Investigating the Protective Effects of a Rhenium (V) Compound with Uracil-Derived Ligands on Liver Damage Associated with Prediabetes in Diet-Induced Prediabetic Rats
Abstract
:1. Introduction
2. Methods and Materials
2.1. Animals
2.1.1. Induction of Prediabetes on Male Sprague Dawley Rats
2.1.2. Study Design for Experiments
2.1.3. Treatment of Prediabetic Animals
2.1.4. Blood Collection and Tissue Harvesting
2.1.5. Relative Liver Weight
2.2. Biochemical Analysis
2.2.1. Quantification of Hepatic Glycogen, Plasma Triglycerides, TNF α and Liver Function Enzymes
2.2.2. Antioxidant Activity Profile
2.3. Statistical Analysis
3. Results
3.1. Fasting Blood Glucose Concentration
3.2. Liver Glycogen Concentration
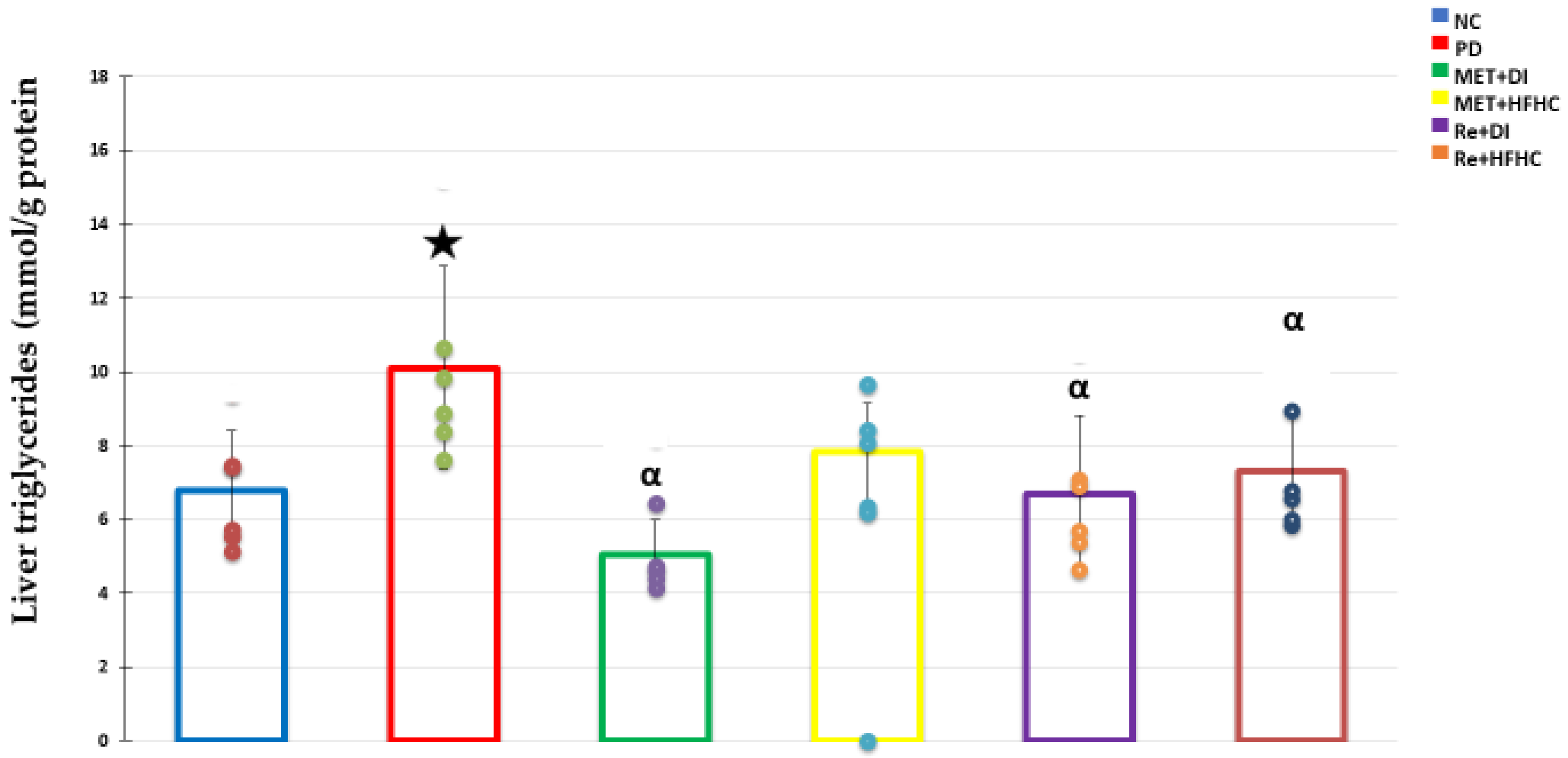
3.3. Liver Triglycerides (TGs) Concentration
3.4. Relative Liver Weight
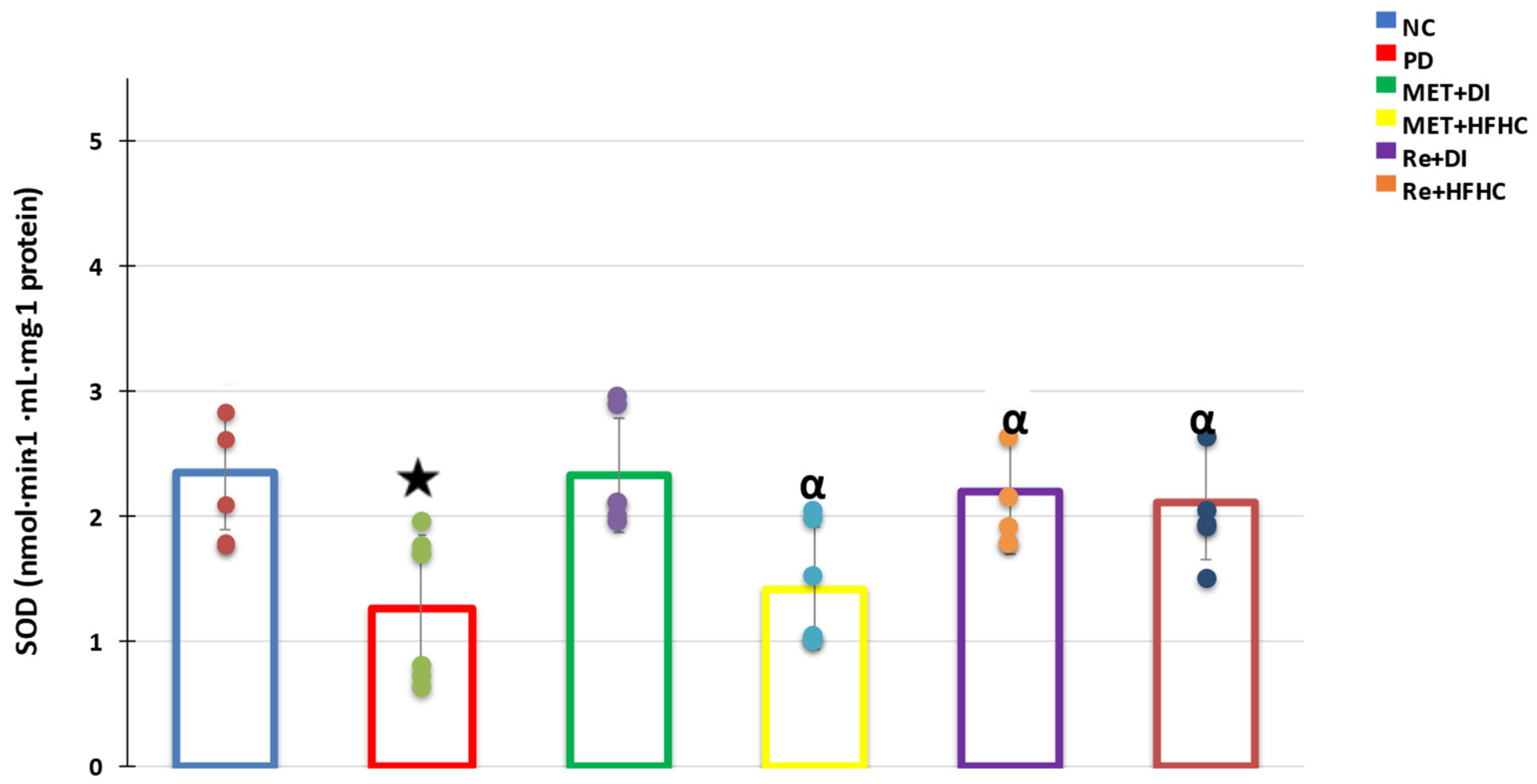
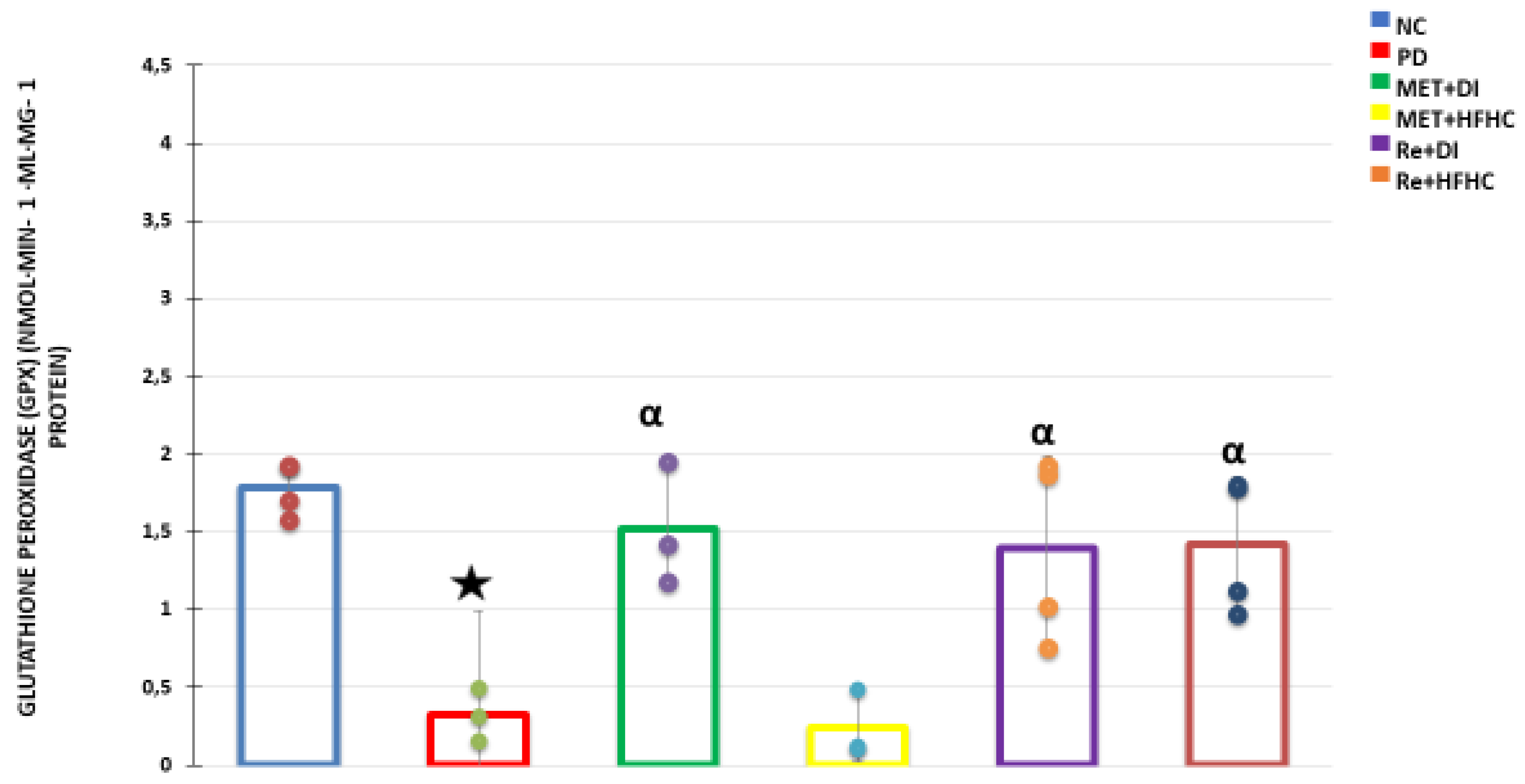
3.5. Liver Antioxidant Activity
3.6. Liver TNF α Concentration
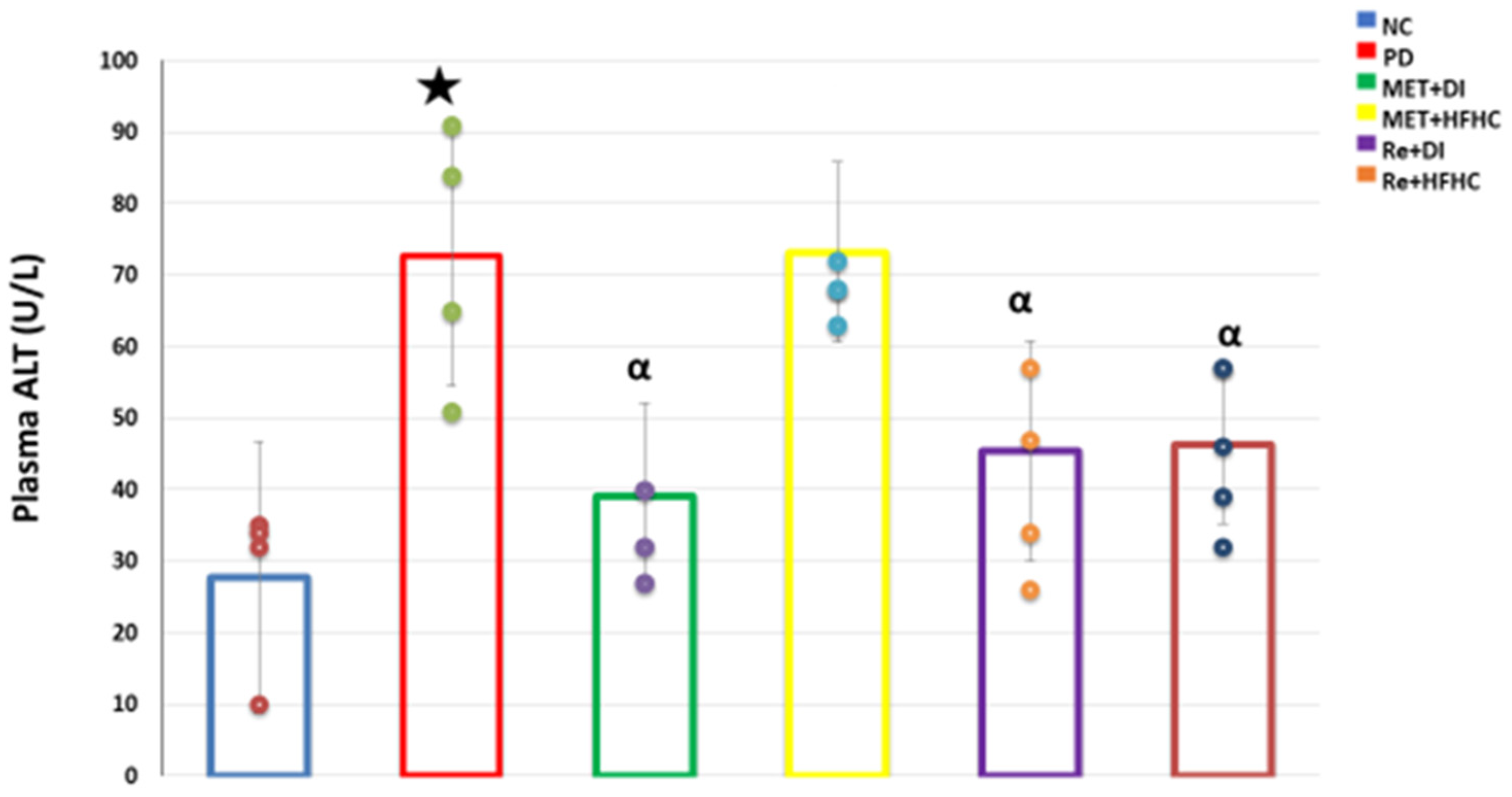
3.7. Plasma ALT Concentration
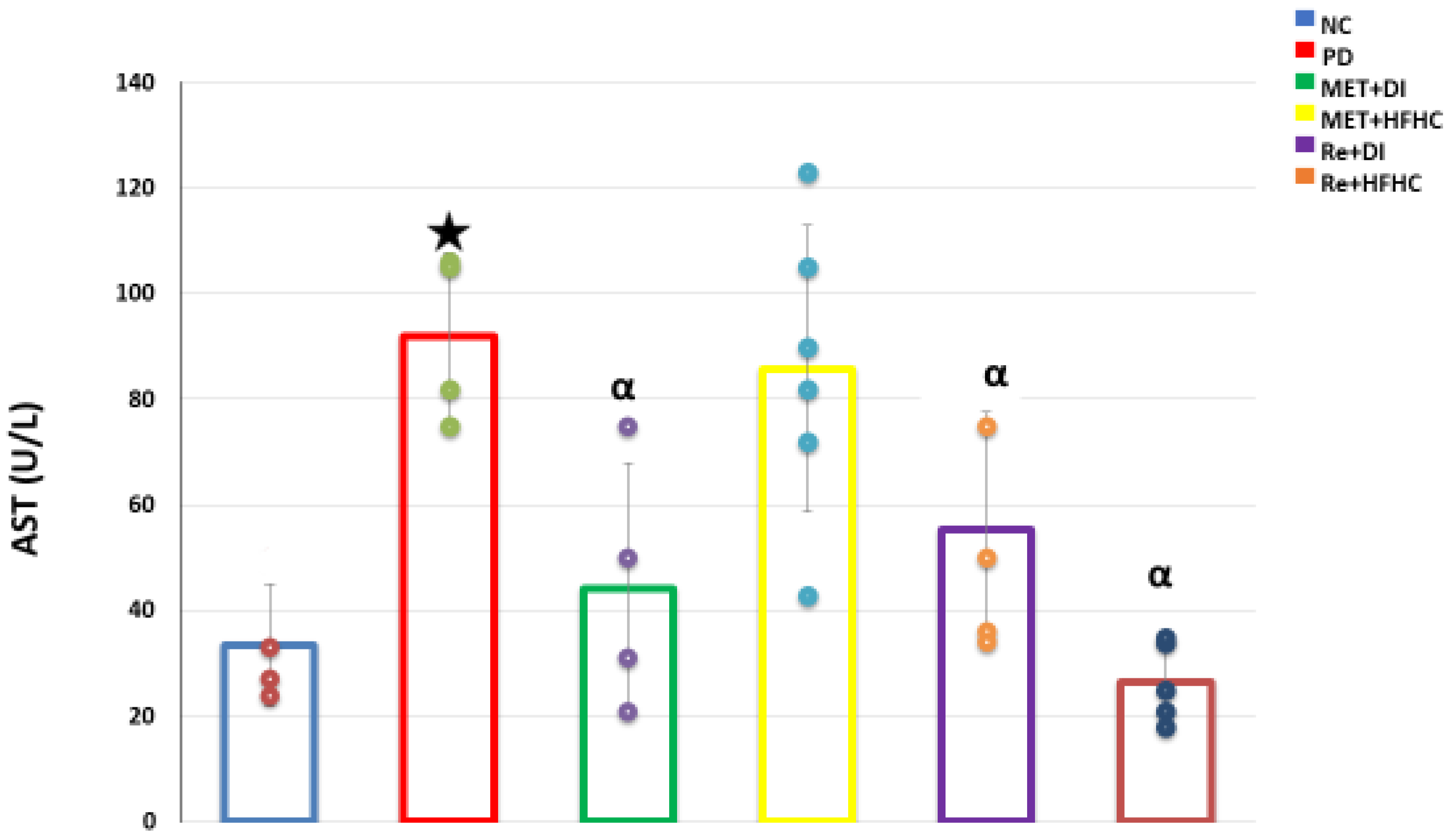
3.8. Plasma AST Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stefan, N.; Häring, H.-U.; Cusi, K. Non-alcoholic fatty liver disease: Causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2018, 7, 313–324. [Google Scholar] [CrossRef]
- Cusi, K.; Sanyal, A.J.; Zhang, S.; Hartman, M.L.; Bue-Valleskey, J.M.; Hoogwerf, B.J.; Haupt, A. Non-alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes. Metab. 2017, 19, 1630–1634. [Google Scholar] [CrossRef] [PubMed]
- Khathi, A.; Luvuno, M.; Mabandla, M. Voluntary Ingestion of a High-Fat High-Carbohydrate Diet: A Model for Prediabetes. Master’s Thesis, University of KwaZulu-Natal, KwaZulu-Natal, South Africa, 2017. [Google Scholar] [CrossRef]
- Maghsoudi, Z.; Ghiasvand, R.; Salehi-Abargouei, A. Empirically derived dietary patterns and incident type 2 diabetes mellitus: A systematic review and meta-analysis on prospective observational studies. Public Health Nutr. 2015, 19, 230–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalender, S.; Apaydin, F.G.; Baş, H.; Kalender, Y. Protective effects of sodium selenite on lead nitrate-induced hepatotoxicity in diabetic and non-diabetic rats. Environ. Toxicol. Pharmacol. 2015, 40, 568–574. [Google Scholar] [CrossRef]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.-H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef] [Green Version]
- Ter Horst, K.W.; Serlie, M.J.J.N. Fructose consumption, lipogenesis, and non-alcoholic fatty liver disease. Nutrients 2017, 9, 981. [Google Scholar] [CrossRef] [Green Version]
- Newton, K.P.; Hou, J.; Crimmins, N.A.; LaVine, J.E.; Barlow, S.E.; Xanthakos, S.A.; Africa, J.; Behling, C.; Donithan, M.; Clark, J.M.; et al. Prevalence of Prediabetes and Type 2 Diabetes in Children With Nonalcoholic Fatty Liver Disease. JAMA Pediatr. 2016, 170, e161971. [Google Scholar] [CrossRef]
- Ortiz-Lopez, C.; Lomonaco, R.; Orsak, B.; Finch, J.; Chang, Z.; Kochunov, V.G.; Hardies, J.; Cusi, K. Prevalence of Prediabetes and Diabetes and Metabolic Profile of Patients With Nonalcoholic Fatty Liver Disease (NAFLD). Diabetes Care 2012, 35, 873–878. [Google Scholar] [CrossRef] [Green Version]
- Sanyal, D.; Mukherjee, P.; Raychaudhuri, M.; Ghosh, S.; Mukherjee, S.; Chowdhury, S. Profile of liver enzymes in non-alcoholic fatty liver disease in patients with impaired glucose tolerance and newly detected untreated type 2 diabetes. Indian J. Endocrinol. Metab. 2015, 19, 597–601. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A.J.B. Non-alcoholic fatty liver disease. BMC medicine. 2017, 15, 45. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, H.B.; Smith, R.J. Fatty liver disease in diabetes mellitus. Hepatobiliary Surg. Nutr. 2015, 4, 101–108. [Google Scholar] [CrossRef]
- Shurrab, N.T.; Arafa, E.-S.A. Metformin: A review of its therapeutic efficacy and adverse effects. Obes. Med. 2020, 17, 100186. [Google Scholar] [CrossRef]
- Gupta, A.; Behl, T.; Sachdeva, M. Key milestones in the diabetes research: A comprehensive update. Obes. Med. 2020, 17, 100183. [Google Scholar] [CrossRef]
- El-Kader, S.M.A.; El-Den Ashmawy, E.M.S. Non-alcoholic fatty liver disease: The diagnosis and management. World J. Hepatol 2015, 7, 846. [Google Scholar] [CrossRef]
- Leite, N.C. Non-alcoholic fatty liver disease and diabetes: From physiopathological interplay to diagnosis and treatment. World J. Gastroenterol. 2014, 20, 8377–8392. [Google Scholar] [CrossRef]
- Malin, S.K.; Gerber, R.; Chipkin, S.R.; Braun, B. Independent and Combined Effects of Exercise Training and Metformin on Insulin Sensitivity in Individuals with Prediabetes. Diabetes Care 2011, 35, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Roberts, S.; Barry, E.; Craig, D.; Airoldi, M.; Bevan, G.; Greenhalgh, T. Preventing type 2 diabetes: Systematic review of studies of cost-effectiveness of lifestyle programmes and metformin, with and without screening, for pre-diabetes. BMJ Open 2017, 7, e017184. [Google Scholar] [CrossRef] [Green Version]
- Mabuza, L.P.; Gamede, M.W.; Maikoo, S.; Booysen, I.N.; Nguban, P.S.; Khathi, A. Hepatoprotective Effects of a Ruthenium(II) Schiff Base Complex in Rats with Diet-Induced Prediabetes. Curr. Ther. Res. 2019, 91, 66–72. [Google Scholar] [CrossRef]
- Siboto, A.; Akinnuga, A.M.; Khumalo, B.N.; Ismail, M.B.; Booysen, I.N.; Sibiya, N.H.; Ngubane, P.S.; Khathi, A. The effects of a [3+1] oxo-free rhenium (V) compound with uracil-derived ligands on selected parameters of glucose homeostasis in diet-induced pre-diabetic rats. Obes. Med. 2020, 19, 100258. [Google Scholar] [CrossRef]
- Mabuza, L.P.; Gamede, M.W.; Maikoo, S.; Booysen, I.N.; Ngubane, P.S.; Khathi, A. Effects of a Ruthenium Schiff Base Complex on Glucose Homeostasis in Diet-Induced Pre-Diabetic Rats. Molecules 2018, 23, 1721. [Google Scholar] [CrossRef] [Green Version]
- Luvuno, M.; Mbongwa, H.; Khathi, A. Development of a novel prediabetes animal model using a high fat high carbohydrate diet: Implications for type 2 diabetes. PLoS ONE 2017, 13, 8–14. [Google Scholar]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- Heikes, K.E.; Eddy, D.M.; Arondekar, B.; Schlessinger, L. Diabetes Risk Calculator: A simple tool for detecting undiagnosed diabetes and pre-diabetes. Diabetes Care 2008, 31, 1040–1045. [Google Scholar] [CrossRef] [Green Version]
- Ngubane, P.S.; Masola, B.; Musabayane, C.T. The Effects of Syzygium aromaticum-Derived Oleanolic Acid on Glycogenic Enzymes in Streptozotocin-Induced Diabetic Rats. Ren. Fail. 2011, 33, 434–439. [Google Scholar] [CrossRef]
- Mkhwanazi, B.N.; Serumula, M.R.; Myburg, R.B.; Van Heerden, F.R.; Musabayane, C.T. Antioxidant effects of maslinic acid in livers, hearts and kidneys of streptozotocin-induced diabetic rats: Effects on kidney function. Ren. Fail. 2013, 36, 419–431. [Google Scholar] [CrossRef]
- Buysschaert, M.; Medina, J.L.; Bergman, M.; Shah, A.; Lonier, J. Prediabetes and associated disorders. Endocrine 2014, 48, 371–393. [Google Scholar] [CrossRef]
- Bungau, S.; Behl, T.; Tit, D.M.; Banica, F.; Bratu, O.G.; Diaconu, C.C.; Nistor-Cseppento, C.D.; Bustea, C.; Aron, R.A.C.; Vesa, C.M. Interactions between leptin and insulin resistance in patients with prediabetes, with and without NAFLD. Exp. Ther. Med. 2020, 20, 1. [Google Scholar] [CrossRef]
- Rajput, R.; Ahlawat, P. Prevalence and predictors of non-alcoholic fatty liver disease in prediabetes. Diabetes Metab. Syndr. 2019, 13, 2957–2960. [Google Scholar] [CrossRef]
- Targher, G.; Corey, K.E.; Byrne, C.D.; Roden, M. The complex link between NAFLD and type 2 diabetes mellitus—Mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 599–612. [Google Scholar] [CrossRef]
- Portillo-Sanchez, P.; Cusi, K. Treatment of Nonalcoholic Fatty Liver Disease (NAFLD) in patients with Type 2 Diabetes Mellitus. Clin. Diabetes Endocrinol. 2016, 2, 9. [Google Scholar] [CrossRef]
- Gaggini, M.; Morelli, M.; Buzzigoli, E.; DeFronzo, R.A.; Bugianesi, E.; Gastaldelli, A. Non-Alcoholic Fatty Liver Disease (NAFLD) and Its Connection with Insulin Resistance, Dyslipidemia, Atherosclerosis and Coronary Heart Disease. Nutrients 2013, 5, 1544–1560. [Google Scholar] [CrossRef] [PubMed]
- Adiels, M.; Taskinen, M.-R.; Borén, J.J.C. Fatty liver, insulin resistance, and dyslipidemia. Curr. Diabetes Rep. 2008, 8, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Chiang, S.-H.; Saltiel, A.R. Insulin Signaling and the Regulation of Glucose Transport. Mol. Med. 2004, 10, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, K.; Tavares, C.D.; Rines, A.K.; Puigserver, P. Molecular pathophysiology of hepatic glucose production. Mol. Asp. Med. 2015, 46, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Katsiki, N.; Mikhailidis, D.P.; Mantzoros, C.S. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism 2016, 65, 1109–1123. [Google Scholar] [CrossRef]
- Sunny, N.E.; Parks, E.J.; Browning, J.D.; Burgess, S.C. Excessive Hepatic Mitochondrial TCA Cycle and Gluconeogenesis in Humans with Nonalcoholic Fatty Liver Disease. Cell Metab. 2011, 14, 804–810. [Google Scholar] [CrossRef] [Green Version]
- Chao, H.-W.; Chao, S.-W.; Lin, H.; Ku, H.-C.; Cheng, C.-F. Homeostasis of Glucose and Lipid in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2019, 20, 298. [Google Scholar] [CrossRef] [Green Version]
- Watterworth, B.; Wright, T.B. Diabetic Peripheral Neuropathy, in Pain; Springer: Berlin/Heidelberg, Germany, 2019; pp. 911–913. [Google Scholar] [CrossRef]
- Bernardes, N.; Ayyappan, P.; De Angelis, K.; Bagchi, A.; Akolkar, G.; Dias, D.D.S.; Belló-Klein, A.; Singal, P.K. Excessive consumption of fructose causes cardiometabolic dysfunctions through oxidative stress and inflammation. Can. J. Physiol. Pharmacol. 2017, 95, 1078–1090. [Google Scholar] [CrossRef] [Green Version]
- Rowell, R.J.; Anstee, Q.M.J.C. An overview of the genetics, mechanisms and management of NAFLD and ALD. Clin. Med. 2015, 15 (Suppl. S6), s77–s82. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathophysiology of prediabetes. Curr. Diabetes Rep. 2009, 9, 193–199. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I.J.T.J. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Sibiya, S.; Msibi, B.; Khathi, A.; Sibiya, N.; Booysen, I.; Ngubane, P. The effect of dioxidovanadium complex (V) on hepatic function in streptozotocin-induced diabetic rats. Can. J. Physiol. Pharmacol. 2019, 97, 1169–1175. [Google Scholar] [CrossRef]
- Kalra, A.; Yetiskul, E.; Wehrle, C.J.; Tuma, F. Physiology, Liver. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Akehi, Y.; Yanase, T.; Motonaga, R.; Umakoshi, H.; Tsuiki, M.; Takeda, Y.; Yoneda, T.; Kurihara, I.; Itoh, H.; Katabami, T.; et al. High Prevalence of Diabetes in Patients With Primary Aldosteronism (PA) Associated With Subclinical Hypercortisolism and Prediabetes More Prevalent in Bilateral Than Unilateral PA: A Large, Multicenter Cohort Study in Japan. Diabetes Care 2019, 42, 938–945. [Google Scholar] [CrossRef] [Green Version]
- Goodpaster, B.H.; DeLany, J.P.; Otto, A.D.; Kuller, L.; Vockley, J.; South-Paul, J.E.; Thomas, S.B.; Brown, J.; McTigue, K.; Hames, K.C.; et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: A randomized trial. JAMA 2010, 304, 1795–1802. [Google Scholar] [CrossRef] [Green Version]
- Manne, V.; Handa, P.; Kowdley, K.V. Pathophysiology of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Clin. Liver Dis. 2018, 22, 23–37. [Google Scholar] [CrossRef]
- Sodum, N.; Kumar, G.; Bojja, S.L.; Kumar, N.; Rao, C.M. Epigenetics in NAFLD/NASH: Targets and therapy. Pharmacol. Res. 2021, 167, 105484. [Google Scholar] [CrossRef]
- Narasimhan, S.; Gokulakrishnan, K.; Sampathkumar, R.; Farooq, S.; Ravikumar, R.; Mohan, V.; Balasubramanyam, M. Oxidative stress is independently associated with non-alcoholic fatty liver disease (NAFLD) in subjects with and without type 2 diabetes. Clin. Biochem. 2010, 43, 815–821. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2015, 24, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef]
- Videla, L.A.; Rodrigo, R.; Araya, J.; Poniachik, J. Insulin resistance and oxidative stress interdependency in non-alcoholic fatty liver disease. Trends Mol. Med. 2006, 12, 555–558. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxidative Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef]
- Clark, J.M.; Brancati, F.L.; Diehl, A.M.J.G. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 122, 1649–1657. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.B.; Booysen, I.N. Complexes of the fac-[Re(CO)3]+ and [ReO]3+ Moieties with Aromatic, Multidentate, NDonor Ligands. Ther. Drug Monit. 2013, 357, 1499–1516. [Google Scholar]
- Ismail, M.B.; Booysen, I.N.; Akerman, M. DNA interaction studies of rhenium compounds with Schiff base chelates encompassing biologically relevant moieties. Nucleosides Nucleotides Nucleic Acids 2019, 38, 950–971. [Google Scholar] [CrossRef]
- Ismail, M.B.; Booysen, I.N.; Akerman, M.P. Rhenium(I) complexes with aliphatic Schiff bases appended to bio-active moieties. Inorg. Chem. Commun. 2017, 78, 78–81. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siboto, A.; Akinnuga, A.M.; Ismail, M.B.; Booysen, I.N.; Sibiya, N.H.; Ngubane, P.; Khathi, A. Investigating the Protective Effects of a Rhenium (V) Compound with Uracil-Derived Ligands on Liver Damage Associated with Prediabetes in Diet-Induced Prediabetic Rats. Diabetology 2022, 3, 524-538. https://doi.org/10.3390/diabetology3040040
Siboto A, Akinnuga AM, Ismail MB, Booysen IN, Sibiya NH, Ngubane P, Khathi A. Investigating the Protective Effects of a Rhenium (V) Compound with Uracil-Derived Ligands on Liver Damage Associated with Prediabetes in Diet-Induced Prediabetic Rats. Diabetology. 2022; 3(4):524-538. https://doi.org/10.3390/diabetology3040040
Chicago/Turabian StyleSiboto, Angezwa, Akinjide Moses Akinnuga, Muhammed Bilaal Ismail, Irvin Noel Booysen, Ntethelelo Hopewell Sibiya, Phikelelani Ngubane, and Andile Khathi. 2022. "Investigating the Protective Effects of a Rhenium (V) Compound with Uracil-Derived Ligands on Liver Damage Associated with Prediabetes in Diet-Induced Prediabetic Rats" Diabetology 3, no. 4: 524-538. https://doi.org/10.3390/diabetology3040040
APA StyleSiboto, A., Akinnuga, A. M., Ismail, M. B., Booysen, I. N., Sibiya, N. H., Ngubane, P., & Khathi, A. (2022). Investigating the Protective Effects of a Rhenium (V) Compound with Uracil-Derived Ligands on Liver Damage Associated with Prediabetes in Diet-Induced Prediabetic Rats. Diabetology, 3(4), 524-538. https://doi.org/10.3390/diabetology3040040





