Acetaminophen-Induced Hepatotoxicity in Obesity and Nonalcoholic Fatty Liver Disease: A Critical Review
Abstract
:1. Introduction
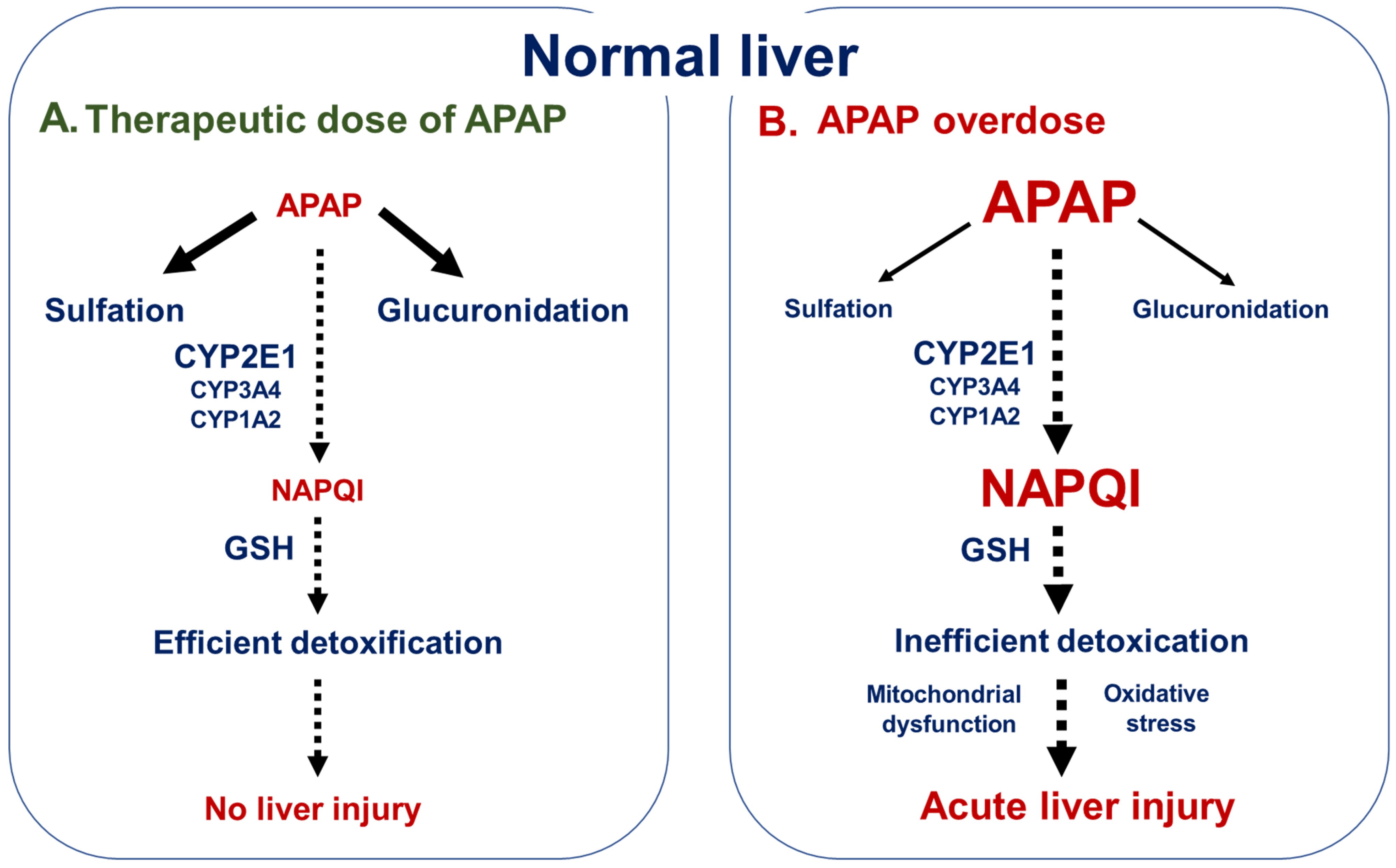
2. APAP Hepatotoxicity
2.1. General Overview
2.2. Predisposing Factors
3. APAP Hepatotoxicity in NAFLD
3.1. Main Features of NAFLD
3.2. Clinical Investigations on Acute APAP Hepatotoxicity in Obesity and NAFLD
3.3. Rodent Studies on Acute APAP Hepatotoxicity in Obesity and NAFLD
3.4. In Vitro Studies on Acute APAP Hepatotoxicity in Models of Fatty Acid Exposure and NAFLD
3.5. Investigations on Chronic APAP Hepatotoxicity in Obesity and NAFLD
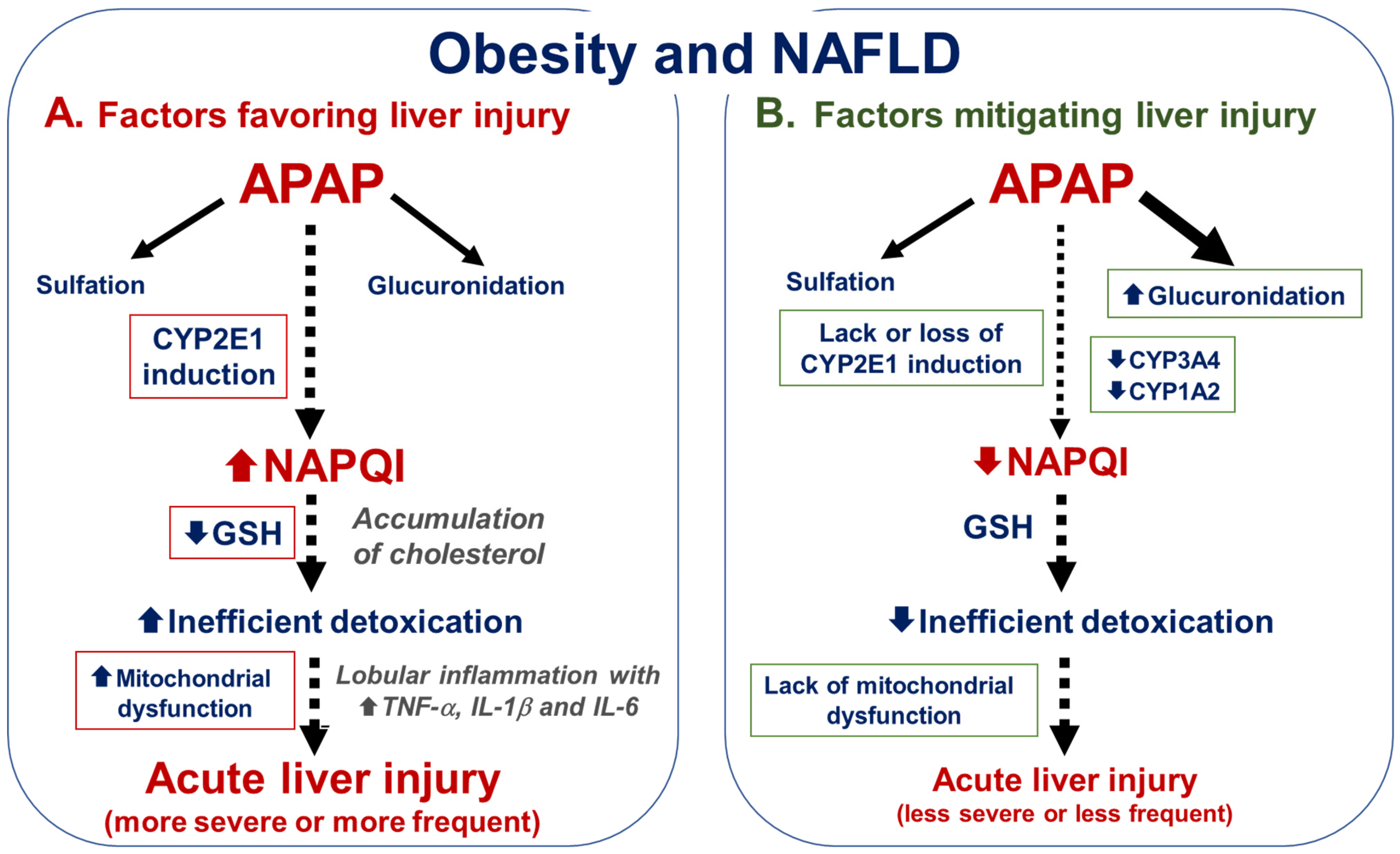
4. Factors Modulating APAP Hepatotoxicity in Obesity and NAFLD
4.1. Factors That Could Favor APAP Hepatotoxicity in Obesity and NAFLD
4.1.1. CYP2E1 Induction
4.1.2. Low Basal Levels of GSH
4.1.3. Extent of Steatosis and Accumulation of Deleterious Fatty Acids and Lipid Species
4.1.4. Mitochondrial Dysfunction
4.1.5. Presence of Lobular Inflammation
4.2. Factors That Could Mitigate APAP Hepatotoxicity in Obesity and NAFLD
4.2.1. Alteration in APAP Absorption and Distribution
4.2.2. Lack of CYP2E1 Induction or CYP2E1 Downregulation
4.2.3. Reduced CYP3A4 and CYP1A2 Activity
4.2.4. Increased APAP Glucuronidation
4.2.5. Exposure and Accumulation of Protective Fatty Acids
5. APAP-Induced Liver Injury after Bariatric Surgery
6. APAP-Hepatotoxicity in Type 1 Diabetes Mellitus
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrett, L.A.; Xing, A.; Sheffler, J.; Steidley, E.; Adam, T.J.; Zhang, R.; He, Z. Assessing the Use of Prescription Drugs and Dietary Supplements in Obese Respondents in the National Health and Nutrition Examination Survey. PLoS ONE 2022, 17, e0269241. [Google Scholar] [CrossRef] [PubMed]
- Pulipati, V.P.; Pannain, S. Pharmacotherapy of Obesity in Complex Diseases. Clin. Obes. 2022, 12, e12497. [Google Scholar] [CrossRef] [PubMed]
- Chew, N.W.S.; Ng, C.H.; Truong, E.; Noureddin, M.; Kowdley, K.V. Nonalcoholic Steatohepatitis Drug Development Pipeline: An Update. Semin. Liver Dis. 2022, 42, 379–400. [Google Scholar] [CrossRef]
- Negi, C.K.; Babica, P.; Bajard, L.; Bienertova-Vasku, J.; Tarantino, G. Insights into the Molecular Targets and Emerging Pharmacotherapeutic Interventions for Nonalcoholic Fatty Liver Disease. Metabolism 2022, 126, 154925. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.A.; Hilmer, S.N.; Reeve, E.; Potter, K.; Le Couteur, D.; Rigby, D.; Gnjidic, D.; Del Mar, C.B.; Roughead, E.E.; Page, A.; et al. Reducing Inappropriate Polypharmacy: The Process of Deprescribing. JAMA Intern. Med. 2015, 175, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A.; Yuen, N.A.; Ilic, K.; Miller, R.T.; Reese, M.J.; Brown, H.R.; Ambroso, J.I.; Falls, J.G.; Hunt, C.M. Comedications Alter Drug-Induced Liver Injury Reporting Frequency: Data Mining in the WHO VigiBaseTM. Regul. Toxicol. Pharm. 2015, 72, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Lammert, C.; Imler, T.; Teal, E.; Chalasani, N. Patients With Chronic Liver Disease Suggestive of Nonalcoholic Fatty Liver Disease May Be at Higher Risk for Drug-Induced Liver Injury. Clin. Gastroenterol. Hepatol. 2019, 17, 2814–2815. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.; Won, S.; Lee, S. Nonalcoholic Fatty Liver Disease for the Incidence of Drug-Induced Liver Injury. Clin. Gastroenterol. Hepatol. 2022, 20, 964–965. [Google Scholar] [CrossRef]
- Michaut, A.; Moreau, C.; Robin, M.-A.; Fromenty, B. Acetaminophen-Induced Liver Injury in Obesity and Nonalcoholic Fatty Liver Disease. Liver Int. 2014, 34, e171–e179. [Google Scholar] [CrossRef]
- Massart, J.; Begriche, K.; Fromenty, B. Cytochrome P450 2E1 Should Not Be Neglected for Acetaminophen-Induced Liver Injury in Metabolic Diseases with Altered Insulin Levels or Glucose Homeostasis. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101470. [Google Scholar] [CrossRef]
- Borlak, J.; Chatterji, B.; Londhe, K.B.; Watkins, P.B. Serum Acute Phase Reactants Hallmark Healthy Individuals at Risk for Acetaminophen-Induced Liver Injury. Genome Med. 2013, 5, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louvet, A.; Ntandja Wandji, L.C.; Lemaître, E.; Khaldi, M.; Lafforgue, C.; Artru, F.; Quesnel, B.; Lassailly, G.; Dharancy, S.; Mathurin, P. Acute Liver Injury With Therapeutic Doses of Acetaminophen: A Prospective Study. Hepatology 2021, 73, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Massart, J.; Begriche, K.; Moreau, C.; Fromenty, B. Role of Nonalcoholic Fatty Liver Disease as Risk Factor for Drug-Induced Hepatotoxicity. J. Clin. Transl. Res. 2017, 3, 212–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licata, M.; Minisalle, M.G.; Stankeviciute, S.; Sanabria-Cabrera, J.; Lucena, M.M.; Andrade, R.J.; Almasio, P.L. N-Acetylcysteine for Preventing Acetaminophen-Induced Liver Injury: A Comprehensive Review. Front. Pharm. 2022, 13, 828565. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.M.; Polson, J.; Fontana, R.J.; Davern, T.J.; Lalani, E.; Hynan, L.S.; Reisch, J.S.; Schiodt, F.V.; Ostapowicz, G.; Shakil, A.O.; et al. Acetaminophen-Induced Acute Liver Failure: Results of a United States Multicenter, Prospective Study. Hepatology 2005, 42, 1364–1372. [Google Scholar] [CrossRef]
- Yoon, E.; Babar, A.; Choudhary, M.; Kutner, M.; Pyrsopoulos, N. Acetaminophen-Induced Hepatotoxicity: A Comprehensive Update. J. Clin. Transl. Hepatol. 2016, 4, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, A.; Jaeschke, H. A Mitochondrial Journey through Acetaminophen Hepatotoxicity. Food Chem. Toxicol. 2020, 140, 111282. [Google Scholar] [CrossRef]
- Biour, M.; Ben Salem, C.; Chazouillères, O.; Grangé, J.-D.; Serfaty, L.; Poupon, R. Drug-induced liver injury; fourteenth updated edition of the bibliographic database of liver injuries and related drugs. Gastroenterol. Clin. Biol. 2004, 28, 720–759. [Google Scholar] [CrossRef]
- Watelet, J.; Laurent, V.; Bressenot, A.; Bronowicki, J.-P.; Larrey, D.; Peyrin-Biroulet, L. Toxicity of Chronic Paracetamol Ingestion. Aliment. Pharm. 2007, 26, 1543–1544. [Google Scholar] [CrossRef]
- Yaghi, C.; Assaf, A. Acetaminophen Toxicity at Therapeutic Doses. Intern. Med. Rev. 2017, 3, 1–13. [Google Scholar]
- McGill, M.R.; Hinson, J.A. The Development and Hepatotoxicity of Acetaminophen: Reviewing over a Century of Progress. Drug Metab. Rev. 2020, 52, 472–500. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H. Acetaminophen Hepatotoxicity: Not as Simple as One Might Think! Introductory Comments on the Special Issue-Recent Advances in Acetaminophen Hepatotoxicity. Livers 2022, 2, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Checa, J.C.; Bagnaninchi, P.; Ye, H.; Sancho-Bru, P.; Falcon-Perez, J.M.; Royo, F.; Garcia-Ruiz, C.; Konu, O.; Miranda, J.; Lunov, O.; et al. Advanced Preclinical Models for Evaluation of Drug-Induced Liver Injury–Consensus Statement by the European Drug-Induced Liver Injury Network [PRO-EURO-DILI-NET]. J. Hepatol. 2021, 75, 935–959. [Google Scholar] [CrossRef] [PubMed]
- Knockaert, L.; Descatoire, V.; Vadrot, N.; Fromenty, B.; Robin, M.-A. Mitochondrial CYP2E1 Is Sufficient to Mediate Oxidative Stress and Cytotoxicity Induced by Ethanol and Acetaminophen. Toxicol. Vitr. 2011, 25, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Massart, J.; Begriche, K.; Hartman, J.H.; Fromenty, B. Role of Mitochondrial Cytochrome P450 2E1 in Healthy and Diseased Liver. Cells 2022, 11, 288. [Google Scholar] [CrossRef]
- Brackett, C.C.; Bloch, J.D. Phenytoin as a Possible Cause of Acetaminophen Hepatotoxicity: Case Report and Review of the Literature. Pharmacotherapy 2000, 20, 229–233. [Google Scholar] [CrossRef]
- McGill, M.R.; Jaeschke, H. Metabolism and Disposition of Acetaminophen: Recent Advances in Relation to Hepatotoxicity and Diagnosis. Pharm. Res. 2013, 30, 2174–2187. [Google Scholar] [CrossRef] [Green Version]
- Caparrotta, T.M.; Antoine, D.J.; Dear, J.W. Are Some People at Increased Risk of Paracetamol-Induced Liver Injury? A Critical Review of the Literature. Eur. J. Clin. Pharm. 2018, 74, 147–160. [Google Scholar] [CrossRef] [Green Version]
- Hidaka, N.; Kaji, Y.; Takatori, S.; Tanaka, A.; Matsuoka, I.; Tanaka, M. Risk Factors for Acetaminophen-Induced Liver Injury: A Single-Center Study from Japan. Clin. Ther. 2020, 42, 704–710. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Sakai, H.; Hirata, A.; Yanai, T. Effects of Food Restriction on the Expression of Genes Related to Acetaminophen-Induced Liver Toxicity in Rats. J. Toxicol. Pathol. 2018, 31, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Court, M.H.; Freytsis, M.; Wang, X.; Peter, I.; Guillemette, C.; Hazarika, S.; Duan, S.X.; Greenblatt, D.J.; Lee, W.M. Acute Liver Failure Study Group The UDP-Glucuronosyltransferase (UGT) 1A Polymorphism c.2042C>G (Rs8330) Is Associated with Increased Human Liver Acetaminophen Glucuronidation, Increased UGT1A Exon 5a/5b Splice Variant MRNA Ratio, and Decreased Risk of Unintentional Acetaminophen-Induced Acute Liver Failure. J. Pharm. Exp. 2013, 345, 297–307. [Google Scholar] [CrossRef]
- Henry, L.; Paik, J.; Younossi, Z.M. Review Article: The Epidemiologic Burden of Non-Alcoholic Fatty Liver Disease across the World. Aliment. Pharm. 2022, 56, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Kleiner, D.E.; Carpenter, D.H.; Rinella, M.; Harrison, S.A.; Loomba, R.; Younossi, Z.; Neuschwander-Tetri, B.A.; Sanyal, A.J. American Association for the Study of Liver Diseases NASH Task Force NAFLD: Reporting Histologic Findings in Clinical Practice. Hepatology 2021, 73, 2028–2038. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.I.; Khan, M.U.; Kodali, S.; Shetty, A.; Bell, S.M.; Victor, D. Hepatocellular Carcinoma Due to Nonalcoholic Fatty Liver Disease: Current Concepts and Future Challenges. J. Hepatocell. Carcinoma 2022, 9, 477–496. [Google Scholar] [CrossRef]
- Koch, L.K.; Yeh, M.M. Nonalcoholic Fatty Liver Disease (NAFLD): Diagnosis, Pitfalls, and Staging. Ann. Diagn. Pathol. 2018, 37, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Tandra, S.; Yeh, M.M.; Brunt, E.M.; Vuppalanchi, R.; Cummings, O.W.; Ünalp-Arida, A.; Wilson, L.A.; Chalasani, N. Presence and Significance of Microvesicular Steatosis in Nonalcoholic Fatty Liver Disease. J. Hepatol. 2011, 55, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begriche, K.; Massart, J.; Robin, M.-A.; Bonnet, F.; Fromenty, B. Mitochondrial Adaptations and Dysfunctions in Nonalcoholic Fatty Liver Disease. Hepatology 2013, 58, 1497–1507. [Google Scholar] [CrossRef]
- Sunny, N.E.; Bril, F.; Cusi, K. Mitochondrial Adaptation in Nonalcoholic Fatty Liver Disease: Novel Mechanisms and Treatment Strategies. Trends Endocrinol. Metab. 2017, 28, 250–260. [Google Scholar] [CrossRef]
- Pafili, K.; Roden, M. Nonalcoholic Fatty Liver Disease (NAFLD) from Pathogenesis to Treatment Concepts in Humans. Mol. Metab. 2021, 50, 101122. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Sam, J.; Thuluvath, P.J. Hepatitis C Is a Predictor of Acute Liver Injury among Hospitalizations for Acetaminophen Overdose in the United States: A Nationwide Analysis. Hepatology 2008, 48, 1336–1341. [Google Scholar] [CrossRef]
- Myers, R.P.; Shaheen, A.A.M. Hepatitis C, Alcohol Abuse, and Unintentional Overdoses Are Risk Factors for Acetaminophen-Related Hepatotoxicity. Hepatology 2009, 49, 1399–1400. [Google Scholar] [CrossRef] [PubMed]
- Chomchai, S.; Chomchai, C. Being Overweight or Obese as a Risk Factor for Acute Liver Injury Secondary to Acute Acetaminophen Overdose. Pharm. Drug Saf. 2018, 27, 19–24. [Google Scholar] [CrossRef] [PubMed]
- van Rongen, A.; Välitalo, P.A.J.; Peeters, M.Y.M.; Boerma, D.; Huisman, F.W.; van Ramshorst, B.; van Dongen, E.P.A.; van den Anker, J.N.; Knibbe, C.A.J. Morbidly Obese Patients Exhibit Increased CYP2E1-Mediated Oxidation of Acetaminophen. Clin. Pharm. 2016, 55, 833–847. [Google Scholar] [CrossRef] [Green Version]
- Rutherford, A.; Davern, T.; Hay, J.E.; Murray, N.G.; Hassanein, T.; Lee, W.M.; Chung, R.T. Acute Liver Failure Study Group Influence of High Body Mass Index on Outcome in Acute Liver Failure. Clin. Gastroenterol. Hepatol. 2006, 4, 1544–1549. [Google Scholar] [CrossRef] [PubMed]
- Radosevich, J.J.; Patanwala, A.E.; Erstad, B.L. Hepatotoxicity in Obese Versus Nonobese Patients With Acetaminophen Poisoning Who Are Treated With Intravenous N-Acetylcysteine. Am. J. Ther. 2016, 23, e714–e719. [Google Scholar] [CrossRef]
- Allard, J.; Le Guillou, D.; Begriche, K.; Fromenty, B. Drug-Induced Liver Injury in Obesity and Nonalcoholic Fatty Liver Disease. Adv. Pharm. 2019, 85, 75–107. [Google Scholar] [CrossRef]
- Corcoran, G.B.; Wong, B.K. Obesity as a Risk Factor in Drug-Induced Organ Injury: Increased Liver and Kidney Damage by Acetaminophen in the Obese Overfed Rat. J. Pharm. Exp. Ther. 1987, 241, 921–927. [Google Scholar]
- Kon, K.; Ikejima, K.; Okumura, K.; Arai, K.; Aoyama, T.; Watanabe, S. Diabetic KK-A(y) Mice Are Highly Susceptible to Oxidative Hepatocellular Damage Induced by Acetaminophen. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G329–G337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubert, J.; Begriche, K.; Delannoy, M.; Morel, I.; Pajaud, J.; Ribault, C.; Lepage, S.; McGill, M.R.; Lucas-Clerc, C.; Turlin, B.; et al. Differences in Early Acetaminophen Hepatotoxicity between Obese Ob/Ob and Db/Db Mice. J. Pharm. Exp. Ther. 2012, 342, 676–687. [Google Scholar] [CrossRef] [Green Version]
- Kučera, O.; Roušar, T.; Staňková, P.; Haňáčková, L.; Lotková, H.; Podhola, M.; Cervinková, Z. Susceptibility of Rat Non-Alcoholic Fatty Liver to the Acute Toxic Effect of Acetaminophen. J. Gastroenterol. Hepatol. 2012, 27, 323–330. [Google Scholar] [CrossRef]
- Piccinin, E.; Ducheix, S.; Peres, C.; Arconzo, M.; Vegliante, M.C.; Ferretta, A.; Bellafante, E.; Villani, G.; Moschetta, A. PGC-1β Induces Susceptibility To Acetaminophen-Driven Acute Liver Failure. Sci. Rep. 2019, 9, 16821. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Xue, W.; Han, B.; Yang, F.; Yin, Y.; Hu, C. Acetaminophen Aggravates Fat Accumulation in NAFLD by Inhibiting Autophagy via the AMPK/MTOR Pathway. Eur. J. Pharm. 2019, 850, 15–22. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, W.; Xin, J.; Xue, W.; Shi, C.; Wen, J.; Huang, Y.; Hu, C. Caveolin-1 Alleviates Acetaminophen-Induced Fat Accumulation in Non-Alcoholic Fatty Liver Disease by Enhancing Hepatic Antioxidant Ability via Activating AMPK Pathway. Front. Pharm. 2021, 12, 717276. [Google Scholar] [CrossRef]
- Tuntaterdtum, S.; Chaudhary, I.P.; Cibull, M.; Robertson, L.W.; Blouin, R.A. Acetaminophen Hepatotoxicity: Influence of Phenobarbital and Beta-Naphthoflavone Treatment in Obese and Lean Zucker Rats. Toxicol. Appl. Pharm. 1993, 123, 219–225. [Google Scholar] [CrossRef]
- Ito, Y.; Abril, E.R.; Bethea, N.W.; McCuskey, M.K.; McCuskey, R.S. Dietary Steatotic Liver Attenuates Acetaminophen Hepatotoxicity in Mice. Microcirculation 2006, 13, 19–27. [Google Scholar] [CrossRef]
- Kim, T.H.; Choi, D.; Kim, J.Y.; Lee, J.H.; Koo, S.-H. Fast Food Diet-Induced Non-Alcoholic Fatty Liver Disease Exerts Early Protective Effect against Acetaminophen Intoxication in Mice. BMC Gastroenterol. 2017, 17, 124. [Google Scholar] [CrossRef] [Green Version]
- Ghallab, A.; Myllys, M.; Friebel, A.; Duda, J.; Edlund, K.; Halilbasic, E.; Vucur, M.; Hobloss, Z.; Brackhagen, L.; Begher-Tibbe, B.; et al. Spatio-Temporal Multiscale Analysis of Western Diet-Fed Mice Reveals a Translationally Relevant Sequence of Events during NAFLD Progression. Cells 2021, 10, 2516. [Google Scholar] [CrossRef]
- Blouin, R.A.; Dickson, P.; McNamara, P.J.; Cibull, M.; McClain, C. Phenobarbital induction and acetaminophen hepatotoxi-city: Resistance in the obese Zucker rodent. J. Pharm. Exp. Ther. 1987, 243, 270–565. [Google Scholar]
- Donthamsetty, S.; Bhave, V.S.; Mitra, M.S.; Latendresse, J.R.; Mehendale, H.M. Nonalcoholic Steatohepatitic (NASH) Mice Are Protected from Higher Hepatotoxicity of Acetaminophen upon Induction of PPARalpha with Clofibrate. Toxicol. Appl. Pharm. 2008, 230, 327–337. [Google Scholar] [CrossRef]
- Jahn, D.; Kircher, S.; Hermanns, H.M.; Geier, A. Animal Models of NAFLD from a Hepatologist’s Point of View. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 943–953. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Yeung, S.F.; Ke, J.-Y.; Antunes, M.M.; Pellizzon, M.A. Considerations When Choosing High-Fat, High-Fructose, and High-Cholesterol Diets to Induce Experimental Nonalcoholic Fatty Liver Disease in Laboratory Animal Models. Curr. Dev. Nutr. 2021, 5, nzab138. [Google Scholar] [CrossRef]
- Carreres, L.; Jilkova, Z.M.; Vial, G.; Marche, P.N.; Decaens, T.; Lerat, H. Modeling Diet-Induced NAFLD and NASH in Rats: A Comprehensive Review. Biomedicines 2021, 9, 378. [Google Scholar] [CrossRef]
- Carmiel-Haggai, M.; Cederbaum, A.I.; Nieto, N. Binge Ethanol Exposure Increases Liver Injury in Obese Rats. Gastroenterology 2003, 125, 1818–1833. [Google Scholar] [CrossRef]
- Cipriani, S.; Mencarelli, A.; Palladino, G.; Fiorucci, S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J. Lipid Res. 2010, 51, 771–784. [Google Scholar] [CrossRef] [Green Version]
- Zaluzny, L.; Farrell, G.C.; Murray, M. Effect of genetic obesity and experimental diabetes on hepatic microsomal mixed function oxidase activities. J. Gastroenterol. Hepatol. 1990, 5, 256–263. [Google Scholar] [CrossRef]
- Trak-Smayra, V.; Paradis, V.; Massart, J.; Nasser, S.; Jebara, V.; Fromenty, B. Pathology of the Liver in Obese and Diabetic Ob/Ob and Db/Db Mice Fed a Standard or High-Calorie Diet. Int. J. Exp. Pathol. 2011, 92, 413–421. [Google Scholar] [CrossRef]
- Robin, M.A.; Demeilliers, C.; Sutton, A.; Paradis, V.; Maisonneuve, C.; Dubois, S.; Poirel, O.; Lettéron, P.; Pessayre, D.; Fromenty, B. Alcohol increases tumor necrosis factor alpha and decreases nuclear factor-kB to activate hepatic apoptosis in genetically obese mice. Hepatology 2005, 42, 1280–1290. [Google Scholar] [CrossRef]
- Enriquez, A.; Leclercq, I.; Farrell, G.C.; Robertson, G. Altered Expression of Hepatic CYP2E1 and CYP4A in Obese, Diabetic Ob/Ob Mice, and Fa/Fa Zucker Rats. Biochem. Biophys. Res. Commun. 1999, 255, 300–306. [Google Scholar] [CrossRef]
- Okumura, K.; Ikejima, K.; Kon, K.; Abe, W.; Yamashina, S.; Enomoto, N.; Takei, Y.; Sato, N. Exacerbation of dietary steatohepatitis and fibrosis in obese, diabetic KK-A(y) mice. Hepatol. Res. 2006, 36, 217–228. [Google Scholar] [CrossRef]
- Teraoka, N.; Mutoh, M.; Takasu, S.; Ueno, T.; Nakano, K.; Takahashi, M.; Imai, T.; Masuda, S.; Sugimura, T.; Wakabayashi, K. High susceptibility to azoxymethane-induced colorectal carcinogenesis in obese KK-Ay mice. Int. J. Cancer 2011, 129, 528–535. [Google Scholar] [CrossRef]
- McDanell, R.E.; Beales, D.; Henderson, L.; Sethi, J.K. Effect of Dietary Fat on the in Vitro Hepatotoxicity of Paracetamol. Biochem. Pharm. 1992, 44, 1303–1306. [Google Scholar] [CrossRef]
- Kučera, O.; Al-Dury, S.; Lotková, H.; Roušar, T.; Rychtrmoc, D.; Červinková, Z. Steatotic Rat Hepatocytes in Primary Culture Are More Susceptible to the Acute Toxic Effect of Acetaminophen. Physiol. Res. 2012, 61, S93–S101. [Google Scholar] [CrossRef]
- Yang, J.; Peng, T.; Huang, J.; Zhang, G.; Xia, J.; Ma, M.; Deng, D.; Gong, D.; Zeng, Z. Effects of Medium- and Long-Chain Fatty Acids on Acetaminophen- or Rifampicin-Induced Hepatocellular Injury. Food Sci. Nutr. 2020, 8, 3590–3601. [Google Scholar] [CrossRef]
- Michaut, A.; Le Guillou, D.; Moreau, C.; Bucher, S.; McGill, M.R.; Martinais, S.; Gicquel, T.; Morel, I.; Robin, M.-A.; Jaeschke, H.; et al. A Cellular Model to Study Drug-Induced Liver Injury in Nonalcoholic Fatty Liver Disease: Application to Acetaminophen. Toxicol. Appl. Pharm. 2016, 292, 40–55. [Google Scholar] [CrossRef] [Green Version]
- Hubel, E.; Fishman, S.; Holopainen, M.; Käkelä, R.; Shaffer, O.; Houri, I.; Zvibel, I.; Shibolet, O. Repetitive Amiodarone Administration Causes Liver Damage via Adipose Tissue ER Stress-Dependent Lipolysis, Leading to Hepatotoxic Free Fatty Acid Accumulation. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G298–G307. [Google Scholar] [CrossRef]
- Mathur, M.; Yeh, Y.-T.; Arya, R.K.; Jiang, L.; Pornour, M.; Chen, W.; Ma, Y.; Gao, B.; He, L.; Ying, Z.; et al. Adipose Lipolysis Is Important for Ethanol to Induce Fatty Liver in the National Institute on Alcohol Abuse and Alcoholism Murine Model of Chronic and Binge Ethanol Feeding. Hepatology 2022. [Google Scholar] [CrossRef]
- Toyoda, T.; Cho, Y.-M.; Akagi, J.-I.; Mizuta, Y.; Matsushita, K.; Nishikawa, A.; Imaida, K.; Ogawa, K. A 13-Week Subchronic Toxicity Study of Acetaminophen Using an Obese Rat Model. J. Toxicol. Sci. 2018, 43, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Kondo, K.; Yamada, N.; Suzuki, Y.; Hashimoto, T.; Toyoda, K.; Takahashi, T.; Kobayashi, A.; Sugai, S.; Yoshinari, K. Enhancement of Acetaminophen-Induced Chronic Hepatotoxicity in Spontaneously Diabetic Torii (SDT) Rats. J. Toxicol. Sci. 2020, 45, 245–260. [Google Scholar] [CrossRef]
- Arconzo, M.; Piccinin, E.; Moschetta, A. Increased Risk of Acute Liver Failure by Pain Killer Drugs in NAFLD: Focus on Nuclear Receptors and Their Coactivators. Dig. Liver Dis. 2021, 53, 26–34. [Google Scholar] [CrossRef]
- Chalasani, N.; Gorski, J.C.; Asghar, M.S.; Asghar, A.; Foresman, B.; Hall, S.D.; Crabb, D.W. Hepatic Cytochrome P450 2E1 Activity in Nondiabetic Patients with Nonalcoholic Steatohepatitis. Hepatology 2003, 37, 544–550. [Google Scholar] [CrossRef]
- Chtioui, H.; Semela, D.; Ledermann, M.; Zimmermann, A.; Dufour, J.-F. Expression and Activity of the Cytochrome P450 2E1 in Patients with Nonalcoholic Steatosis and Steatohepatitis. Liver Int. 2007, 27, 764–771. [Google Scholar] [CrossRef]
- Aubert, J.; Begriche, K.; Knockaert, L.; Robin, M.A.; Fromenty, B. Increased Expression of Cytochrome P450 2E1 in Nonalcoholic Fatty Liver Disease: Mechanisms and Pathophysiological Role. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 630–637. [Google Scholar] [CrossRef]
- Brill, M.J.E.; Diepstraten, J.; van Rongen, A.; van Kralingen, S.; van den Anker, J.N.; Knibbe, C.A.J. Impact of Obesity on Drug Metabolism and Elimination in Adults and Children. Clin. Pharm. 2012, 51, 277–304. [Google Scholar] [CrossRef]
- Aljomah, G.; Baker, S.S.; Liu, W.; Kozielski, R.; Oluwole, J.; Lupu, B.; Baker, R.D.; Zhu, L. Induction of CYP2E1 in Non-Alcoholic Fatty Liver Diseases. Exp. Mol. Pathol. 2015, 99, 677–681. [Google Scholar] [CrossRef] [Green Version]
- Gade, C.; Dalhoff, K.; Petersen, T.S.; Riis, T.; Schmeltz, C.; Chabanova, E.; Christensen, H.R.; Mikus, G.; Burhenne, J.; Holm, J.C.; et al. Higher Chlorzoxazone Clearance in Obese Children Compared with Nonobese Peers. Br. J. Clin. Pharm. 2018, 84, 1738–1747. [Google Scholar] [CrossRef] [Green Version]
- Raucy, J.L.; Lasker, J.M.; Kraner, J.C.; Salazar, D.E.; Lieber, C.S.; Corcoran, G.B. Induction of Cytochrome P450IIE1 in the Obese Overfed Rat. Mol. Pharm. 1991, 39, 275–280. [Google Scholar]
- Baumgardner, J.N.; Shankar, K.; Hennings, L.; Badger, T.M.; Ronis, M.J.J. A New Model for Nonalcoholic Steatohepatitis in the Rat Utilizing Total Enteral Nutrition to Overfeed a High-Polyunsaturated Fat Diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G27–G38. [Google Scholar] [CrossRef]
- Begriche, K.; Lettéron, P.; Abbey-Toby, A.; Vadrot, N.; Robin, M.-A.; Bado, A.; Pessayre, D.; Fromenty, B. Partial Leptin Deficiency Favors Diet-Induced Obesity and Related Metabolic Disorders in Mice. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E939–E951. [Google Scholar] [CrossRef]
- Abdelmegeed, M.A.; Banerjee, A.; Yoo, S.-H.; Jang, S.; Gonzalez, F.J.; Song, B.-J. Critical Role of Cytochrome P450 2E1 (CYP2E1) in the Development of High Fat-Induced Non-Alcoholic Steatohepatitis. J. Hepatol. 2012, 57, 860–866. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.-S.; Lee, W.-C.; Lin, Y.-E.; Ho, C.-T.; Lu, K.-H.; Lin, S.-H.; Panyod, S.; Chu, Y.-L.; Sheen, L.-Y. Ginger Essential Oil Ameliorates Hepatic Injury and Lipid Accumulation in High Fat Diet-Induced Nonalcoholic Fatty Liver Disease. J. Agric. Food Chem. 2016, 64, 2062–2071. [Google Scholar] [CrossRef]
- Liu, W.; Shang, J.; Deng, Y.; Han, X.; Chen, Y.; Wang, S.; Yang, R.; Dong, F.; Shang, H. Network Pharmacology Analysis on Mechanism of Jian Pi Qing Gan Yin Decoction Ameliorating High Fat Diet-Induced Non-Alcoholic Fatty Liver Disease and Validated in Vivo. J. Ethnopharmacol. 2022, 295, 115382. [Google Scholar] [CrossRef]
- Seth, R.K.; Das, S.; Pourhoseini, S.; Dattaroy, D.; Igwe, S.; Ray, J.B.; Fan, D.; Michelotti, G.A.; Diehl, A.M.; Chatterjee, S. M1 Polarization Bias and Subsequent Nonalcoholic Steatohepatitis Progression Is Attenuated by Nitric Oxide Donor DETA NONOate via Inhibition of CYP2E1-Induced Oxidative Stress in Obese Mice. J. Pharm. Exp. Ther. 2015, 352, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Correia, M.A.; Kwon, D. Why Hepatic CYP2E1-Elevation by Itself Is Insufficient for Inciting NAFLD/NASH: Inferences from Two Genetic Knockout Mouse Models. Biology 2020, 9, 419. [Google Scholar] [CrossRef]
- Leclercq, I.A.; Farrell, G.C.; Field, J.; Bell, D.R.; Gonzalez, F.J.; Robertson, G.R. CYP2E1 and CYP4A as Microsomal Catalysts of Lipid Peroxides in Murine Nonalcoholic Steatohepatitis. J. Clin. Investig. 2000, 105, 1067–1075. [Google Scholar] [CrossRef]
- Abdelmegeed, M.A.; Choi, Y.; Godlewski, G.; Ha, S.-K.; Banerjee, A.; Jang, S.; Song, B.-J. Cytochrome P450-2E1 Promotes Fast Food-Mediated Hepatic Fibrosis. Sci. Rep. 2017, 7, 39764. [Google Scholar] [CrossRef] [Green Version]
- Leung, T.-M.; Nieto, N. CYP2E1 and Oxidant Stress in Alcoholic and Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2013, 58, 395–398. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Tan, W.; Liu, X.; Deng, L.; Huang, L.; Wang, X.; Gao, X. New Insight and Potential Therapy for NAFLD: CYP2E1 and Flavonoids. Biomed. Pharm. 2021, 137, 111326. [Google Scholar] [CrossRef]
- Raucy, J.L.; Lasker, J.; Ozaki, K.; Zoleta, V. Regulation of CYP2E1 by Ethanol and Palmitic Acid and CYP4A11 by Clofibrate in Primary Cultures of Human Hepatocytes. Toxicol. Sci. 2004, 79, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Qi, J.; Lim, C.W.; Kim, J.-W.; Kim, B. Dual TBK1/IKKε Inhibitor Amlexanox Mitigates Palmitic Acid-Induced Hepatotoxicity and Lipoapoptosis in Vitro. Toxicology 2020, 444, 152579. [Google Scholar] [CrossRef]
- Achterbergh, R.; Lammers, L.A.; Klümpen, H.-J.; Mathôt, R.A.A.; Romijn, J.A. Short-Term High-Fat Diet Alters Acetaminophen Metabolism in Healthy Individuals. Ther. Drug Monit. 2022, 44, 797–804. [Google Scholar] [CrossRef]
- Videla, L.A.; Rodrigo, R.; Orellana, M.; Fernandez, V.; Tapia, G.; Quiñones, L.; Varela, N.; Contreras, J.; Lazarte, R.; Csendes, A.; et al. Oxidative Stress-Related Parameters in the Liver of Non-Alcoholic Fatty Liver Disease Patients. Clin. Sci. 2004, 106, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Hardwick, R.N.; Fisher, C.D.; Canet, M.J.; Lake, A.D.; Cherrington, N.J. Diversity in Antioxidant Response Enzymes in Progressive Stages of Human Nonalcoholic Fatty Liver Disease. Drug Metab. Dispos. 2010, 38, 2293–2301. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Cai, F.; Lin, N.; Ye, J.; Zheng, Q.; Ding, G. Effects of Glutamine on Oxidative Stress and Nuclear Factor-ΚB Expression in the Livers of Rats with Nonalcoholic Fatty Liver Disease. Exp. Ther. Med. 2014, 7, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Wu, B.; Zhang, L.; Jin, X.; Wang, K.; Xu, W.; Zhang, B.; Wang, H. The Protective Effects of Trelagliptin on High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease in Mice. J. Biochem. Mol. Toxicol. 2021, 35, e22696. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.-F.; Lee, K.; Lopez, R.; Previs, S.F.; Willard, B.; McCullough, A.; Kasumov, T. A Western Diet Induced NAFLD in LDLR(-/)(-) Mice Is Associated with Reduced Hepatic Glutathione Synthesis. Free Radic. Biol. Med. 2016, 96, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Staňková, P.; Kučera, O.; Peterová, E.; Lotková, H.; Maseko, T.E.; Nožičková, K.; Červinková, Z. Adaptation of Mitochondrial Substrate Flux in a Mouse Model of Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 1101. [Google Scholar] [CrossRef] [Green Version]
- Smirne, C.; Croce, E.; Di Benedetto, D.; Cantaluppi, V.; Comi, C.; Sainaghi, P.P.; Minisini, R.; Grossini, E.; Pirisi, M. Oxidative Stress in Non-Alcoholic Fatty Liver Disease. Livers 2022, 2, 30–76. [Google Scholar] [CrossRef]
- Fromenty, B.; Roden, M. Mitochondrial Alterations in Fatty Liver Diseases. J. Hepatol. 2022, Oct 7. 22, S0168–S8278. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Xie, X.; Yin, A.; Yin, Y.; Liu, Y.; Dong, L.; Zhu, Z.; Zhou, J.; Zeng, Q.; et al. Gender Difference on the Effect of Omega-3 Polyunsaturated Fatty Acids on Acetaminophen-Induced Acute Liver Failure. Oxid. Med. Cell Longev. 2020, 2020, 8096847. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, J.; Chen, Y.; Li, Z.; Zhou, J.; Lu, X.; Chen, Z.; Zuo, D. Omega-3 Polyunsaturated Fatty Acids Inhibit IL-11/STAT3 Signaling in Hepatocytes during Acetaminophen Hepatotoxicity. Int. J. Mol. Med. 2021, 48, 190. [Google Scholar] [CrossRef]
- Teratani, T.; Tomita, K.; Suzuki, T.; Furuhashi, H.; Irie, R.; Hida, S.; Okada, Y.; Kurihara, C.; Ebinuma, H.; Nakamoto, N.; et al. Free Cholesterol Accumulation in Liver Sinusoidal Endothelial Cells Exacerbates Acetaminophen Hepatotoxicity via TLR9 Signaling. J. Hepatol. 2017, 67, 780–790. [Google Scholar] [CrossRef]
- Marí, M.; Caballero, F.; Colell, A.; Morales, A.; Caballeria, J.; Fernandez, A.; Enrich, C.; Fernandez-Checa, J.C.; García-Ruiz, C. Mitochondrial Free Cholesterol Loading Sensitizes to TNF- and Fas-Mediated Steatohepatitis. Cell Metab. 2006, 4, 185–198. [Google Scholar] [CrossRef]
- Ribas, V.; García-Ruiz, C.; Fernández-Checa, J.C. Glutathione and Mitochondria. Front Pharm. 2014, 5, 151. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Lu, J.; Guo, Y.; Zhang, Y.; Liu, J.; Huang, S.; Zhang, Y.; Gao, L.; Wang, X. Hypercholesterolemia Reduces the Expression and Function of Hepatic Drug Metabolizing Enzymes and Transporters in Rats. Toxicol. Lett. 2022, 364, 1–11. [Google Scholar] [CrossRef]
- Karkucinska-Wieckowska, A.; Simoes, I.C.M.; Kalinowski, P.; Lebiedzinska-Arciszewska, M.; Zieniewicz, K.; Milkiewicz, P.; Górska-Ponikowska, M.; Pinton, P.; Malik, A.N.; Krawczyk, M.; et al. Mitochondria, Oxidative Stress and Nonalcoholic Fatty Liver Disease: A Complex Relationship. Eur. J. Clin. Investig. 2022, 52, e13622. [Google Scholar] [CrossRef]
- Pérez-Carreras, M.; Del Hoyo, P.; Martín, M.A.; Rubio, J.C.; Martín, A.; Castellano, G.; Colina, F.; Arenas, J.; Solis-Herruzo, J.A. Defective Hepatic Mitochondrial Respiratory Chain in Patients with Nonalcoholic Steatohepatitis. Hepatology 2003, 38, 999–1007. [Google Scholar] [CrossRef]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Knoefel, W.T.; et al. Adaptation of Hepatic Mitochondrial Function in Humans with Non-Alcoholic Fatty Liver Is Lost in Steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, R.R.; Rashed, M.S.; Nelson, S.D. In Vitro Effects of Acetaminophen Metabolites and Analogs on the Respiration of Mouse Liver Mitochondria. Arch. Biochem. Biophys. 1989, 273, 449–457. [Google Scholar] [CrossRef]
- Chrøis, K.M.; Larsen, S.; Pedersen, J.S.; Rygg, M.O.; Boilsen, A.E.B.; Bendtsen, F.; Dela, F. Acetaminophen Toxicity Induces Mitochondrial Complex I Inhibition in Human Liver Tissue. Basic Clin. Pharm. Toxicol. 2020, 126, 86–91. [Google Scholar] [CrossRef]
- Fromenty, B. Alteration of Mitochondrial DNA Homeostasis in Drug-Induced Liver Injury. Food Chem. Toxicol. 2020, 135, 110916. [Google Scholar] [CrossRef]
- Farrell, G.; Schattenberg, J.M.; Leclercq, I.; Yeh, M.M.; Goldin, R.; Teoh, N.; Schuppan, D. Mouse Models of Nonalcoholic Steatohepatitis: Toward Optimization of Their Relevance to Human Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2241–2257. [Google Scholar] [CrossRef] [Green Version]
- Schuster, S.; Cabrera, D.; Arrese, M.; Feldstein, A.E. Triggering and Resolution of Inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 349–364. [Google Scholar] [CrossRef]
- Kazankov, K.; Jørgensen, S.M.D.; Thomsen, K.L.; Møller, H.J.; Vilstrup, H.; George, J.; Schuppan, D.; Grønbæk, H. The Role of Macrophages in Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 145–159. [Google Scholar] [CrossRef]
- Schneider, K.M.; Elfers, C.; Ghallab, A.; Schneider, C.V.; Galvez, E.J.C.; Mohs, A.; Gui, W.; Candels, L.S.; Wirtz, T.H.; Zuehlke, S.; et al. Intestinal Dysbiosis Amplifies Acetaminophen-Induced Acute Liver Injury. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 909–933. [Google Scholar] [CrossRef]
- Levy, M.; Shapiro, H.; Thaiss, C.A.; Elinav, E. NLRP6: A Multifaceted Innate Immune Sensor. Trends Immunol. 2017, 38, 248–260. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut Microbiota and Human NAFLD: Disentangling Microbial Signatures from Metabolic Disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Breton, J.; Galmiche, M.; Déchelotte, P. Dysbiotic Gut Bacteria in Obesity: An Overview of the Metabolic Mechanisms and Therapeutic Perspectives of Next-Generation Probiotics. Microorganisms 2022, 10, 452. [Google Scholar] [CrossRef]
- Sneed, R.A.; Grimes, S.D.; Schultze, A.E.; Brown, A.P.; Ganey, P.E. Bacterial Endotoxin Enhances the Hepatotoxicity of Allyl Alcohol. Toxicol. Appl. Pharm. 1997, 144, 77–87. [Google Scholar] [CrossRef]
- Shaw, P.J.; Ganey, P.E.; Roth, R.A. Idiosyncratic Drug-Induced Liver Injury and the Role of Inflammatory Stress with an Emphasis on an Animal Model of Trovafloxacin Hepatotoxicity. Toxicol. Sci. 2010, 118, 7–18. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, L.; Yang, P.; Zhou, W.; Li, B.; Moorhead, J.F.; Varghese, Z.; Ruan, X.Z.; Chen, Y. Inflammatory Stress Sensitizes the Liver to Atorvastatin-Induced Injury in ApoE-/- Mice. PLoS ONE 2016, 11, e0159512. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.H.; Kramer, W.G.; Granville, G.E. The Effect of Obesity on Acetaminophen Pharmacokinetics in Man. J. Clin. Pharm. 1981, 21, 284–287. [Google Scholar] [CrossRef]
- Abernethy, D.R.; Divoll, M.; Greenblatt, D.J.; Ameer, B. Obesity, Sex, and Acetaminophen Disposition. Clin. Pharm. Ther. 1982, 31, 783–790. [Google Scholar] [CrossRef]
- Emery, M.G.; Fisher, J.M.; Chien, J.Y.; Kharasch, E.D.; Dellinger, E.P.; Kowdley, K.V.; Thummel, K.E. CYP2E1 Activity before and after Weight Loss in Morbidly Obese Subjects with Nonalcoholic Fatty Liver Disease. Hepatology 2003, 38, 428–435. [Google Scholar] [CrossRef]
- Leclercq, I.A.; Field, J.; Enriquez, A.; Farrell, G.C.; Robertson, G.R. Constitutive and Inducible Expression of Hepatic CYP2E1 in Leptin-Deficient Ob/Ob Mice. Biochem. Biophys. Res. Commun. 2000, 268, 337–344. [Google Scholar] [CrossRef]
- Abdel-Razzak, Z.; Loyer, P.; Fautrel, A.; Gautier, J.C.; Corcos, L.; Turlin, B.; Beaune, P.; Guillouzo, A. Cytokines Down-Regulate Expression of Major Cytochrome P-450 Enzymes in Adult Human Hepatocytes in Primary Culture. Mol. Pharm. 1993, 44, 707–715. [Google Scholar]
- Wang, J.; Hu, Y.; Nekvindova, J.; Ingelman-Sundberg, M.; Neve, E.P.A. IL-4-Mediated Transcriptional Regulation of Human CYP2E1 by Two Independent Signaling Pathways. Biochem. Pharm. 2010, 80, 1592–1600. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Kang, X.; Li, X.; Wang, T.; Liu, F.; Jia, J.; Jin, Z.; Xue, Y. NF-ΚB-Mediated Regulation of Rat CYP2E1 by Two Independent Signaling Pathways. PLoS ONE 2019, 14, e0225531. [Google Scholar] [CrossRef]
- Drozdzik, M.; Lapczuk-Romanska, J.; Wenzel, C.; Szelag-Pieniek, S.; Post, M.; Skalski, Ł.; Kurzawski, M.; Oswald, S. Gene Expression and Protein Abundance of Hepatic Drug Metabolizing Enzymes in Liver Pathology. Pharmaceutics 2021, 13, 1334. [Google Scholar] [CrossRef]
- Nakai, K.; Tanaka, H.; Hanada, K.; Ogata, H.; Suzuki, F.; Kumada, H.; Miyajima, A.; Ishida, S.; Sunouchi, M.; Habano, W.; et al. Decreased Expression of Cytochromes P450 1A2, 2E1, and 3A4 and Drug Transporters Na+-Taurocholate-Cotransporting Polypeptide, Organic Cation Transporter 1, and Organic Anion-Transporting Peptide-C Correlates with the Progression of Liver Fibrosis in Chronic Hepatitis C Patients. Drug Metab. Dispos. 2008, 36, 1786–1793. [Google Scholar] [CrossRef] [Green Version]
- Müller, G.F.; Döhr, O.; El-Bahay, C.; Kahl, R.; Abel, J. Effect of Transforming Growth Factor-Beta1 on Cytochrome P450 Expression: Inhibition of CYP1 MRNA and Protein Expression in Primary Rat Hepatocytes. Arch. Toxicol. 2000, 74, 145–152. [Google Scholar] [CrossRef]
- Ciuclan, L.; Ehnert, S.; Ilkavets, I.; Weng, H.-L.; Gaitantzi, H.; Tsukamoto, H.; Ueberham, E.; Meindl-Beinker, N.M.; Singer, M.V.; Breitkopf, K.; et al. TGF-Beta Enhances Alcohol Dependent Hepatocyte Damage via down-Regulation of Alcohol Dehydrogenase I. J. Hepatol. 2010, 52, 407–416. [Google Scholar] [CrossRef]
- Kolwankar, D.; Vuppalanchi, R.; Ethell, B.; Jones, D.R.; Wrighton, S.A.; Hall, S.D.; Chalasani, N. Association between Nonalcoholic Hepatic Steatosis and Hepatic Cytochrome P-450 3A Activity. Clin. Gastroenterol. Hepatol. 2007, 5, 388–393. [Google Scholar] [CrossRef]
- Chiba, T.; Noji, K.; Shinozaki, S.; Suzuki, S.; Umegaki, K.; Shimokado, K. Diet-Induced Non-Alcoholic Fatty Liver Disease Affects Expression of Major Cytochrome P450 Genes in a Mouse Model. J. Pharm. Pharm. 2016, 68, 1567–1576. [Google Scholar] [CrossRef]
- Woolsey, S.J.; Mansell, S.E.; Kim, R.B.; Tirona, R.G.; Beaton, M.D. CYP3A Activity and Expression in Nonalcoholic Fatty Liver Disease. Drug Metab. Dispos. 2015, 43, 1484–1490. [Google Scholar] [CrossRef] [Green Version]
- Cobbina, E.; Akhlaghi, F. Non-Alcoholic Fatty Liver Disease (NAFLD)—Pathogenesis, Classification, and Effect on Drug Metabolizing Enzymes and Transporters. Drug Metab. Rev. 2017, 49, 197–211. [Google Scholar] [CrossRef]
- Jamwal, R.; de la Monte, S.M.; Ogasawara, K.; Adusumalli, S.; Barlock, B.B.; Akhlaghi, F. Nonalcoholic Fatty Liver Disease and Diabetes Are Associated with Decreased CYP3A4 Protein Expression and Activity in Human Liver. Mol. Pharm. 2018, 15, 2621–2632. [Google Scholar] [CrossRef]
- Smit, C.; De Hoogd, S.; Brüggemann, R.J.M.; Knibbe, C.A.J. Obesity and Drug Pharmacology: A Review of the Influence of Obesity on Pharmacokinetic and Pharmacodynamic Parameters. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 275–285. [Google Scholar] [CrossRef]
- Zeng, H.; Lin, Y.; Gong, J.; Lin, S.; Gao, J.; Li, C.; Feng, Z.; Zhang, H.; Zhang, J.; Li, Y.; et al. CYP3A Suppression during Diet-Induced Nonalcoholic Fatty Liver Disease Is Independent of PXR Regulation. Chem. Biol. Interact. 2019, 308, 185–193. [Google Scholar] [CrossRef]
- Krogstad, V.; Peric, A.; Robertsen, I.; Kringen, M.K.; Vistnes, M.; Hjelmesæth, J.; Sandbu, R.; Johnson, L.K.; Angeles, P.C.; Jansson-Löfmark, R.; et al. Correlation of Body Weight and Composition With Hepatic Activities of Cytochrome P450 Enzymes. J. Pharm. Sci. 2021, 110, 432–437. [Google Scholar] [CrossRef]
- Wiese, M.D.; Meakin, A.S.; Varcoe, T.J.; Darby, J.R.T.; Sarr, O.; Kiser, P.; Bradshaw, E.L.; Regnault, T.R.H.; Morrison, J.L. Hepatic Cytochrome P450 Function Is Reduced by Life-Long Western Diet Consumption in Guinea Pig Independent of Birth Weight. Life Sci. 2021, 287, 120133. [Google Scholar] [CrossRef]
- Fisher, C.D.; Lickteig, A.J.; Augustine, L.M.; Ranger-Moore, J.; Jackson, J.P.; Ferguson, S.S.; Cherrington, N.J. Hepatic Cytochrome P450 Enzyme Alterations in Humans with Progressive Stages of Nonalcoholic Fatty Liver Disease. Drug Metab. Dispos. 2009, 37, 2087–2094. [Google Scholar] [CrossRef] [Green Version]
- Lake, A.D.; Novak, P.; Fisher, C.D.; Jackson, J.P.; Hardwick, R.N.; Billheimer, D.D.; Klimecki, W.T.; Cherrington, N.J. Analysis of Global and Absorption, Distribution, Metabolism, and Elimination Gene Expression in the Progressive Stages of Human Nonalcoholic Fatty Liver Disease. Drug Metab. Dispos. 2011, 39, 1954–1960. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Clarke, J.D.; Dzierlenga, A.L.; Bear, J.; Goedken, M.J.; Cherrington, N.J. In Vivo Cytochrome P450 Activity Alterations in Diabetic Nonalcoholic Steatohepatitis Mice. J. Biochem. Mol. Toxicol. 2017, 31, e21840. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.; Feng, W.; Wei, S.; Wang, Y.; Xu, X.; Wei, J.; Ma, Z.; Du, Y.; Guo, J.; He, Y.; et al. Bioinformatics Analysis of Key Differentially Expressed Genes in Nonalcoholic Fatty Liver Disease Mice Models. Gene Expr. 2018, 19, 25–35. [Google Scholar] [CrossRef]
- Gabbia, D.; Roverso, M.; Guido, M.; Sacchi, D.; Scaffidi, M.; Carrara, M.; Orso, G.; Russo, F.P.; Floreani, A.; Bogialli, S.; et al. Western Diet-Induced Metabolic Alterations Affect Circulating Markers of Liver Function before the Development of Steatosis. Nutrients 2019, 11, 1602. [Google Scholar] [CrossRef]
- Al Nebaihi, H.M.; Al Batran, R.; Ussher, J.R.; Maayah, Z.H.; El-Kadi, A.O.S.; Brocks, D.R. Dietary-Induced Obesity, Hepatic Cytochrome P450, and Lidocaine Metabolism: Comparative Effects of High-Fat Diets in Mice and Rats and Reversibility of Effects With Normalization of Diet. J. Pharm. Sci. 2020, 109, 1199–1210. [Google Scholar] [CrossRef]
- Wu, J.; Lou, Y.-G.; Yang, X.; Wang, R.; Zhang, R.; Aa, J.-Y.; Wang, G.-J.; Xie, Y. Silybin Regulates P450s Activity by Attenuating Endoplasmic Reticulum Stress in Mouse Nonalcoholic Fatty Liver Disease. Acta Pharm. Sin. 2022, 44, 133–144. [Google Scholar] [CrossRef]
- Xiang, L.; Jiao, Y.; Qian, Y.; Li, Y.; Mao, F.; Lu, Y. Comparison of Hepatic Gene Expression Profiles between Three Mouse Models of Nonalcoholic Fatty Liver Disease. Genes Dis. 2022, 9, 201–215. [Google Scholar] [CrossRef]
- Wahlang, B.; Song, M.; Beier, J.I.; Cameron Falkner, K.; Al-Eryani, L.; Clair, H.B.; Prough, R.A.; Osborne, T.S.; Malarkey, D.E.; States, J.C.; et al. Evaluation of Aroclor 1260 Exposure in a Mouse Model of Diet-Induced Obesity and Non-Alcoholic Fatty Liver Disease. Toxicol. Appl. Pharm. 2014, 279, 380–390. [Google Scholar] [CrossRef] [Green Version]
- Abernethy, D.R.; Greenblatt, D.J.; Divoll, M.; Shader, R.I. Enhanced Glucuronide Conjugation of Drugs in Obesity: Studies of Lorazepam, Oxazepam, and Acetaminophen. J. Lab. Clin. Med. 1983, 101, 873–880. [Google Scholar]
- Barshop, N.J.; Capparelli, E.V.; Sirlin, C.B.; Schwimmer, J.B.; Lavine, J.E. Acetaminophen Pharmacokinetics in Children with Nonalcoholic Fatty Liver Disease. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 198–202. [Google Scholar] [CrossRef] [Green Version]
- Mazaleuskaya, L.L.; Sangkuhl, K.; Thorn, C.F.; FitzGerald, G.A.; Altman, R.B.; Klein, T.E. PharmGKB Summary: Pathways of Acetaminophen Metabolism at the Therapeutic versus Toxic Doses. Pharm. Genom. 2015, 25, 416–426. [Google Scholar] [CrossRef] [Green Version]
- Hardwick, R.N.; Ferreira, D.W.; More, V.R.; Lake, A.D.; Lu, Z.; Manautou, J.E.; Slitt, A.L.; Cherrington, N.J. Altered UDP-Glucuronosyltransferase and Sulfotransferase Expression and Function during Progressive Stages of Human Nonalcoholic Fatty Liver Disease. Drug Metab. Dispos. 2013, 41, 554–561. [Google Scholar] [CrossRef] [Green Version]
- Feng, R.; Wang, Y.; Liu, C.; Yan, C.; Zhang, H.; Su, H.; Kang, J.X.; Shang, C.-Z.; Wan, J.-B. Acetaminophen-Induced Liver Injury Is Attenuated in Transgenic Fat-1 Mice Endogenously Synthesizing Long-Chain n-3 Fatty Acids. Biochem. Pharm. 2018, 154, 75–88. [Google Scholar] [CrossRef]
- Eraky, S.M.; Abo El-Magd, N.F. Omega-3 Fatty Acids Protect against Acetaminophen-Induced Hepatic and Renal Toxicity in Rats through HO-1-Nrf2-BACH1 Pathway. Arch. Biochem. Biophys. 2020, 687, 108387. [Google Scholar] [CrossRef]
- Speck, R.F.; Lauterburg, B.H. Fish Oil Protects Mice against Acetaminophen Hepatotoxicity in Vivo. Hepatology 1991, 13, 557–561. [Google Scholar] [CrossRef]
- Maksymchuk, O.; Shysh, A.; Stroy, D. Treatment with Omega-3 PUFAs Does Not Increase the Risk of CYP2E1-Dependent Oxidative Stress and Diabetic Liver Pathology. Front. Endocrinol. 2022, 13, 1004564. [Google Scholar] [CrossRef]
- Jain, P.; Hejjaji, V.; Thomas, M.B.; Garcia, R.A.; Kennedy, K.F.; Goyal, A.; Sperling, L.; Das, S.R.; Hafida, S.; Enriquez, J.R.; et al. Use of Primary Bariatric Surgery among Patients with Obesity and Diabetes. Insights from the Diabetes Collaborative Registry. Int. J. Obes. 2022, 46, 2163–2167. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, P.; Li, P.; Wang, G.; Yi, X.; Fu, Z.; Sun, X.; Cui, B.; Zhu, L.; Zhu, S. Bariatric Surgery Improves Nonalcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Obes. Surg. 2022, 32, 1872–1883. [Google Scholar] [CrossRef]
- Holt, E.W.; DeMartini, S.; Davern, T.J. Acute Liver Failure Due to Acetaminophen Poisoning in Patients With Prior Weight Loss Surgery: A Case Series. J. Clin. Gastroenterol. 2015, 49, 790–793. [Google Scholar] [CrossRef]
- Abusabeib, A.; El Ansari, W.; Alobaidan, J.; Elhag, W. First Case Report of Fulminant Hepatitis After Laparoscopic Sleeve Gastrectomy Associated with Concomitant Maximal Therapeutic Dose of Acetaminophen Use, Protein Calorie Malnutrition, and Vitamins A and D, Selenium, and Glutathione Deficiencies. Obes. Surg. 2021, 31, 899–903. [Google Scholar] [CrossRef]
- Puris, E.; Pasanen, M.; Ranta, V.-P.; Gynther, M.; Petsalo, A.; Käkelä, P.; Männistö, V.; Pihlajamäki, J. Laparoscopic Roux-En-Y Gastric Bypass Surgery Influenced Pharmacokinetics of Several Drugs given as a Cocktail with the Highest Impact Observed for CYP1A2, CYP2C8 and CYP2E1 Substrates. Basic. Clin. Pharm. Toxicol. 2019, 125, 123–132. [Google Scholar] [CrossRef]
- Porat, D.; Markovic, M.; Zur, M.; Fine-Shamir, N.; Azran, C.; Shaked, G.; Czeiger, D.; Vaynshtein, J.; Replyanski, I.; Sebbag, G.; et al. Increased Paracetamol Bioavailability after Sleeve Gastrectomy: A Crossover Pre- vs. Post-Operative Clinical Trial. J. Clin. Med. 2019, 8, 1949. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.-F.; Chan, L.-N.; Senn, T.D.; Oelschlager, B.K.; Flum, D.R.; Shen, D.D.; Horn, J.R.; Lin, Y.S. The Impact of Proximal Roux-En-Y Gastric Bypass Surgery on Acetaminophen Absorption and Metabolism. Pharmacotherapy 2020, 40, 191–203. [Google Scholar] [CrossRef]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 Diabetes Mellitus. Nat. Rev. Dis. Prim. 2017, 3, 17016. [Google Scholar] [CrossRef]
- Zaccardi, F.; Webb, D.R.; Yates, T.; Davies, M.J. Pathophysiology of Type 1 and Type 2 Diabetes Mellitus: A 90-Year Perspective. Postgrad. Med. J. 2016, 92, 63–69. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic β-Cells in Type 1 and Type 2 Diabetes Mellitus: Different Pathways to Failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef]
- de Vries, M.; Westerink, J.; Kaasjager, K.H.A.H.; de Valk, H.W. Prevalence of Nonalcoholic Fatty Liver Disease (NAFLD) in Patients With Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2020, 105, 3842–3853. [Google Scholar] [CrossRef]
- Mertens, J.; De Block, C.; Spinhoven, M.; Driessen, A.; Francque, S.M.; Kwanten, W.J. Hepatopathy Associated With Type 1 Diabetes: Distinguishing Non-Alcoholic Fatty Liver Disease From Glycogenic Hepatopathy. Front. Pharm. 2021, 12, 768576. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Everhart, J.E. Diabetes Increases the Risk of Acute Hepatic Failure. Gastroenterology 2002, 122, 1822–1828. [Google Scholar] [CrossRef]
- Chalasani, N.; Fontana, R.J.; Bonkovsky, H.L.; Watkins, P.B.; Davern, T.; Serrano, J.; Yang, H.; Rochon, J. Drug Induced Liver Injury Network (DILIN) Causes, Clinical Features, and Outcomes from a Prospective Study of Drug-Induced Liver Injury in the United States. Gastroenterology 2008, 135, 1924–1934, 1934.e1-4. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.-J.; Zhang, Y.; Tang, F.-L.; Zheng, Z.-W.; Fan, Z.-D.; Zhu, S.-M.; Qian, X.-F.; Liu, N.-N. Clinical Characteristics of Drug-Induced Liver Injury and Related Risk Factors. Exp. Ther. Med. 2016, 12, 2606–2616. [Google Scholar] [CrossRef] [Green Version]
- Deeds, M.C.; Anderson, J.M.; Armstrong, A.S.; Gastineau, D.A.; Hiddinga, H.J.; Jahangir, A.; Eberhardt, N.L.; Kudva, Y.C. Single Dose Streptozotocin-Induced Diabetes: Considerations for Study Design in Islet Transplantation Models. Lab. Anim. 2011, 45, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wei, S.; Zhou, H.; Shen, G.; Gan, X.; Zhou, S.; Qiu, J.; Shi, C.; Lu, L. Hyperglycemia Exacerbates Acetaminophen-Induced Acute Liver Injury by Promoting Liver-Resident Macrophage Proinflammatory Response via AMPK/PI3K/AKT-Mediated Oxidative Stress. Cell Death Discov. 2019, 5, 119. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Cederbaum, A.I. Combined Effects of Streptozotocin-Induced Diabetes plus 4-Methylpyrazole Treatment on Rat Liver Cytochrome P4502E1. Arch. Biochem. Biophys. 1993, 302, 175–182. [Google Scholar] [CrossRef]
- Raza, H.; Prabu, S.K.; Robin, M.-A.; Avadhani, N.G. Elevated Mitochondrial Cytochrome P450 2E1 and Glutathione S-Transferase A4-4 in Streptozotocin-Induced Diabetic Rats: Tissue-Specific Variations and Roles in Oxidative Stress. Diabetes 2004, 53, 185–194. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Koo, J.R.; Sindhu, K.K.; Ehdaie, A.; Farmand, F.; Roberts, C.K. Differential Regulation of Hepatic Cytochrome P450 Monooxygenases in Streptozotocin-Induced Diabetic Rats. Free Radic. Res. 2006, 40, 921–928. [Google Scholar] [CrossRef]
- Maksymchuk, O.; Shysh, A.; Rosohatska, I.; Chashchyn, M. Quercetin Prevents Type 1 Diabetic Liver Damage through Inhibition of CYP2E1. Pharmacol. Rep. 2017, 69, 1386–1392. [Google Scholar] [CrossRef]
- Price, V.F.; Jollow, D.J. Increased Resistance of Diabetic Rats to Acetaminophen-Induced Hepatotoxicity. J. Pharm. Exp. Ther. 1982, 220, 504–513. [Google Scholar]
- Shankar, K.; Vaidya, V.S.; Apte, U.M.; Manautou, J.E.; Ronis, M.J.J.; Bucci, T.J.; Mehendale, H.M. Type 1 Diabetic Mice Are Protected from Acetaminophen Hepatotoxicity. Toxicol. Sci. 2003, 73, 220–234. [Google Scholar] [CrossRef]
- Shankar, K.; Vaidya, V.S.; Corton, J.C.; Bucci, T.J.; Liu, J.; Waalkes, M.P.; Mehendale, H.M. Activation of PPAR-Alpha in Streptozotocin-Induced Diabetes Is Essential for Resistance against Acetaminophen Toxicity. FASEB J. 2003, 17, 1748–1750. [Google Scholar] [CrossRef] [Green Version]
- Boyle, M.; Masson, S.; Anstee, Q.M. The Bidirectional Impacts of Alcohol Consumption and the Metabolic Syndrome: Cofactors for Progressive Fatty Liver Disease. J. Hepatol. 2018, 68, 251–267. [Google Scholar] [CrossRef] [Green Version]
- Di Ciaula, A.; Bonfrate, L.; Krawczyk, M.; Frühbeck, G.; Portincasa, P. Synergistic and Detrimental Effects of Alcohol Intake on Progression of Liver Steatosis. Int. J. Mol. Sci. 2022, 23, 2636. [Google Scholar] [CrossRef]
- Massart, J.; Begriche, K.; Corlu, A.; Fromenty, B. Xenobiotic-Induced Aggravation of Metabolic-Associated Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 1062. [Google Scholar] [CrossRef]
- Akakpo, J.Y.; Ramachandran, A.; Jaeschke, H. Novel strategies for the treatment of acetaminophen hepatotoxicity. Expert. Opin. Drug Metab. Toxicol. 2020, 16, 1039–1050. [Google Scholar] [CrossRef]
- Subramanya, S.B.; Venkataraman, B.; Meeran, M.F.N.; Goyal, S.N.; Patil, C.R.; Ojha, S. Therapeutic Potential of Plants and Plant Derived Phytochemicals against Acetaminophen-Induced Liver Injury. Int. J. Mol. Sci. 2018, 19, 3776. [Google Scholar] [CrossRef] [Green Version]
- Jaeschke, H.; Adelusi, O.B.; Ramachandran, A. Ferroptosis and Acetaminophen Hepatotoxicity: Are We Going Down Another Rabbit Hole? Gene Expr. 2021, 20, 169–178. [Google Scholar] [CrossRef]
- Liao, Y.; Yang, Y.; Wang, X.; Wei, M.; Guo, Q.; Zhao, L. Oroxyloside ameliorates acetaminophen-induced hepatotoxicity by inhibiting JNK related apoptosis and necroptosis. J. Ethnopharmacol. 2020, 258, 112917. [Google Scholar] [CrossRef]
- Hwang, K.A.; Hwang, Y.; Hwang, H.J.; Park, N. Hepatoprotective Effects of Radish (Raphanus sativus L.) on Acetaminophen-Induced Liver Damage via Inhibiting Oxidative Stress and Apoptosis. Nutrients 2022, 14, 5082. [Google Scholar] [CrossRef]
- Du, K.; Ramachandran, A.; McGill, M.R.; Mansouri, A.; Asselah, T.; Farhood, A.; Woolbright, B.L.; Ding, W.X.; Jaeschke, H. Induction of mitochondrial biogenesis protects against acetaminophen hepatotoxicity. Food Chem. Toxicol. 2017, 108, 339–350. [Google Scholar]
| Authors, Year [References] | Design of the Study | Presence of NAFLD | Hepatic CYP2E1 Activity | APAP Overdose | APAP-Induced Acute Liver Injury |
|---|---|---|---|---|---|
| Rutherford et al., 2006 [44] | Prospective | Not reported in this study 1 | Not reported in this study | Yes | Lower incidence (but poorer outcomes) in obese patients |
| Nguyen et al., 2008 [40] | Retrospective | Yes | Not reported in this study | Yes | Higher prevalence in patients with NAFLD |
| Myers and Shaheen, 2009 [41] | Retrospective | Yes | Not reported in this study | Yes | Higher prevalence in patients with NAFLD |
| Radosevich et al., 2016 [45] | Retrospective | Not reported in this study 1 | Not reported in this study | Yes | Equal prevalence between obese and nonobese patients |
| Van Rongen et al., 2016 [43] | Prospective | Not reported in this study 1 | Increased | No (4 to 5 g) | Increased plasma ALT and AST in morbidly obese patients but not in nonobese individuals |
| Chomchai and Chomchai, 2018 [42] | Retrospective | Not reported in this study 1 | Not reported in this study | Yes | Higher prevalence in overweight and obese patients |
| Authors, Year [References] | Rodent Models of Obesity and NAFLD | Presence of NAFLD | Hepatic CYP2E1 Activity | Dose of APAP | APAP-Induced Hepatotoxicity |
|---|---|---|---|---|---|
| Corcoran and Wong, 1987 [47] | Male Sprague–Dawley rats fed a high-fat diet for 24 weeks | Not reported in this study 1 | Not reported in this study | 710 mg/kg (i.p.) | Higher hepatotoxicity after 48 h, compared to rats fed a standard diet |
| Blouin et al., 1987 [58] | Male obese Zucker fa/fa rats | Not reported in this study 2 | Not reported in this study 2 | 1300 mg (p.o.) | Similar hepatotoxicity after 48 h, compared to lean rats |
| Tuntaterdtum et al., 1993 [54]. | Male obese Zucker fa/fa rats | Not reported in this study 2 | Not reported in this study 2 | 3000 mg/kg (p.o) | Lower hepatotoxicity after 48 h, compared to lean rats |
| Ito et al., 2006 [55] | Male C57Bl/6 mice fed a Western-style diet for 16 weeks Male ob/ob mice | Yes Not reported in this study 3 | Not reported in this study Not reported in this study 3 | 300 mg/kg (p.o.) 300 mg/kg (p.o.) | Lower hepatotoxicity after 6 h, compared to mice fed a standard diet Lower hepatotoxicity after 6 h, compared to wild-type mice |
| Donthamsetty et al., 2008 [59] | Male Swiss Webster mice fed a MCD diet for 1 month 4 | Yes | Unchanged | 360 mg/kg (i.p.) | Higher hepatotoxicity from 6 to 48 h after overdose, compared to mice fed a standard diet |
| Kon et al., 2010 [48] | Male KK-Ay mice | Yes | Not reported in this study 5 | 300 or 600 mg/kg (p.o.) | Higher hepatotoxicity after 6 h, compared to wild-type mice |
| Kucera et al., 2012 [50] | Male Sprague-Dawley rats fed a high-fat diet for 6 weeks | Yes | Not reported in this study | 1 g/kg (p.o) | Higher hepatotoxicity after 24 and 48 h, compared to rats fed a standard diet |
| Aubert et al., 2012 [49] | Female db/db mice Female ob/ob mice | Yes Yes | Increased Unchanged | 500 mg/kg (p.o.) 500 mg/kg (p.o.) | Higher hepatotoxicity after 8 h, compared to wild-type mice Similar hepatotoxicity after 8 h, compared to wild-type mice |
| Kim et al., 2017 [56] | Male C57Bl/6 mice fed a fast food diet for 14 weeks | Yes | Not reported in this study (but higher CYP2E1 protein levels) | 200 mg/kg (i.p.) | Lower hepatotoxicity compared to wild-type mice (timing not specified) |
| Piccinin et al., 2019 [51] | Male FVB/N mice fed a high-fat diet for 1 month | Yes | Not reported in this study | 300 mg/kg (i.p.) | Higher hepatotoxicity after 6 h, compared to wild-type mice |
| Shi et al., 2019 [52] | Male C57Bl/6 mice fed a high-fat diet for 8 weeks | Yes | Not reported in this study | 50, 100 or 200 mg/kg (p.o.) | Significant hepatotoxicity after 24 h but no comparison with wild-type mice |
| Wang et al., 2021 [53] | Male C57Bl/6J mice fed a high-fat diet for 8 weeks | Yes | Not reported in this study | 100 mg/kg (p.o.) | Significant hepatotoxicity after 24 h but no comparison with wild-type mice |
| Ghallab et al., 2021 [57] | Male C57Bl/6N mice fed a Western diet for 48 to 50 weeks | Yes | Not reported in this study (but lower CYP2E1 immunostaining) | 300 mg/kg (i.p.) | Lower hepatotoxicity compared to wild-type mice (timing not specified) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begriche, K.; Penhoat, C.; Bernabeu-Gentey, P.; Massart, J.; Fromenty, B. Acetaminophen-Induced Hepatotoxicity in Obesity and Nonalcoholic Fatty Liver Disease: A Critical Review. Livers 2023, 3, 33-53. https://doi.org/10.3390/livers3010003
Begriche K, Penhoat C, Bernabeu-Gentey P, Massart J, Fromenty B. Acetaminophen-Induced Hepatotoxicity in Obesity and Nonalcoholic Fatty Liver Disease: A Critical Review. Livers. 2023; 3(1):33-53. https://doi.org/10.3390/livers3010003
Chicago/Turabian StyleBegriche, Karima, Clémence Penhoat, Pénélope Bernabeu-Gentey, Julie Massart, and Bernard Fromenty. 2023. "Acetaminophen-Induced Hepatotoxicity in Obesity and Nonalcoholic Fatty Liver Disease: A Critical Review" Livers 3, no. 1: 33-53. https://doi.org/10.3390/livers3010003
APA StyleBegriche, K., Penhoat, C., Bernabeu-Gentey, P., Massart, J., & Fromenty, B. (2023). Acetaminophen-Induced Hepatotoxicity in Obesity and Nonalcoholic Fatty Liver Disease: A Critical Review. Livers, 3(1), 33-53. https://doi.org/10.3390/livers3010003






