Lights and Shadows of Paracentesis: Is an Ultrasound Guided Approach Enough to Prevent Bleeding Complications?
Abstract
1. Introduction
2. Methods
3. Case Reports
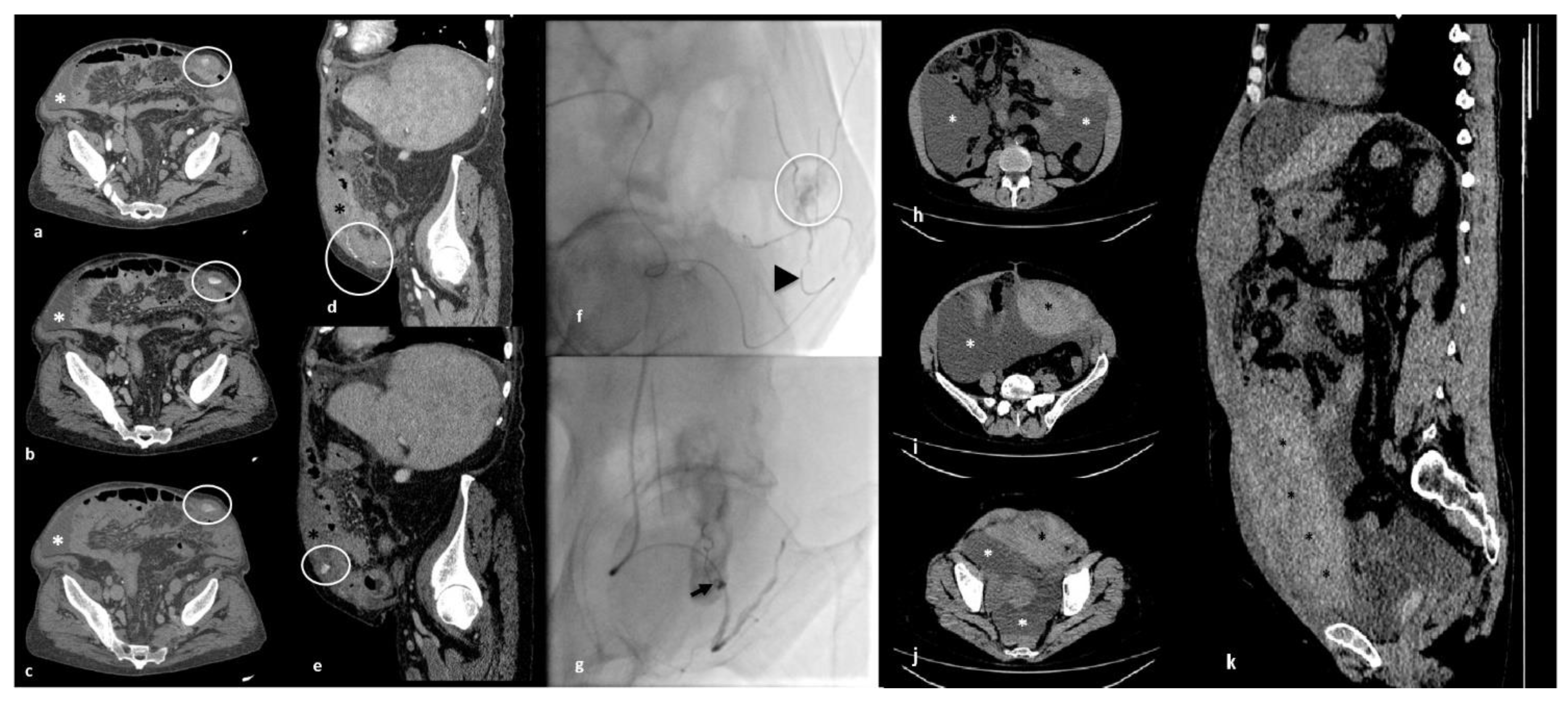
3.1. First Case
3.2. Second Case
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angeli, P.; Bernardi, M.; Villanueva, C.; Francoz, C.; Mookerjee, R.P.; Trebicka, J.; Krag, A.; Laleman, W.; Gines, P. EASL Clinical Practice Guidelines for the Management of Patients with Decompensated Cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [PubMed]
- Senousy, B.E.; Draganov, P.V. Evaluation and management of patients with refractory ascites. World J. Gastroenterol. 2009, 15, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, D.; Neong, S.F.; Wong, F. Refractory Ascites in Liver Cirrhosis. Am. J. Gastroenterol. 2019, 114, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Will, V.; Rodrigues, S.G.; Berzigotti, A. Current treatment options of refractory ascites in liver cirrhosis–A systematic review and meta-analysis. Dig. Liver Dis. 2022, 54, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Sharzehi, K.; Jain, V.; Naveed, A.; Schreibman, I. Hemorrhagic Complications of Paracentesis: A Systematic Review of the Literature. Gastroenterol. Res. Pract. 2014, 2014, 985141. [Google Scholar] [CrossRef] [PubMed]
- Millington, S.J.; Koenig, S. Better with Ultrasound: Paracentesis. Chest 2018, 154, 177–184. [Google Scholar] [CrossRef]
- Runyon, B.A. Paracentesis of Ascitic Fluid. A Safe Procedure. Arch. Intern. Med. 1986, 146, 2259–2261. [Google Scholar] [CrossRef]
- Sobkin, P.R.; Bloom, A.I.; Wilson, M.W.; LaBerge, J.M.; Hastings, G.S.; Gordon, R.L.; Brody, L.A.; Sawhney, R.; Kerlan, R.K. Massive Abdominal Wall Hemorrhage from Injury to the Inferior Epigastric Artery: A Retrospective Review. J. Vasc. Interv. Radiol. 2008, 19, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Afreen, S.; Deonarine, U.; Ogundipe, F.; Thomas, A. A Case of Large, Hemodynamically Significant Abdominal Wall Hematoma Following Paracentesis in a Cirrhotic Patient. Cureus 2017, 9, e1483. [Google Scholar] [CrossRef]
- Lin, S.; Wang, M.; Zhu, Y.; Dong, J.; Weng, Z.; Shao, L.; Chen, J.; Jiang, J. Hemorrhagic Complications Following Abdominal Paracentesis in Acute on Chronic Liver Failure: A Propensity Score Analysis. Medicine 2015, 94, e2225. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.C. Hemorrhagic Complications of Paracentesis: Aberrant Anatomy Versus Aberrant Technique-A Fatal Case of Abdominal Hemoperitoneum. Cureus 2020, 12, e8827. [Google Scholar] [CrossRef] [PubMed]
- Seidler, M.; Sayegh, K.; Roy, A.; Mesurolle, B. A fatal complication of ultrasound-guided abdominal paracentesis. J. Clin. Ultrasound 2013, 41, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Day, R.W.; Huettl, E.A.; Naidu, S.G.; Eversman, W.G.; Douglas, D.D.; O’Donnell, M.E. Successful Coil Embolization of Circumflex Iliac Artery Pseudoaneurysms Following Paracentesis. Vasc. Endovasc. Surg. 2014, 48, 262–266. [Google Scholar] [CrossRef]
- Naqvi, I.; Mahmood, K.; Talib, A. Haemorrhagic versus non haemorrhagic ascites in cirrhosis: Their relationship and impact on prognosis of liver cirrhosis. Pak. J. Med. Sci. 2020, 36, 603–608. [Google Scholar] [CrossRef] [PubMed]
- De Gottardi, A.; Thévenot, T.; Spahr, L.; Morard, I.; Bresson-Hadni, S.; Torres, F.; Giostra, E.; Hadengue, A. Risk of Complications after Abdominal Paracentesis in Cirrhotic Patients: A Prospective Study. Clin. Gastroenterol. Hepatol. 2009, 7, 906–909. [Google Scholar] [CrossRef]
- Katz, M.J.; Peters, M.N.; Wysocki, J.D.; Chakraborti, C. Diagnosis and management of delayed hemoperitoneum following therapeutic paracentesis. Bayl. Univ. Med Cent. Proc. 2013, 26, 185–186. [Google Scholar] [CrossRef]
- Hung, A.; Garcia-Tsao, G. Acute kidney injury, but not sepsis, is associated with higher procedure-related bleeding in patients with decompensated cirrhosis. Liver Int. 2018, 38, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Sabath, B.F.; Khan, S. A 54-Year-Old Woman with a History of Alcoholic Cirrhosis and Recurrent Ascites Presenting with Abdominal Pain and Increasing Abdominal Girth. Chest 2020, 157, e95–e97. [Google Scholar] [CrossRef]
- Buliarca, A.; Horhat, A.; Mocan, T.; Craciun, R.; Procopet, B.; Sparchez, Z. Viscoelastic tests in liver disease: Where do we stand now? World J. Gastroenterol. 2021, 27, 3290–3302. [Google Scholar] [CrossRef]
- Cho, J.; Jensen, T.P.; Reierson, K.; Mathews, B.K.; Bhagra, A.; Franco-Sadud, R.; Grikis, L.; Mader, M.; Dancel, R.; Lucas, B.P.; et al. Recommendations on the Use of Ultrasound Guidance for Adult Abdominal Paracentesis: A Position Statement of the Society of Hospital Medicine. J. Hosp. Med. 2019, 14, E7–E15. [Google Scholar] [CrossRef] [PubMed]
- Mercaldi, C.J.; Lanes, S.F. Ultrasound Guidance Decreases Complications and Improves the Cost of Care Among Patients Undergoing Thoracentesis and Paracentesis. Chest 2013, 143, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Lee, S.Y.; Kim, S.H.; Kim, I.H.; Kim, S.W.; Lee, S.O. Transcatheter Coil Embolization of the Inferior Epigastric Artery in a Huge Abdominal Wall Hematoma Caused by Paracentesis in a Patient with Liver Cirrhosis. Korean J. Hepatol. 2011, 17, 233–237. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Leite, T.F. Superselective Transcatheter Arterial Embolization of Iatrogenic Inferior Epigastric Artery after Paracentesis: Unusual Manifestation of Hemoperitoneum. Int. J. Surg. Case Rep. 2020, 74, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, J.; Nashed, M.H.; Smith, J.C. Post paracentesis deep circumflex iliac artery injury identified at angiography, an underreported complication. CVIR Endovasc. 2019, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Webster, S.T.; Brown, K.L.; Lucey, M.R.; Nostrant, T.T. Hemorrhagic complications of large volume abdominal paracentesis. Am. J. Gastroenterol. 1996, 91, 366–368. [Google Scholar]
- Guzman Rojas, P.; Sachdeva, R.; Blonski, W. Delayed Retroperitoneal Hemorrhage as a Complication of Large-Volume Paracentesis. Cureus 2019, 11, e4167. [Google Scholar] [CrossRef]
- Funaki, B. Embolization Iatrogenic Hemorrhage after Paracentesis. Semin. Interv. Radiol. 2008, 25, 329–333. [Google Scholar] [CrossRef]
- Grabau, C.M.; Crago, S.F.; Hoff, L.K.; Simon, J.A.; Melton, C.A.; Ott, B.J.; Kamath, P.S. Performance standards for therapeutic abdominal paracentesis. Hepatology 2004, 40, 484–488. [Google Scholar] [CrossRef]
- Fyson, J.; Chapman, L.; Tatton, M.; Raos, Z. Abdominal Paracentesis: Use of a Standardised Procedure Checklist and Equipment Kit Improves Procedural Quality and Reduces Complications. Intern. Med. J. 2018, 48, 572–579. [Google Scholar] [CrossRef]
- Siau, K.; Robson, N.; Bollipo, S. Where Should Ascitic Drains Be Placed? Revisiting Anatomical Landmarks for Paracentesis. Gut 2021, 70, 2216–2217. [Google Scholar] [CrossRef]
- Moon, S.N. Transarterial embolization for incorrectable abdominal wall hematoma after abdominal paracentesis. Korean J. Intern. Med. 2019, 34, 938–939. [Google Scholar] [CrossRef] [PubMed]
- La Mura, V.; Salerno, F. Therapy of the refractory ascites: Total paracentesis vs. TIPS. Gastroenterol. Hepatol. 2016, 39, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.; Haag, K.; Blum, H.E.; Rössle, M. Acute hemoperitoneum after large-volume paracentesis. Gastroenterology 1997, 113, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, F.; Khalid, A.; Tow, C. A Diagnostic Paracentesis Leading to Intraabdominal Hematoma and Small Bowel Obstruction. Cureus 2022, 14, e23472. [Google Scholar] [PubMed]
| Patient 1 | Patient 2 | |
|---|---|---|
| Sex | Male | Female |
| Age | 84 years | 51 years |
| Liver disease | Cryptogenic advanced chronic liver disease Child–Pugh score C10 at admission | Cryptogenic advanced chronic liver disease Child–Pugh score B9 at admission |
| Portal hypertension-associated complications | Moderate portal hypertensive gastropathy; splenomegaly; isolated gastric varices (IGV1); refractory ascites | Mild portal hypertensive gastropathy; splenomegaly: esophageal varices (F2 RWM-); refractory ascites |
| Comorbidities | Obesity; Hypertensive heart disease; Jak-2-positive Philadelphia-negative chronic myeloproliferative disorder; permanent atrial fibrillation; Stage 3b chronic kidney disease | No relevant comorbidities |
| Ascitic fluid appearance and volume | Sero-hematic—6 L | Yellow citrine—6.5 L |
| Hemodynamic parameters Before paracentesis After paracentesis | Regular No alterations found | Regular Mild drop in blood pressure and increase in heart rate |
| Hemoglobin Value (g/dL) Before paracentesis After paracentesis (30 min) After paracentesis (2 h) | 14.2 13.5 12.9 | 11.9 9.7 7.3 |
| Abdominal ultrasonography findings | A voluminous (7 × 2 cm) abdominal wall hematoma | A bulky abdominal wall hematoma, apparently replenished |
| Abdomen and pelvis CT scan angiography findings | A coarse collection of blood density in the context of the muscular planes of the left iliac fossa, showing contextual active bleeding strictly contiguous to the distal third of the inferior epigastric artery | A large blood collection in the context of the anterolateral wall of the abdomen in the subfascial area measuring about 14 × 7.5 cm in the axial section |
| Therapeutic approach | Percutaneous transcatheter arterial embolization | Conservative approach |
| Adverse events during hospitalization | No relevant adverse events | Anemia requiring multiple transfusions (6 units of packed red blood cells); esophageal variceal bleeding treated with endoscopic ligation and subsequent TIPS placement; episode of stage 1 acute kidney injury (AKI), treated with endovenous idratation, albumin infusion, and diuretic therapy suspension with the restoration of normal kidney function within 48 h; hospital-acquired bacterial pneumonia treated with an empirical antimicrobial therapy (after TIPS placement) |
| Hospital length-of-stay (days) | 5 | 27 |
| TIPS placement | Yes (1 month after the bleeding complication) | Yes (14 days after the bleeding complication, during hospitalization) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patturelli, M.; Pignata, L.; Venetucci, P.; Guarino, M. Lights and Shadows of Paracentesis: Is an Ultrasound Guided Approach Enough to Prevent Bleeding Complications? Livers 2023, 3, 54-62. https://doi.org/10.3390/livers3010004
Patturelli M, Pignata L, Venetucci P, Guarino M. Lights and Shadows of Paracentesis: Is an Ultrasound Guided Approach Enough to Prevent Bleeding Complications? Livers. 2023; 3(1):54-62. https://doi.org/10.3390/livers3010004
Chicago/Turabian StylePatturelli, Marta, Luca Pignata, Pietro Venetucci, and Maria Guarino. 2023. "Lights and Shadows of Paracentesis: Is an Ultrasound Guided Approach Enough to Prevent Bleeding Complications?" Livers 3, no. 1: 54-62. https://doi.org/10.3390/livers3010004
APA StylePatturelli, M., Pignata, L., Venetucci, P., & Guarino, M. (2023). Lights and Shadows of Paracentesis: Is an Ultrasound Guided Approach Enough to Prevent Bleeding Complications? Livers, 3(1), 54-62. https://doi.org/10.3390/livers3010004







