A Real-World Comparison of Three Deep Learning Systems for Diabetic Retinopathy in Remote Australia
Abstract
1. Introduction
2. Materials and Methods
2.1. Development of the DLSs
2.2. Ethical Approval
2.3. Statistical Analysis
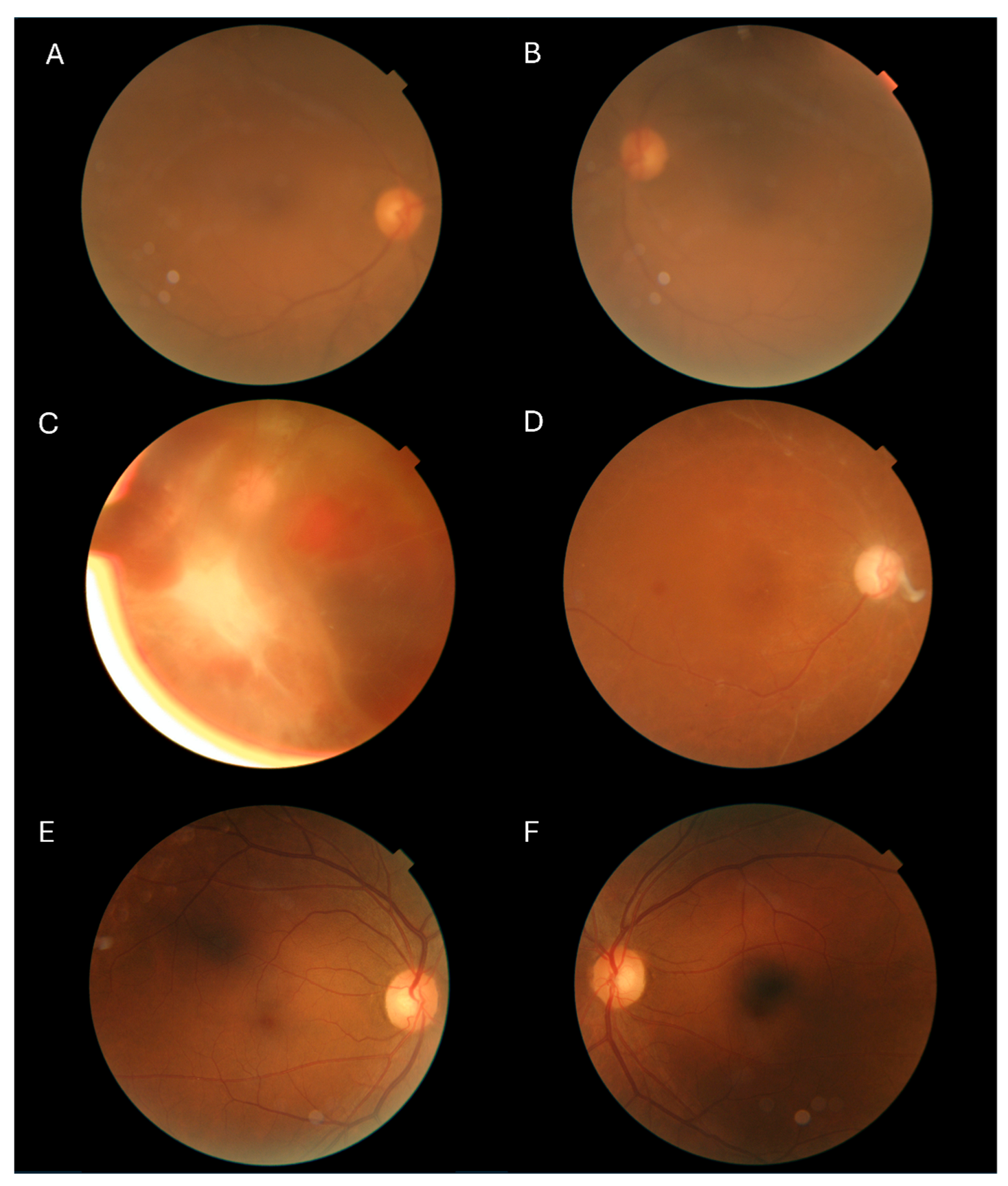
3. Results
4. Discussion
4.1. Strengths and Limitations
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourne, R.R.A.; Jonas, J.B.; Flaxman, S.R.; Keeffe, J.; Leasher, J.; Naidoo, K.; Parodi, M.B.; Pesudovs, K.; Price, H.; A White, R.; et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990–2010. Br. J. Ophthalmol. 2014, 98, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Indigenous Eye Health Measures 2021. AIHW 261; Australian Institute of Health and Welfare: Canberra, Australia, 2021. Available online: https://www.aihw.gov.au/reports/indigenous-australians/indigenous-eye-health-measures-2021 (accessed on 20 January 2025).
- Teo, Z.L.; Tham, Y.-C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Sun, J.; Kawasaki, R.; Ruamviboonsuk, P.; Gupta, N.; Lansingh, V.C.; Maia, M.; Mathenge, W.; Moreker, S.; Muqit, M.M.; et al. Guidelines on diabetic eye care: The International Council of Ophthalmology recommen-dations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology 2018, 125, 1608–1622. [Google Scholar] [CrossRef]
- Mitchell, P.; Foran, S.; Wong, T.; Chua, B.; Patel, I.; Ojaimi, E. Guidelines for the Management of Diabetic Retinopathy; National Health and Medical Research Council: Canberra, Australia, 2008; pp. 1–183. [Google Scholar]
- Foreman, J.; Keel, S.; Xie, J.; Van Wijngaarden, P.; Taylor, H.R.; Dirani, M. Adherence to diabetic eye examination guidelines in Australia: The National Eye Health Survey. Med. J. Aust. 2017, 206, 402–406. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Diabetes: Australian Facts; Australian Institute of Health and Welfare: Canberra, Australia, 2023. Available online: https://www.aihw.gov.au/reports/diabetes/diabetes (accessed on 26 August 2024).
- White, C.S.; Seear, K.; Anderson, L.; Griffiths, E. Use of a primary care dataset to describe ‘the real picture’ of diabetes in Kimberley Aboriginal communities. J. Aust. Indig. Heal. 2024, 5, 4. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. 2021 Census Aboriginal and/or Torres Strait Islander People Quickstats; Australian Bureau of Statistics: Canberra, Australia, 2021. Available online: https://abs.gov.au/census/find-census-data/quickstats/2021/IQS51002 (accessed on 25 August 2024).
- Khou, V.; Khan, M.A.; Jiang, I.W.; Katalinic, P.; Agar, A.; Zangerl, B. Evaluation of the initial implementation of a nationwide diabetic retinopathy screening programme in primary care: A multimethod study. BMJ Open 2021, 11, e044805. [Google Scholar] [CrossRef]
- Hu, W.; Joseph, S.; Li, R.; Woods, E.; Sun, J.; Shen, M.; Jan, C.L.; Zhu, Z.; He, M.; Zhang, L. Population impact and cost-effectiveness of artificial intelligence-based diabetic retinopathy screening in people living with diabetes in Australia: A cost effectiveness analysis. eClinicalMedicine 2024, 67, 102387. [Google Scholar] [CrossRef]
- Joseph, S.; Selvaraj, J.; Mani, I.; Kumaragurupari, T.; Shang, X.; Mudgil, P.; Ravilla, T.; He, M. Diagnostic accuracy of artificial intelligence-based automated diabetic retinopathy screening in real-world settings: A systematic review and meta-analysis. Arch. Ophthalmol. 2024, 263, 214–230. [Google Scholar] [CrossRef]
- Chia, M.A.; Hersch, F.; Sayres, R.; Bavishi, P.; Tiwari, R.; Keane, P.A.; Turner, A.W. Validation of a deep learning system for the detection of diabetic retinopathy in Indigenous Australians. Br. J. Ophthalmol. 2023, 108, 268–273. [Google Scholar] [CrossRef]
- Quinn, N.; Brazionis, L.; Zhu, B.; Ryan, C.; D’aloisio, R.; Tang, H.L.; Peto, T.; Jenkins, A. The Centre of Research Excellence in Diabetic Retinopathy Study, TEAMSnet Study Groups. Facilitating diabetic retinopathy screening using automated retinal image analysis in underresourced settings. Diabet. Med. 2021, 38, e14582. [Google Scholar] [CrossRef]
- Scheetz, J.; Koca, D.; McGuinness, M.; Holloway, E.; Tan, Z.; Zhu, Z.; O’Day, R.; Sandhu, S.; MacIsaac, R.J.; Gilfillan, C.; et al. Real-world artificial intelligence-based opportunistic screening for diabetic retinopathy in endocrinology and indigenous healthcare settings in Australia. Sci. Rep. 2021, 11, 15808. [Google Scholar] [CrossRef]
- Li, Z.; Keel, S.; Liu, C.; He, Y.; Meng, W.; Scheetz, J.; Lee, P.Y.; Shaw, J.; Ting, D.; Wong, T.Y.; et al. An automated grading system for detection of vision-threatening referable diabetic retinopathy on the basis of color fundus photographs. Diabetes Care 2018, 41, 2509–2516. [Google Scholar] [CrossRef]
- Google Health. Using AI to Prevent Blindness. Available online: https://health.google/caregivers/arda/ (accessed on 19 February 2025).
- Thirona Retina. Artificial Intelligence for High Performance Eye Disease Screening. Available online: https://retcad.eu/ (accessed on 19 February 2025).
- EyRIS. Revolutionizing the Detection of Eye Diseases. Available online: https://www.eyris.io/index.cfm (accessed on 19 February 2025).
- Arora, A.; Alderman, J.E.; Palmer, J.; Ganapathi, S.; Laws, E.; McCradden, M.D.; Oakden-Rayner, L.; Pfohl, S.R.; Ghassemi, M.; McKay, F.; et al. The value of standards for health datasets in artificial intelligence-based applications. Nat. Med. 2023, 29, 2929–2938. [Google Scholar] [CrossRef]
- Ktena, I.; Wiles, O.; Albuquerque, I.; Rebuffi, S.-A.; Tanno, R.; Roy, A.G.; Azizi, S.; Belgrave, D.; Kohli, P.; Cemgil, T.; et al. Generative models improve fairness of medical classifiers under distribution shifts. Nat. Med. 2024, 30, 1166–1173. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Gichoya, J.W.; Katabi, D.; Ghassemi, M. The limits of fair medical imaging AI in real-world generalization. Nat. Med. 2024, 30, 2838–2848. [Google Scholar] [CrossRef]
- Li, Q.; Drinkwater, J.J.; Woods, K.; Douglas, E.; Ramirez, A.; Turner, A.W. Implementation of a new, mobile diabetic retinopathy screening model incorporating artificial intelligence in remote western australia. Aust. J. Rural. Heal. 2025, 33, e70031. [Google Scholar] [CrossRef]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 2016, 316, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.W.; Cheung, C.Y.-L.; Lim, G.; Tan, G.S.W.; Quang, N.D.; Gan, A.; Hamzah, H.; Garcia-Franco, R.; Yeo, I.Y.S.; Lee, S.Y.; et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA 2017, 318, 2211–2223. [Google Scholar] [CrossRef] [PubMed]
- RetCAD, Version 2.2; White Paper, 2023. Available online: https://delft.care/retcad/ (accessed on 12 May 2025).
- Wilkinson, C.; Ferris, F.L.; E Klein, R.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Arifin, W.N. Sample Size Calculator (Web). Available online: http://wnarifin.github.io (accessed on 29 July 2024).
- Ibrahim, H.; Liu, X.; Zariffa, N.; Morris, A.D.; Denniston, A.K. Health data poverty: An assailable barrier to equitable digital health care. Lancet Digit. Health 2021, 3, e260–e265. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.M.; Channa, R.; Liu, T.Y.A.; Zehra, A.; Bromberger, L.; Patel, D.; Ananthakrishnan, A.; Brown, E.A.; Prichett, L.; Lehmann, H.P.; et al. Autonomous artificial intelligence increases screening and follow-up for diabetic retinopathy in youth: The ACCESS randomized control trial. Nat. Commun. 2024, 15, 421. [Google Scholar] [CrossRef]
- Ruamviboonsuk, P.; Tiwari, R.; Sayres, R.; Nganthavee, V.; Hemarat, K.; Kongprayoon, A.; Raman, R.; Levinstein, B.; Liu, Y.; Schaekermann, M.; et al. Real-time diabetic retinopathy screening by deep learning in a multisite national screening programme: A prospective interventional cohort study. Lancet Digit. Health 2022, 4, e235–e244. [Google Scholar] [CrossRef]
- Gulshan, V.; Rajan, R.P.; Widner, K.; Wu, D.; Wubbels, P.; Rhodes, T.; Whitehouse, K.; Coram, M.; Corrado, G.; Ramasamy, K.; et al. Performance of a deep-learning algorithm vs manual grading for detecting diabetic retinopathy in India. JAMA Ophthalmol. 2019, 137, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Piatti, A.; Rui, C.; Gazzina, S.; Tartaglino, B.; Romeo, F.; Manti, R.; Doglio, M.; Nada, E.; Giorda, C. Diabetic retinopathy screening with confocal fundus camera and artificial intelligence—Assisted grading. Eur. J. Ophthalmol. 2024, 35, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Meredith, S.; van Grinsven, M.; Engelberts, J.; Clarke, D.; Prior, V.; Vodrey, J.; Hammond, A.; Muhammed, R.; Kirby, P. Performance of an artificial intelligence automated system for diabetic eye screening in a large English population. Diabet. Med. 2023, 40, e15055. [Google Scholar] [CrossRef]
- Taylor, J.R.; Drinkwater, J.; Sousa, D.C.; Shah, V.; Turner, A.W. Real-world evaluation of RetCAD deep-learning system for the detection of referable diabetic retinopathy and age-related macular degeneration. Clin. Exp. Optom. 2024, 108, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Skevas, C.; Weindler, H.; Levering, M.; Engelberts, J.; van Grinsven, M.; Katz, T. Simultaneous screening and classification of diabetic retinopathy and age-related macular degeneration based on fundus photos—A prospective analysis of the RetCAD system. Int. J. Ophthalmol. 2022, 15, 1985–1993. [Google Scholar] [CrossRef]
- Bellemo, V.; Lim, Z.W.; Lim, G.; Nguyen, Q.D.; Xie, Y.; Yip, M.Y.T.; Hamzah, H.; Ho, J.; Lee, X.Q.; Hsu, W.; et al. Artificial intelligence using deep learning to screen for referable and vision-threatening diabetic retinopathy in Africa: A clinical validation study. Lancet Digit. Health 2019, 1, e35–e44. [Google Scholar] [CrossRef]
- Kanagasingam, Y.; Xiao, D.; Vignarajan, J.; Preetham, A.; Tay-Kearney, M.-L.; Mehrotra, A. Evaluation of artificial intelligence–based grading of diabetic retinopathy in primary care. JAMA Netw. Open 2018, 1, e182665. [Google Scholar] [CrossRef] [PubMed]
- Chu, K. An introduction to sensitivity, specificity, predictive values and likelihood ratios. Emerg. Med. 1999, 11, 175–181. [Google Scholar] [CrossRef]
- Cheung, R.; Ly, A. A survey of eyecare affordability among patients seen in collaborative care in Australia and factors contributing to cost barriers. Public Health Res. Pract. 2024, 34, e3422415. [Google Scholar] [CrossRef] [PubMed]
- Drinkwater, J.J.; Kalantary, A.; Turner, A.W. A systematic review of diabetic retinopathy screening intervals. Acta Ophthalmol. 2023, 102, e473–e484. [Google Scholar] [CrossRef] [PubMed]
- Ta, A.W.A.; Goh, H.L.; Ang, C.; Koh, L.Y.; Poon, K.; Miller, S.M. Two Singapore public healthcare AI applications for national screening programs and other examples. Health Care Sci. 2022, 1, 41–57. [Google Scholar] [CrossRef] [PubMed]
| Deep Learning System | Google ARDA | Thirona RetCADTM | EyRIS SELENA+ | |
|---|---|---|---|---|
| Eyes with diabetes | Number of images analysed | 174/188 (92.6) | 172/188 (91.5) | 161/188 (85.6) |
| Ungradable images (total) | 4/188 (2.1) | 9/188 (4.8) | 24/188 (12.8) | |
| Ungradable images that were gradable by ophthalmologists | 0/4 (0) | 2/9 (22.2) | 13/24 (54.2) | |
| Sensitivity | 100 (91.03–100) | 97.37 (86.50–99.53) | 91.67 (78.17–97.13) | |
| Specificity | 94.81 (89.68–97.47) | 97.01 (92.58–98.83) | 80.80 (73.02–86.74) | |
| Diagnostic accuracy | 95.98 (91.93–98.04) | 97.09 (93.38–98.75) | 83.23 (76.70–88.21) | |
| PPV | 84.78 (71.78–92.43) | 90.24 (77.45–96.14) | 57.89 (44.98–69.81) | |
| NPV | 100 (97.09–100) | 99.24 (95.80–99.87) | 97.12 (91.86–99.01) | |
| First Nations, eyes | Number of images analysed | 122/132 (92.4) | 120/132 (90.9) | 111/132 (84.1) |
| Ungradable images (total) | 4/132 (3.0) | 7/132 (5.3) | 19/132 (14.4) | |
| Ungradable images that were gradable by ophthalmologists | 0/4 (0) | 2/7(28.6) | 11/19 (57.9) | |
| Sensitivity | 100 (90.59–100) | 97.22 (85.83–99.51) | 91.18 (77.04–96.95) | |
| Specificity | 96.47 (90.13–98.79) | 97.62 (91.73–99.34) | 89.61 (80.82–94.64) | |
| Diagnostic accuracy | 97.54 (93.02–99.16) | 97.50 (92.91–99.15) | 90.09 (83.12–94.38) | |
| PPV | 92.50 (80.14–97.42) | 94.59 (82.30–98.50) | 79.49 (64.47–89.22) | |
| NPV | 100 (95.52–100) | 98.80 (93.49–99.79) | 95.83 (88.45–98.57) | |
| Other Australians, eyes | Number of images analysed | 52/56 (92.9) | 52/56 (92.9) | 50/56 (89.3) |
| Ungradable images (total) | 0/56 (0) | 2/56 (3.6) | 5/56 (8.9) | |
| Ungradable images that were gradable by ophthalmologists | 0 (0) | 0/2 (0) | 2/5 (40.0) | |
| Sensitivity ** | 100 (34.24–100) | 100 (34.24–100) | 100 (34.24–100) | |
| Specificity | 92.00 (81.16–96.85) | 96.00 (86.54–98.90) | 66.67 (52.54–78.32) | |
| Diagnostic accuracy | 92.31 (81.83–96.97) | 96.15 (87.02–98.94) | 68.00 (54.19–79.24) | |
| PPV | 33.33 (9.68–70.00) | 50.00 (15.00–85.00) | 11.11 (3.10–32.80) | |
| NPV | 100 (92.29–100) | 100 (92.59–100) | 100 (89.28–100) | |
| People with diabetes | Number of people analysed | 91/94 (96.8) | 91/94 (96.8) | 87/94 (92.6) |
| Ungradable people’s images (total) | 1/94 (1.1) | 2/94 (2.1) | 7/94 (7.4) | |
| Ungradable people’s images that were gradable by ophthalmologists | 0 | 0 | 4/7 (57.1) | |
| Sensitivity ** | 100 (85.69–100) | 100 (85.69–100) | 95.45 (78.20–99.19) | |
| Specificity | 92.65 (83.91–96.82) | 97.06 (89.90–99.19) | 72.31 (60.42–81.71) | |
| Diagnostic accuracy | 94.51 (87.78–97.63) | 97.80 (92.34–99.40) | 78.16 (68.39–85.55) | |
| PPV | 82.14 (64.41–92.12) | 92.00 (75.03–97.78) | 53.85 (38.57–68.43) | |
| NPV | 100 (94.25–100) | 100 (94.50–100) | 97.92 (89.10–99.63) | |
| Deep Learning System | Google ARDA | Thirona RetCADTM | EyRIS SELENA+ |
|---|---|---|---|
| Sensitivity | 100 (91.03–100) | 97.44 (86.82–99.55) | 92.31 (79.68–97.35) |
| Specificity | 94.81 (89.68–97.47) | 96.30 (91.62–98.41) | 74.81 (66.88–81.38) |
| Diagnostic accuracy | 95.98 (91.93–98.04) | 96.55 (92.68–98.41) | 78.74 (72.07–84.16) |
| PPV | 84.78 (71.78–92.43) | 88.37 (77.52–94.93) | 51.43 (39.95–62.75) |
| NPV | 100 (97.09–100) | 99.24 (95.80–99.87) | 97.12 (91.86–99.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drinkwater, J.J.; Li, Q.; Woods, K.; Douglas, E.; Chia, M.; Zhou, Y.; Bartnik, S.; Shah, Y.; Shah, V.; Keane, P.A.; et al. A Real-World Comparison of Three Deep Learning Systems for Diabetic Retinopathy in Remote Australia. Diabetology 2025, 6, 146. https://doi.org/10.3390/diabetology6120146
Drinkwater JJ, Li Q, Woods K, Douglas E, Chia M, Zhou Y, Bartnik S, Shah Y, Shah V, Keane PA, et al. A Real-World Comparison of Three Deep Learning Systems for Diabetic Retinopathy in Remote Australia. Diabetology. 2025; 6(12):146. https://doi.org/10.3390/diabetology6120146
Chicago/Turabian StyleDrinkwater, Jocelyn J., Qiang Li, Kerry Woods, Emma Douglas, Mark Chia, Yukun Zhou, Steve Bartnik, Yachana Shah, Vaibhav Shah, Pearse A. Keane, and et al. 2025. "A Real-World Comparison of Three Deep Learning Systems for Diabetic Retinopathy in Remote Australia" Diabetology 6, no. 12: 146. https://doi.org/10.3390/diabetology6120146
APA StyleDrinkwater, J. J., Li, Q., Woods, K., Douglas, E., Chia, M., Zhou, Y., Bartnik, S., Shah, Y., Shah, V., Keane, P. A., & Turner, A. W. (2025). A Real-World Comparison of Three Deep Learning Systems for Diabetic Retinopathy in Remote Australia. Diabetology, 6(12), 146. https://doi.org/10.3390/diabetology6120146







