Chitosan Combined with Methanolic Plants Extracts: Antifungal Activity, Phytotoxicity and Acute Toxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Biological Samples
2.3. Preparation of Methanolic Extracts
2.4. Chitosan Solution and ExB-CS/ExJ-CS Combinations
2.5. Evaluation of Antifungal Activity
2.5.1. Preparation of Conidial Suspension
2.5.2. Radial Growth Kinetics
2.5.3. Cell Viability Test
2.5.4. Cell Integrity Damage Analysis
2.6. Phytotoxicity Bioassay
2.7. Acute Toxicity Test in Artemia Salina
2.8. Statistical Analysis
3. Results
3.1. Evaluation of Antifungal Activity
3.1.1. Radial Growth Kinetics
3.1.2. Cell Viability Test
3.1.3. Cell Integrity Damage Analysis
3.2. Phytotoxicity Bioassay
3.3. Acute Toxicity Test in Artemia Salina
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, R.A.; Najeeb, S.; Hussain, S.; Li, Y. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms 2020, 8, 817. [Google Scholar] [CrossRef]
- Herrera-González, J.A.; Bautista-Baños, S.; Serrano, M.; Ramos-Bell, S.; Gutiérrez-Martínez, P. Colletotrichum siamense causing anthracnose in postharvest of ‘Hass’ avocado. Rev. Mex. Cienc. Agric. 2024, 15, 5. [Google Scholar]
- Tovar-Pedraza, J.M.; Solano-Báez, A.R.; Leyva-Mir, S.G.; Tlapal-Bolaños, B.; Camacho-Tapia, M.; García-León, E.; Ayala-Escobar, V.; Nava-Díaz, C.; Quezada-Salinas, A.; Santiago-Santiago, V.; et al. The need and opportunity to update the inventory of plant pathogenic fungi and oomycetes in Mexico. J. Fungi 2024, 10, 395. [Google Scholar] [CrossRef]
- Fuentes-Aragón, D.; Silva-Rojas, H.V.; Guarnaccia, V.; Mora-Aguilera, J.A.; Aranda-Ocampo, S.; Bautista-Martínez, N.; Téliz-Ortíz, D. Colletotrichum species causing anthracnose on avocado fruit in Mexico: Current status. Plant Pathol. 2020, 69, 1513–1528. [Google Scholar] [CrossRef]
- García-Estrada, R.S.; Cruz-Lachica, I.; Osuna-García, L.A.; Márquez-Zequera, I. First report of papaya (Carica papaya) anthracnose caused by Colletotrichum plurivorum in Mexico. Plant Dis. 2020, 104, 589. [Google Scholar] [CrossRef]
- Pineda-Vaca, D.; Flores-Méndez, G.; Fernández-Pavía, S.P.; Santillán-Mendoza, R.; Montero-Castro, J.C.; Garay-Serrano, E.; Ortega-Arreola, R.; Rodríguez-Alvarado, G. Genetic diversity of Colletotrichum siamense causing mango anthracnose in the state of Jalisco, Mexico, based on ISSR markers. Can. J. Plant Pathol. 2025, 47, 1–17. [Google Scholar] [CrossRef]
- Ciofini, A.; Negrini, F.; Baroncelli, R.; Baraldi, E. Management of post-harvest anthracnose: Current approaches and future perspectives. Plants 2022, 11, 1856. [Google Scholar] [CrossRef]
- Azeem, S.; Agha, S.; Jamil, N.; Tabassum, B.; Ahmed, S.; Raheem, A.; Khan, A. Characterization and survival of broad-spectrum biocontrol agents against phytopathogenic fungi. Rev. Argent. Microbiol. 2022, 54, 233–242. [Google Scholar] [CrossRef] [PubMed]
- El Khetabi, A.; Lahlali, R.; Ezrari, S.; Radouane, N.; Lyousfi, N.; Banani, H.; Askarne, L.; Tahiri, A.; El Ghadraoui, L.; Belmalha, S.; et al. Role of plant extracts and essential oils in fighting against postharvest fruit pathogens and extending fruit shelf life: A review. Trends Food Sci. Technol. 2022, 120, 402–417. [Google Scholar] [CrossRef]
- Poznanski, P.; Hameed, A.; Orczyk, W. Chitosan and chitosan nanoparticles: Parameters enhancing antifungal activity. Molecules 2023, 28, 2996. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras-Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, processing and modification techniques. Gels 2022, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Dash, P.; Chang, Y.H.; Yang, J.M. Improvement of chitosan water solubility by fumaric acid modification. Mater. Lett. 2022, 316, 132046. [Google Scholar] [CrossRef]
- Li, Z.; Bi, X.; Dai, Y.; Ren, R. Enhancing mango anthracnose control and quality maintenance through chitosan and iturin A coating. LWT 2024, 198, 115955. [Google Scholar] [CrossRef]
- Nascimento, J.I.G.; Stamford, T.C.M.; Melo, N.F.C.B.; Nunes, I.D.S.; Lima, M.A.B.; Pintado, M.M.E.; Stamford-Arnaud, T.M.; Stamford, N.P.; Stamford, T.L.M. Chitosan–citric acid edible coating to control Colletotrichum gloeosporioides and maintain quality parameters of fresh-cut guava. Int. J. Biol. Macromol. 2020, 163, 1127–1135. [Google Scholar] [CrossRef]
- Rosas-Burgos, E.C.; Cortez-Rocha, M.O.; Cinco-Moroyoqui, F.J.; Robles-Zepeda, R.E.; López-Cervantes, J.; Sánchez-Machado, D.I.; Lares-Villa, F. Antifungal activity in vitro of Baccharis glutinosa and Ambrosia confertiflora extracts on Aspergillus flavus, Aspergillus parasiticus and Fusarium verticillioides. World J. Microbiol. Biotechnol. 2009, 25, 2257–2261. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, G.V.; Rosas-Burgos, E.C.; Cinco-Moroyoqui, F.J.; Burgos-Hernández, A.; Plascencia-Jatomea, M.; Cortez-Rocha, M.O.; Gálvez-Ruiz, J.C. In vitro effect of antifungal fractions from the plants Baccharis glutinosa and Jacquinia macrocarpa on chitin and β-1,3-glucan hydrolysis of maize phytopathogenic fungi and on the fungal β-1,3-glucanase and chitinase activities. J. Food Saf. 2013, 33, 526–535. [Google Scholar] [CrossRef]
- López-Bermúdez, L.S.; Quintana-Obregón, E.A.; Rosas-Burgos, E.C.; Gálvez-Iriqui, A.C.; Gutiérrez-Martínez, P.; Lizardi-Mendoza, J.; Plascencia-Jatomea, M. Acute phytotoxicity and antifungal effect of nanochitosan particles on Colletotrichum fructicola with low susceptibility to chitosan. Curr. Microbiol. 2024, 81, 445. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Magdaleno, M.E.; Luque-Alcaraz, A.G.; Gutiérrez-Martínez, P.; Cortez-Rocha, M.O.; Burgos-Hernández, A.; Lizardi-Mendoza, J.; Plascencia-Jatomea, M. Effect of chitosan-pepper tree (Schinus molle) essential oil biocomposites on the growth kinetics, viability and membrane integrity of Colletotrichum gloeosporioides. Rev. Mex. Ing. Quim. 2018, 17, 29–45. [Google Scholar] [CrossRef]
- Zhang, N.; Fan, Y.; Li, C.; Wang, Q.; Leksawasdi, N.; Li, F.; Wang, S. Cell Permeability and nuclear DNA staining by propidium iodide in Basidiomycetous yeasts. Appl. Microbiol. Biotechnol. 2018, 102, 4183–4191. [Google Scholar] [CrossRef]
- Oliveira, B.F.; Reis, A.; Costa, C.A.; Moita, A.W.; Pilon, L. Evaluation of chitosan for in vitro control of Colletotrichum tamarilloi and anthracnose on scarlet eggplant fruit. Hortic. Bras. 2023, 41, e2621. [Google Scholar] [CrossRef]
- Gálvez-Marroquín, L.A.; Martínez-Bolaños, M.; Cruz-Chávez, M.A.; Ariza-Flores, R.; Cruz-López, J.A.; Magaña-Lira, N.; Cruz-de-la-Cruz, L.L.; Ariza-Hernández, F.J. Inhibition of mycelial growth and conidium germination of Colletotrichum sp. for organic and inorganic products. Agro. Prod. 2022, 15, 25–32. [Google Scholar] [CrossRef]
- López-Zazueta, B.A.; Ayón-Reyna, L.E.; Gutiérrez-Dorado, R.; Rodríguez-Gómez, F.A.; López-López, M.E.; López-Velázquez, J.G.; Vega-García, M.O. Effect of chitosan with different molecular weights on the antifungal activity against Colletotrichum gloeosporioides and activation of the non-enzymatic antioxidant system on infected papaya. J. Food Sci. 2023, 88, 1979–1993. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Parra, E.; Cristóbal-Alejo, J.; Magaña-Alvarez, A.; Medina-Baizabal, I.L.; Gamboa-Angulo, M. Antifungal effect of Bonellia flammea extracts against Colletotrichum magnum in postharvest fruits of Carica papaya cv. maradol. J. Plant Dis. Prot. 2024, 131, 1685–1694. [Google Scholar] [CrossRef]
- El Ghaouth, A.; Arul, J.; Grenier, J.; Asselin, A. Antifungal activity of chitosan on two postharvest pathogens of strawberry fruits. Phytopathology 1992, 82, 398–402. [Google Scholar] [CrossRef]
- Moo-Koh, F.A.; Cristóbal-Alejo, J.; Tun-Suárez, J.M.; Medina-Baizabal, I.L.; Arjona-Cruz, A.A.; Gamboa-Angulo, M. Activity of aqueous extracts from native plants of the Yucatan peninsula against fungal pathogens of tomato in vitro and from Croton chichenensis against Corynespora cassiicola on tomato. Plants 2022, 11, 2821. [Google Scholar] [CrossRef] [PubMed]
- García-Sosa, K.; Sánchez-Medina, A.; Álvarez, S.L.; Zacchino, S.; Veitch, N.C.; Simá-Polanco, P.; Peña-Rodríguez, L.M. Antifungal activity of sakurasosaponin from the root extract of Jacquinia flammea. Nat. Prod. Res. 2011, 25, 1185–1189. [Google Scholar] [CrossRef]
- Muñoz-Ochoa, I.J.; Plascencia-Jatomea, M.; Cinco-Moroyoqui, F.J.; Valenzuela-Cota, D.F.; Buitimea-Cantúa, G.V.; Martínez-Higuera, A.A.; Rosas-Burgos, E.C. Persistence of the antifungal capacity of a fraction of Jacquinia macrocarpa plant against Fusarium verticillioides after continuous exposure. Indian J. Microbiol. 2020, 60, 458–467. [Google Scholar] [CrossRef]
- Valenzuela-Cota, D.F.; Buitimea-Cantúa, G.V.; Plascencia-Jatomea, M.; Cinco-Moroyoqui, F.J.; Martínez-Higuera, A.A.; Rosas-Burgos, E.C. Inhibition of the antioxidant activity of catalase and superoxide dismutase from Fusarium verticillioides exposed to a Jacquinia macrocarpa antifungal fraction. J. Environ. Sci. Health B 2019, 54, 647–654. [Google Scholar] [CrossRef]
- Amiel-Pérez, J.; Fukusaki, A.; Enciso, N.; Altamirano, C.; Mayanga-Herrera, A.; Marcelo, Á.; Marin-Sánchez, O. Interferencia de pigmentos vegetales al aplicar la técnica XTT a extractos de Buddleja globosa, Senecio tephrosiodes Turcz. y Equisetum giganteum. Científica 2016, 13, 9–29. [Google Scholar]
- Xing, K.; Xing, Y.; Liu, Y.; Zhang, Y.; Shen, X.; Li, X.; Qin, S. Fungicidal effect of chitosan via inducing membrane disturbance against Ceratocystis fimbriata. Carbohydr. Polym. 2018, 192, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Hernández-López, N.A.; Plascencia-Jatomea, M.; Del-Toro-Sánchez, C.L.; López-Saiz, C.M.; Morales-Rodríguez, S.; Martínez-Téllez, M.Á.; Quintana-Obregón, E.A. Antifungal activity of nanochitosan in Colletotrichum musae and Colletotrichum chrysophillum. Polysaccharides 2025, 6, 4. [Google Scholar] [CrossRef]
- Lam-Gutiérrez, A.; Ayora-Talavera, T.R.; Garrido-Ramírez, E.R.; Gutiérrez-Miceli, F.A.; Montes-Molina, J.A.; Lagunas-Rivera, S.; Ruíz-Valdiviezo, V.M. Phytochemical profile of methanolic extracts from chilca (Baccharis glutinosa) roots and its activity against Aspergillus ochraceus and Fusarium moniliforme. J. Environ. Biol. 2019, 40, 302–308. [Google Scholar] [CrossRef]
- Eseyin, A.E.; Steele, P.H. An overview of the applications of furfural and its derivatives. Int. J. Adv. Chem. 2015, 3, 42–47. [Google Scholar] [CrossRef]
- Yadav, R.P.; Huo, C.; Budhathoki, R.; Budthapa, P.; Bhattarai, B.R.; Rana, M.; Kim, K.H.; Parajuli, N. Antibacterial, antifungal, and cytotoxic effects of endophytic Streptomyces species isolated from the Himalayan regions of Nepal and their metabolite study. Biomedicines 2024, 12, 2192. [Google Scholar] [CrossRef]
- Mendes, P.M.; Ribeiro, J.A.; Martins, G.A.; Lucia, T.; Araujo, T.R.; Fuentes-Guevara, M.D.; Corrêa, L.B.; Corrêa, É.K. Phytotoxicity test in check: Proposition of methodology for comparison of different method adaptations usually used worldwide. J. Environ. Manag. 2021, 291, 112698. [Google Scholar] [CrossRef]
- Dias, M.P.; Nozari, R.M.; Santarém, E.R. Herbicidal activity of natural compounds from Baccharis spp. on the germination and seedlings growth of Lactuca sativa and Bidens pilosa. Allelopathy J. 2017, 42, 21–36. [Google Scholar] [CrossRef]
- Miranda-Arámbula, M.; Reyes-Chilpa, R.; Anaya-Lang, A.L. Phytotoxic activity of aqueous extracts of ruderal plants and its potential application to tomato crop. Bot. Sci. 2021, 99, 487–498. [Google Scholar] [CrossRef]
- Nurliana, S.; Fachriza, S.; Hemelda, N.M. Chitosan application for maintaining the growth of lettuce (Lactuca sativa) under drought condition. In Proceedings of the 4th International Conference on Food and Agriculture (ICOFA), Jember, Indonesia, 6–7 November 2021. IOP Conf. Ser. Earth Environ. Sci. 2022, 980, 012013. [Google Scholar] [CrossRef]
- Kocięcka, J.; Liberacki, D. The potential of using chitosan on cereal crops in the face of climate change. Plants 2021, 10, 1160. [Google Scholar] [CrossRef]
- Regueiras, A.; Pereira, S.; Costa, M.S.; Vasconcelos, V. Differential toxicity of cyanobacteria isolated from marine sponges towards echinoderms and crustaceans. Toxins 2018, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.R.; Antunes, A.; Welker, M.; Martíns, R.; Vasconcelos, V. Morphological, toxicological and molecular characterization of a benthic Nodularia isolated from Atlantic estuarine environments. Res. Microbiol. 2010, 161, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.S.L.; Jarvis, B.B. Livestock Intoxication by Baccharis. In Baccharis: From Evolutionary and Ecological Aspects to Social Uses and Medicinal Applications; Fernandes, G.W., Oki, Y., Barbosa, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-45373-7. [Google Scholar]
- Bhoopathy, S.; Inbakandan, D.; Thirugnanasambandam, R.; Kumar, C.; Sampath, P.; Bethunaickan, R.; Raguraman, V.; Vijayakumar, G.K. A comparative study on chitosan nanoparticle synthesis methodologies for application in aquaculture through toxicity studies. IET Nanobiotechnol. 2021, 15, 418–426. [Google Scholar] [CrossRef] [PubMed]
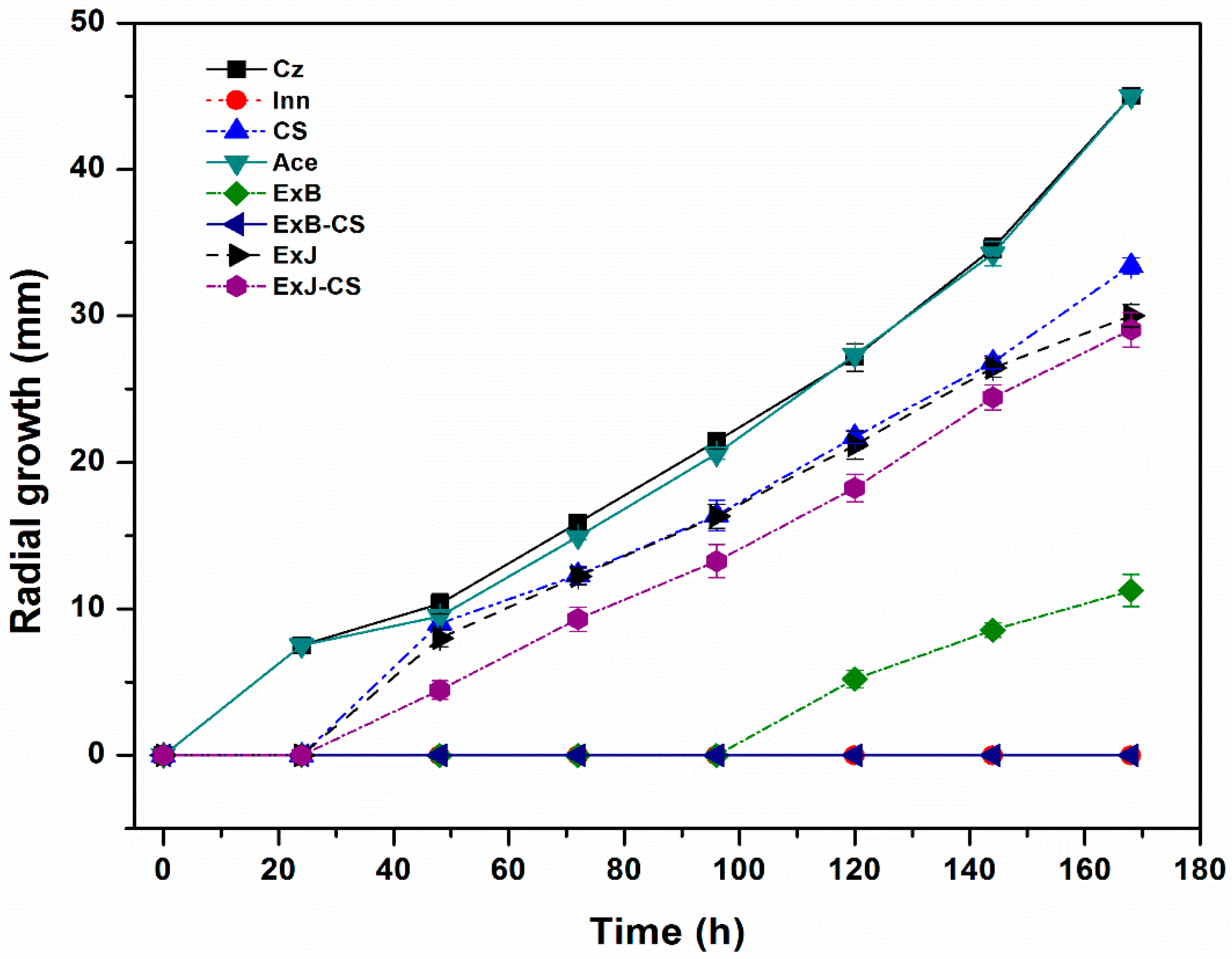
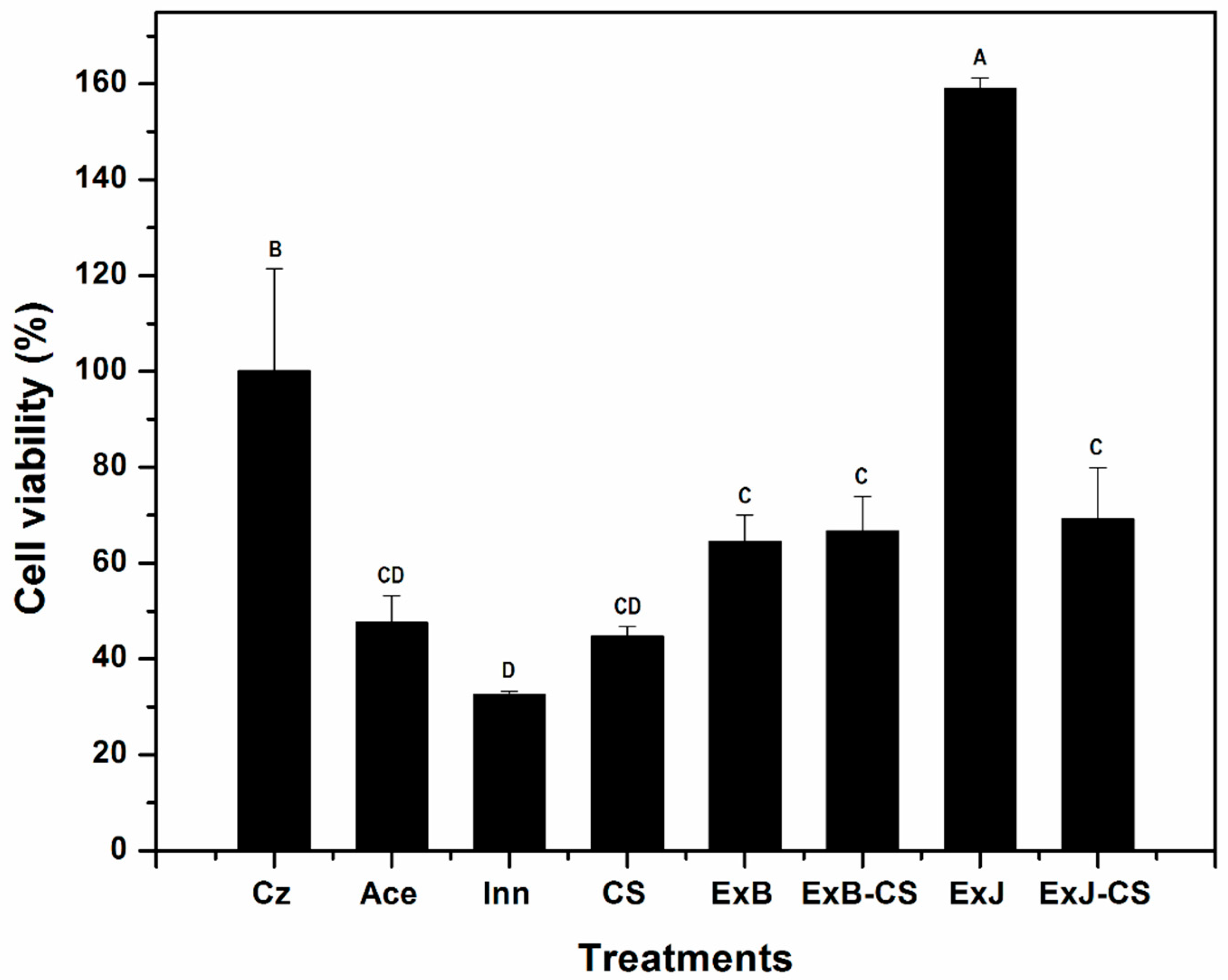
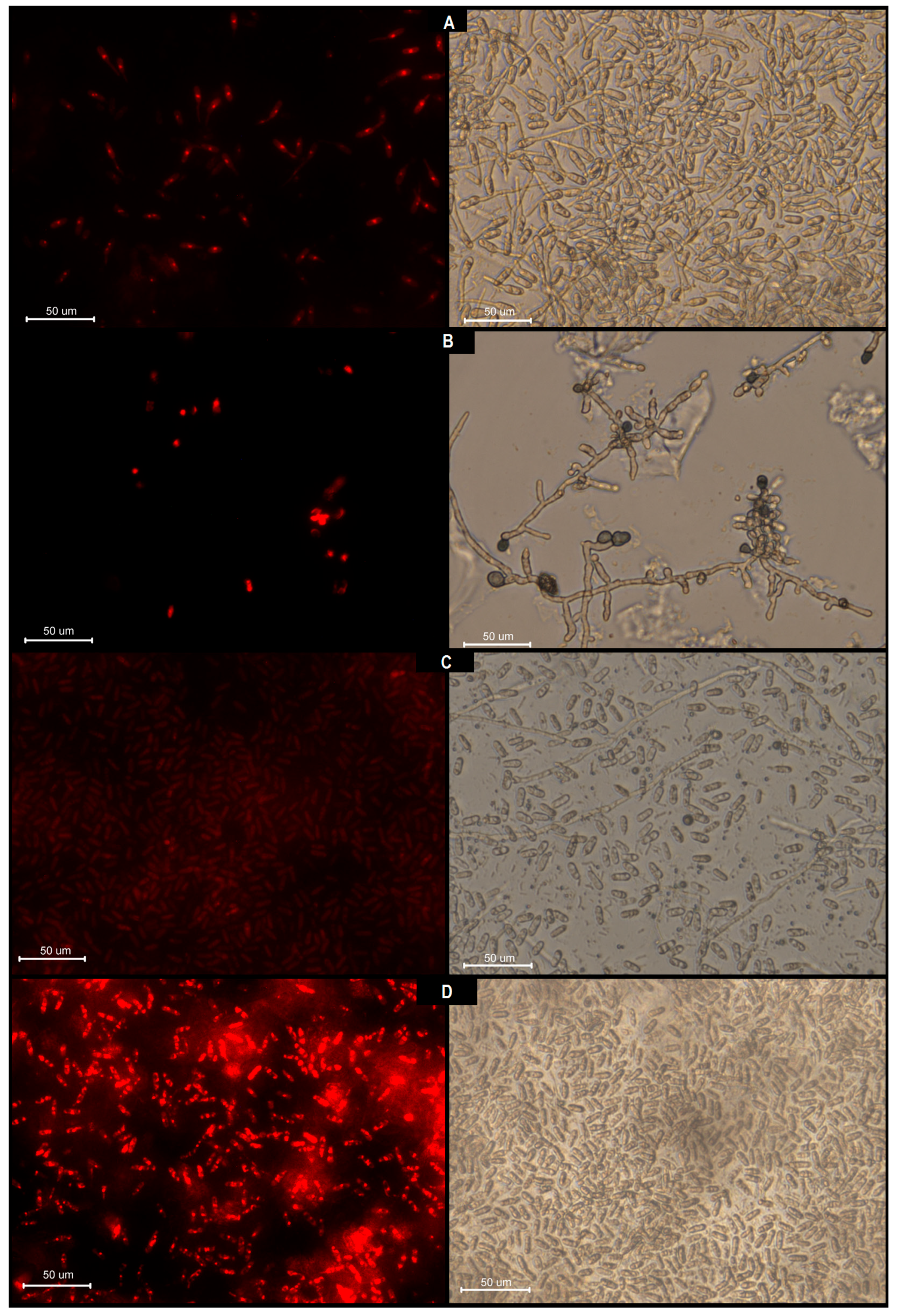
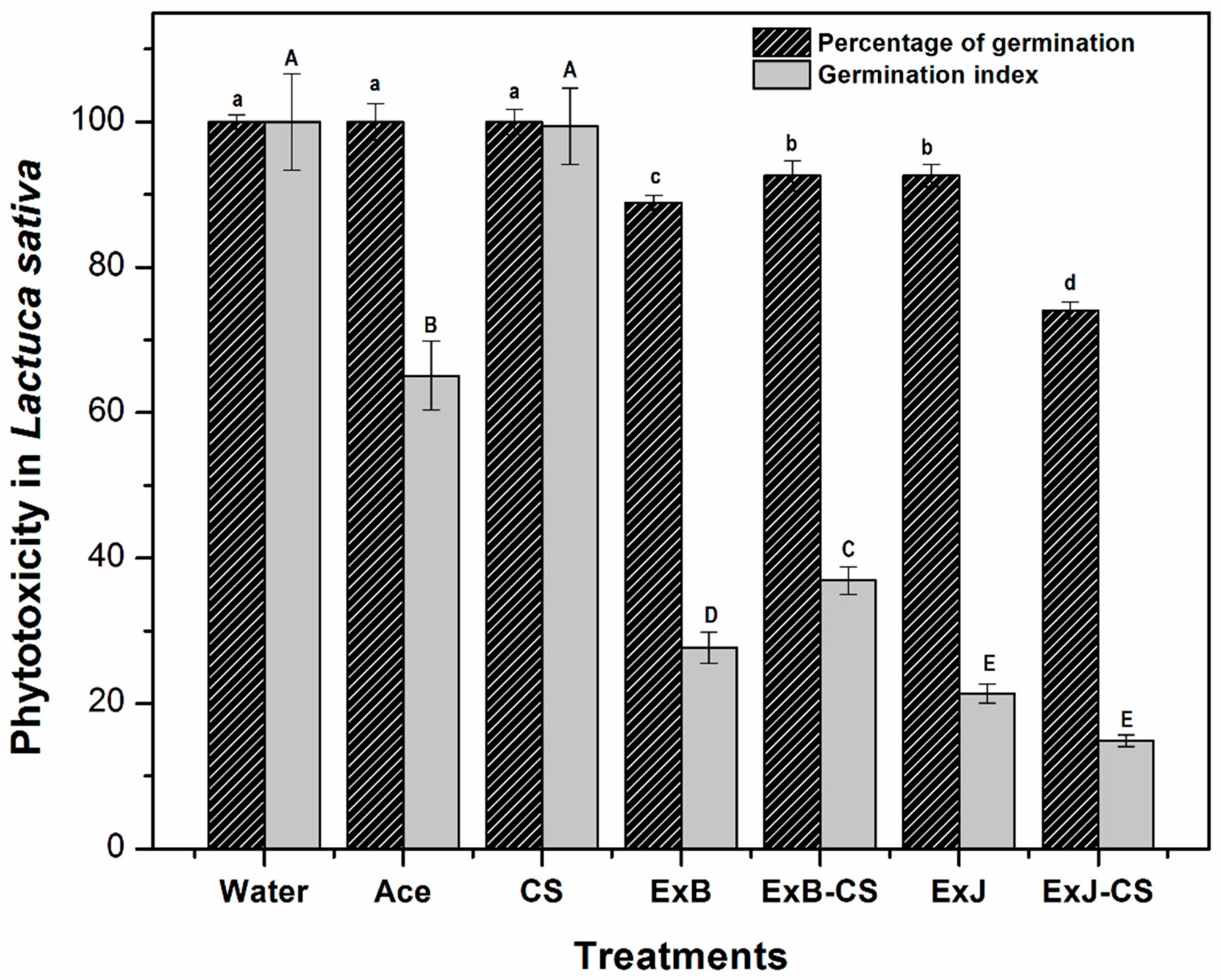

| Time (h) | Chitosan (CS) | B. glutinosa (ExB) | J. macrocarpa (ExJ) | |||
|---|---|---|---|---|---|---|
| 2 mg/mL | 4 mg/mL | 2 mg/mL | 4 mg/mL | 2 mg/mL | 4 mg/mL | |
| 24 | 100 ± 0.0 a | 100 ± 0.0 a | 100 ± 0.0 a | 100 ± 0.0 a | 100 ± 0.0 a | 100 ± 0.0 a |
| 48 | 60.9 ± 2.7 b | 62.4 ± 2.3 b | 100 ± 0.0 a | 100 ± 0.0 a | 43.7 ± 2.3 c | 42.1 ± 2.7 c |
| 72 | 29.5 ± 0.8 d | 47.0 ± 3.7 b | 79.6 ± 2.5 a | 81.1 ± 1.5 a | 45.1 ± 5.9 bc | 38.3 ± 1.7 c |
| 96 | 26.6 ± 1.4 d | 46.3 ± 1.8 b | 71.8 ± 0.7 a | 72.6 ± 1.8 a | 42.3 ± 6.0 bc | 33.8 ± 5.6 cd |
| 120 | 22.4 ± 1.5 d | 42.5 ± 2.6 bc | 63.3 ± 1.5 a | 67.2 ± 2.3 a | 41.8 ± 3.9 b | 33.7 ± 2.6 c |
| 144 | 25.7 ± 1.0 d | 39.8 ± 11.2 c | 62.5 ± 0.8 a | 66.8 ± 2.5 a | 45.4 ± 1.2 b | 38.2 ± 2.3 c |
| 168 | 27.6 ± 0.0 f | 38.6 ± 0.9 e | 63.5 ± 0.6 b | 67.6 ± 0.4 a | 47.4 ± 0.6 c | 43.3 ± 0.4 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Gante-de la Maza, S.; Plascencia-Jatomea, M.; Cortez-Rocha, M.O.; Sánchez-Mariñez, R.I.; Meneses-Sagrero, S.E.; Gálvez-Iriqui, A.C.; López-Meneses, A.K. Chitosan Combined with Methanolic Plants Extracts: Antifungal Activity, Phytotoxicity and Acute Toxicity. Polysaccharides 2025, 6, 52. https://doi.org/10.3390/polysaccharides6020052
de Gante-de la Maza S, Plascencia-Jatomea M, Cortez-Rocha MO, Sánchez-Mariñez RI, Meneses-Sagrero SE, Gálvez-Iriqui AC, López-Meneses AK. Chitosan Combined with Methanolic Plants Extracts: Antifungal Activity, Phytotoxicity and Acute Toxicity. Polysaccharides. 2025; 6(2):52. https://doi.org/10.3390/polysaccharides6020052
Chicago/Turabian Stylede Gante-de la Maza, Sofía, Maribel Plascencia-Jatomea, Mario Onofre Cortez-Rocha, Reyna Isabel Sánchez-Mariñez, Salvador Enrique Meneses-Sagrero, Alma Carolina Gálvez-Iriqui, and Ana Karenth López-Meneses. 2025. "Chitosan Combined with Methanolic Plants Extracts: Antifungal Activity, Phytotoxicity and Acute Toxicity" Polysaccharides 6, no. 2: 52. https://doi.org/10.3390/polysaccharides6020052
APA Stylede Gante-de la Maza, S., Plascencia-Jatomea, M., Cortez-Rocha, M. O., Sánchez-Mariñez, R. I., Meneses-Sagrero, S. E., Gálvez-Iriqui, A. C., & López-Meneses, A. K. (2025). Chitosan Combined with Methanolic Plants Extracts: Antifungal Activity, Phytotoxicity and Acute Toxicity. Polysaccharides, 6(2), 52. https://doi.org/10.3390/polysaccharides6020052









