglutide and liraglutide on kidneyoutcomes in patients with type 2 diabetes: Pooled analysis of SUS-TAIN 6 and LEADER. Circulation 2022, 145, 575–585. [Google Scholar] [CrossRef]
- Michos, E.D.; Bakris, G.L.; Rodbard, H.W.; Tuttle, K.R. Glucagon-like peptide-1 receptor agonists in diabetic kidney disease: A review of their kidney and heart protection. Am. J. Prev. Cardiol. 2023, 14, 100502. [Google Scholar] [CrossRef]
- Currie, G.; Taylor, A.H.M.; Fujita, T.; Ohtsu, H.; Lindhardt, M.; Rossing, P.; Boesby, L.; Edwards, N.C.; Ferro, C.J.; Townend, J.N.; et al. Effect of mineralocorticoid receptor antagonists on proteinuria and progression of chronic kidney disease: A systematic review and meta-analysis. BMC Nephrol. 2016, 17, 127. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Nigwekar, S.U.; Sehgal, A.R.; Strippoli, G.F.M. Aldosterone antagonists for preventing the progression of chronic kidney disease: A systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 2009, 4, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, J.; Jaisser, F.; Toto, R. Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension 2015, 65, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; Bittman, R.; Hurley, S.; Kleiman, J.; Gatlin, M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 2003, 348, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; McMurray, J.J.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef]
- Alexandrou, M.-E.; Papagianni, A.; Tsapas, A.; Loutradis, C.; Boutou, A.; Piperidou, A.; Papadopoulou, D.; Ruilope, L.; Bakris, G.; Sarafidis, P. Effects of mineralocorticoid receptor antagonists in proteinuric kidney disease: A systematic review and meta-analysis of randomized controlled trials. J. Hypertens. 2019, 37, 2307–2324. [Google Scholar] [CrossRef]
- Agarwal, R.; Joseph, A.; Anker, S.D.; Filippatos, G.; Rossing, P.; Ruilope, L.M.; Pitt, B.; Kolkhof, P.; Scott, C.; Lawatscheck, R.; et al. Hyperkalemia Risk with Finerenone: Results from the FIDELIO-DKD Trial. J. Am. Soc. Nephrol. 2022, 33, 225–237. [Google Scholar] [CrossRef]
- Filippatos, G.; Anker, S.D.; Agarwal, R.; Ruilope, L.M.; Rossing, P.; Bakris, G.L.; Tasto, C.; Joseph, A.; Kolkhof, P.; Lage, A.; et al. Finerenone Reduces Risk of Incident Heart Failure in Patients with Chronic Kidney Disease and Type 2 Diabetes: Analyses from the FIGARO-DKD Trial. Circulation 2022, 145, 437–447. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- Agarwal, R.; Filippatos, G.; Pitt, B.; Anker, S.D.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Gebel, M.; Ruilope, L.M.; et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis. Eur. Heart J. 2022, 43, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kashihara, N.; Shikata, K.; Nangaku, M.; Wada, T.; Okuda, Y.; Sawanobori, T. Esaxerenone (CS-3150) in Patients with Type 2 Diabetes and Microalbuminuria (ESAX-DN): Phase 3 Randomized Controlled Clinical Trial. Clin. J. Am. Soc. Nephrol. 2020, 15, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Hauske, S.J.; Canziani, M.E.; Caramori, M.L.; Cherney, D.; Cronin, L.; Heerspink, H.J.L.; Hugo, C.; Nangaku, M.; Rotter, R.C.; et al. Efficacy and safety of aldosterone synthase inhibition with and without empagliflozin for chronic kidney disease: A randomised, controlled, phase 2 trial. Lancet 2024, 403, 379–390. [Google Scholar] [CrossRef]
- Judge, P.K.; Tuttle, K.R.; Staplin, N.; Hauske, S.J.; Zhu, D.; Sardell, R.; Cronin, L.; Green, J.B.; Agrawal, N.; Arimoto, R.; et al. The potential for improving cardio-renal outcomes in chronic kidney disease with the aldosterone synthase inhibitor vicadrostat (BI 690517): A rationale for the EASi-KIDNEY trial. Nephrol. Dial. Transplant. 2025, 40, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Oe, Y.; Vallon, V. The Pathophysiological Basis of Diabetic Kidney Protection by Inhibition of SGLT2 and SGLT1. Kidney Dial. 2022, 2, 349–368. [Google Scholar] [CrossRef]
- Aggarwal, R.; Bhatt, D.L.; Szarek, M.; Cannon, C.P.; Leiter, L.A.; Inzucchi, S.E.; Lopes, R.D.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; et al. Effect of sotagliflozin on major adverse cardiovascular events: A prespecified secondary analysis of the SCORED randomised trial. Lancet Diabetes Endocrinol. 2025, 13, 321–332. [Google Scholar] [CrossRef]
- Sridhar, V.S.; Bhatt, D.L.; Odutayo, A.; Szarek, M.; Davies, M.J.; Banks, P.; Pitt, B.; Steg, P.G.; Cherney, D.Z. Sotagliflozin and Kidney Outcomes, Kidney Function, and Albuminuria in Type 2 Diabetes and CKD: A Secondary Analysis of the SCORED Trial. Clin. J. Am. Soc. Nephrol. 2024, 19, 557–564. [Google Scholar] [CrossRef]
- Empitu, M.A.; Rinastiti, P.; Kadariswantiningsih, I.N. Targeting endothelin signaling in podocyte injury and diabetic nephropathy-diabetic kidney disease. J. Nephrol. 2025, 38, 49–60. [Google Scholar] [CrossRef]
- Khurana, N.; James, S.; Coughlan, M.T.; MacIsaac, R.J.; Ekinci, E.I. Novel Therapies for Kidney Disease in People with Diabetes. J. Clin. Endocrinol. Metab. 2022, 107, e1–e24. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Parving, H.-H.; Andress, D.L.; Correa-Rotter, R.; Hou, F.-F.; Kitzman, D.W.; Kohan, D.; Makino, H.; McMurray, J.J.V.; Melnick, J.Z.; et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): A double-blind, randomised, placebo-controlled trial. Lancet 2019, 393, 1937–1947. [Google Scholar] [CrossRef]
- de Zeeuw, D.; Coll, B.; Andress, D.; Brennan, J.J.; Tang, H.; Houser, M.; Correa-Rotter, R.; Kohan, D.; Heerspink, H.J.L.; Makino, H.; et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 2014, 25, 1083–1093. [Google Scholar] [CrossRef]
- Andress, D.L.; Coll, B.; Pritchett, Y.; Brennan, J.; Molitch, M.; Kohan, D.E. Clinical efficacy of the selective endothelin A receptor antagonist, atrasentan, in patients with diabetes and chronic kidney disease (CKD). Life Sci. 2012, 91, 739–742. [Google Scholar] [CrossRef]
- Shetty, S.; Suvarna, R.; Awasthi, A.; Bhojaraja, M.V.; Pappachan, J.M. Emerging Biomarkers and Innovative Therapeutic Strategies in Diabetic Kidney Disease: A Pathway to Precision Medicine. Diagnostics 2025, 15, 973. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Culliford, A.; Phagura, N.; Evison, F.; Gallier, S.; Sharif, A. Comparing survival outcomes for kidney transplant recipients with pre-existing diabetes versus those who develop post-transplantation diabetes. Diabet. Med. 2022, 39, e14707. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, C.; Campioli, E.; Fiorina, P.; Orsi, E.; Grancini, V.; Regalia, A.; Campise, M.; Verdesca, S.; Delfrate, N.W.; Molinari, P.; et al. Post-Transplant Diabetes Mellitus in Kidney-Transplanted Patients: Related Factors and Impact on Long-Term Outcome. Nutrients 2024, 16, 1520. [Google Scholar] [CrossRef] [PubMed]
- Mourad, G.; Glyda, M.; Albano, L.; Viklický, O.; Merville, P.; Tydén, G.; Mourad, M.; Lõhmus, A.; Witzke, O.; Christiaans, M.H.L.; et al. Incidence of Posttransplantation Diabetes Mellitus in de Novo Kidney Transplant Recipients Receiving Prolonged-Release Tacrolimus-Based Immunosuppression with 2 Different Corticosteroid Minimization Strategies: ADVANCE, A Randomized Controlled Trial. Transplantation 2017, 101, 1924–1934. [Google Scholar] [CrossRef]
- Kanbay, M.; Copur, S.; Topçu, A.U.; Guldan, M.; Ozbek, L.; Gaipov, A.; Ferro, C.; Cozzolino, M.; Cherney, D.Z.I.; Tuttle, K.R. An update review of post-transplant diabetes mellitus: Concept, risk factors, clinical implications and management. Diabetes Obes. Metab. 2024, 26, 2531–2545. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Radek, M.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. Diabetes and cardiovascular risk in renal transplant patients. Int. J. Mol. Sci. 2021, 22, 3422. [Google Scholar] [CrossRef]
- Bonato, V.; Barni, R.; Cataldo, D.; Collini, A.; Ruggieri, G.; De Bartolomeis, C.; Dotta, F.; Carmellini, M. Analysis of Posttransplant Diabetes Mellitus Prevalence in a Population of Kidney Transplant Recipients. Transplant. Proc. 2008, 40, 1888–1890. [Google Scholar] [CrossRef]
- Laghrib, Y.; Hilbrands, L.; Oniscu, G.C.; Crespo, M.; Gandolfini, I.; Mariat, C.; Mjøen, G.; Sever, M.S.; Watschinger, B.; Velioglu, A.; et al. Current practices in prevention, screening, and treatment of diabetes in kidney transplant recipients: European survey highlights from the ERA DESCARTES Working Group. Clin. Kidney J. 2025, 18, sfae367. [Google Scholar] [CrossRef]
- Sridhar, V.S.; Ambinathan, J.P.N.; Gillard, P.; Mathieu, C.; Cherney, D.Z.; Lytvyn, Y.; Singh, S.K. Cardiometabolic and Kidney Protection in Kidney Transplant Recipients With Diabetes: Mechanisms, Clinical Applications, and Summary of Clinical Trials. Transplantation 2022, 106, 734–748. [Google Scholar] [CrossRef]
- Lim, J.-H.; Kwon, S.; Jeon, Y.; Kim, Y.H.; Kwon, H.; Kim, Y.S.; Lee, H.; Kim, Y.-L.; Kim, C.-D.; Park, S.-H.; et al. The Efficacy and Safety of SGLT2 Inhibitor in Diabetic Kidney Transplant Recipients. Transplantation 2022, 106, E404–E412. [Google Scholar] [CrossRef]
- Sharif, A.; Chakkera, H.; de Vries, A.P.J.; Eller, K.; Guthoff, M.; Haller, M.C.; Hornum, M.; Nordheim, E.; Kautzky-Willer, A.; Krebs, M.; et al. International consensus on post-transplantation diabetes mellitus. In Nephrology Dialysis Transplantation; Oxford University Press: Oxford, UK, 2024; Volume 39, pp. 531–549. [Google Scholar] [CrossRef]
- Bellos, I.; Lagiou, P.; Benetou, V.; Marinaki, S. Safety and Efficacy of Sodium-Glucose Transport Protein 2 Inhibitors and Glucagon-like Peptide-1 Receptor Agonists in Diabetic Kidney Transplant Recipients: Synthesis of Evidence. J. Clin. Med. 2024, 13, 6181. [Google Scholar] [CrossRef]
- Siehler, J.; Blöchinger, A.K.; Meier, M.; Lickert, H. Engineering islets from stem cells for advanced therapies of diabetes. Nat. Rev. Drug Discov. 2021, 20, 920–940. [Google Scholar] [CrossRef] [PubMed]
- Mikłosz, A.; Chabowski, A. Adipose-derived Mesenchymal Stem Cells Therapy as a new Treatment Option for Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2023, 108, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.T.; Dadheech, N.; Tan, E.H.P.; Ng, N.H.J.; Koh, M.B.C.; Shapiro, J.; Teo, A.K.K. Stem cell therapies for diabetes. Nat. Med. 2025, 31, 2147–2160. [Google Scholar] [CrossRef] [PubMed]
- Madkor, H.R.; Abd El-Aziz, M.K.; El-Maksoud, M.S.A.; Ibrahim, I.M.; Ali, F.E.M. Stem Cells Reprogramming in Diabetes Mellitus and Diabetic Complications: Recent Advances. Curr. Diabetes Rev. 2025, 21, e010124225101. [Google Scholar] [CrossRef]
- Abdalla, M.M.I. Advancing diabetes management: Exploring pancreatic beta-cell restoration’s potential and challenges. World J. Gastroenterol. 2024, 30, 4339–4353. [Google Scholar] [CrossRef]
- Mou, L.; Wang, T.B.; Wang, X.; Pu, Z. Advancing diabetes treatment: The role of mesenchymal stem cells in islet transplantation. Front. Immunol. 2024, 15, 1389134. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F.; Lightstone, L.; Jha, V.; Pollock, C.; Tuttle, K.; Kotanko, P.; Wiecek, A.; Anders, H.J.; Remuzzi, G.; et al. A new era in the science and care of kidney diseases. Nat. Rev. Nephrol. 2024, 20, 460–472. [Google Scholar] [CrossRef]
| Values | Dialysis Start | Dialysis End |
|---|---|---|
| Serum creatinine (mg/dL) | 7.41 ± 2.97 | 3.42 ± 1.83 |
| GFR (mL/min/1.73 mq, EPI-CKD) | 6.52 ± 2.93 | |
| Serum metformin (mg/L, ref. value < 2) | 24.39 ± 16.35 | 9.81 ± 5.57 |
| Blood pH | 7.10 ± 0.2 | 7.35 ± 0.05 |
| Serum lactate (mg/dL) | 103.15 ± 60.51 | 28.7 ± 19.81 |
| HCO3-(mmol/L) | 11.1 ± 5.6 | 19.36 ± 3.88 |
| Serum potassium (mEq/L) | 6 ± 1.25 | 4.5 ± 0.26 |
| Trial | Treatment | Population | Follow Up | Results |
|---|---|---|---|---|
| FIGARO-DKD (2021) [88] | Finerenone |
| 3.4 years | Reduction in risk of cardiovascular events compared with placebo and occurrence of a trend toward reduced kidney-related outcomes in patients with T2D and early to moderate stages of CKD |
| FIDELIO-DKD (2020) [89] | Finerenone |
| 2.6 years | Reduction in risk of a composite of kidney failure, sustained ≥40% decrease in eGFR from baseline, or renal death, compared with placebo |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentile, M.; D’Angelo, M.; Varì, M.R.; Bolla, A.M.; Bianco, M.; Scarpioni, R. From Classic to Contemporary, Evolving Therapies in Diabetic Kidney Disease: The Point of View of the Nephrologist and the Diabetologist. Diabetology 2025, 6, 144. https://doi.org/10.3390/diabetology6120144
Gentile M, D’Angelo M, Varì MR, Bolla AM, Bianco M, Scarpioni R. From Classic to Contemporary, Evolving Therapies in Diabetic Kidney Disease: The Point of View of the Nephrologist and the Diabetologist. Diabetology. 2025; 6(12):144. https://doi.org/10.3390/diabetology6120144
Chicago/Turabian StyleGentile, Micaela, Marta D’Angelo, Maria Rosaria Varì, Andrea Mario Bolla, Maurizio Bianco, and Roberto Scarpioni. 2025. "From Classic to Contemporary, Evolving Therapies in Diabetic Kidney Disease: The Point of View of the Nephrologist and the Diabetologist" Diabetology 6, no. 12: 144. https://doi.org/10.3390/diabetology6120144
APA StyleGentile, M., D’Angelo, M., Varì, M. R., Bolla, A. M., Bianco, M., & Scarpioni, R. (2025). From Classic to Contemporary, Evolving Therapies in Diabetic Kidney Disease: The Point of View of the Nephrologist and the Diabetologist. Diabetology, 6(12), 144. https://doi.org/10.3390/diabetology6120144







