Cellular Antioxidant Potential and Cytotoxic Activities of Extracellular Polysaccharides Isolated from Lactobacillus graminis Strain KNUAS018
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Consumables
2.2. Culture Condition and Extraction of Crude EPS
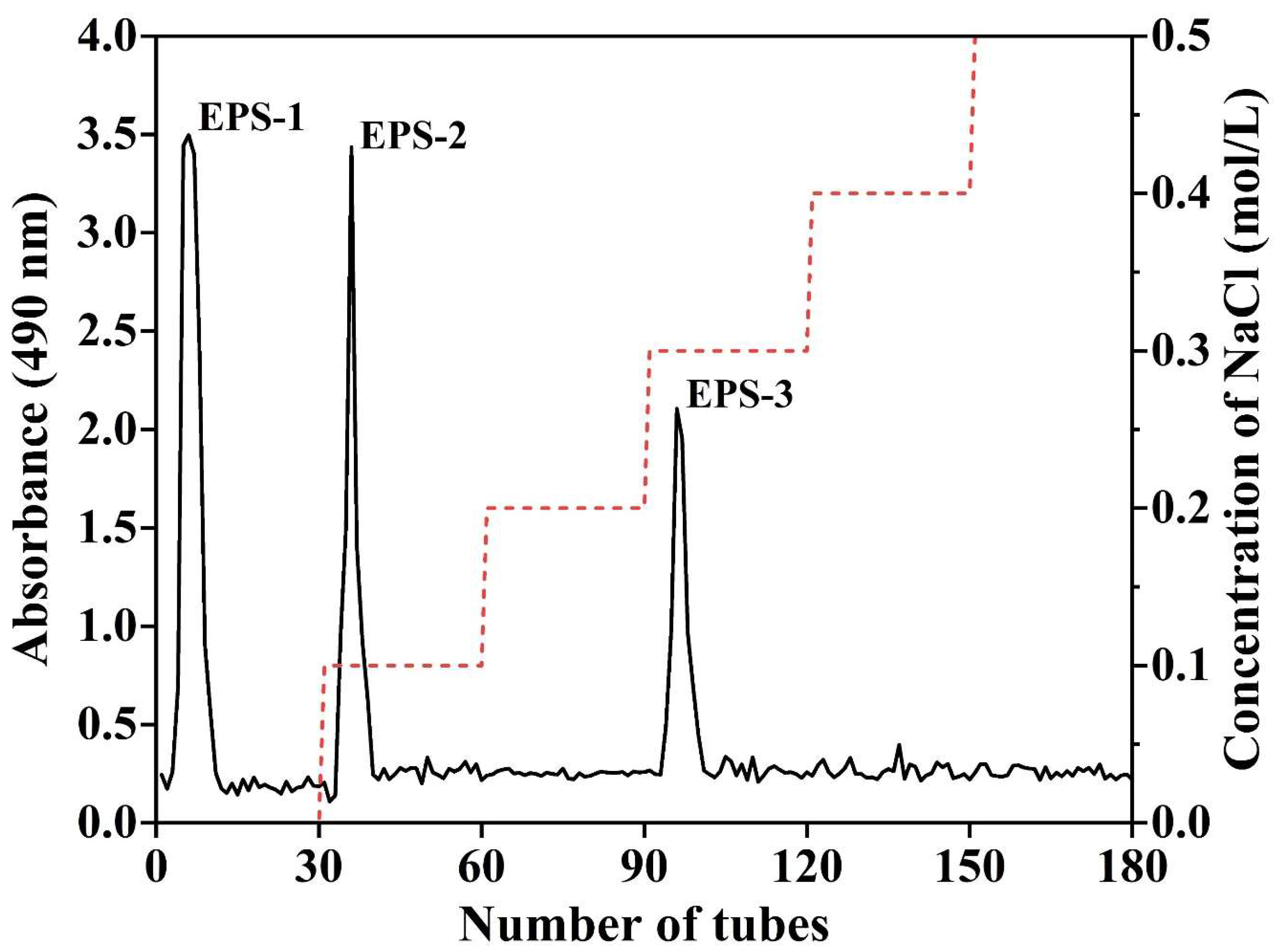
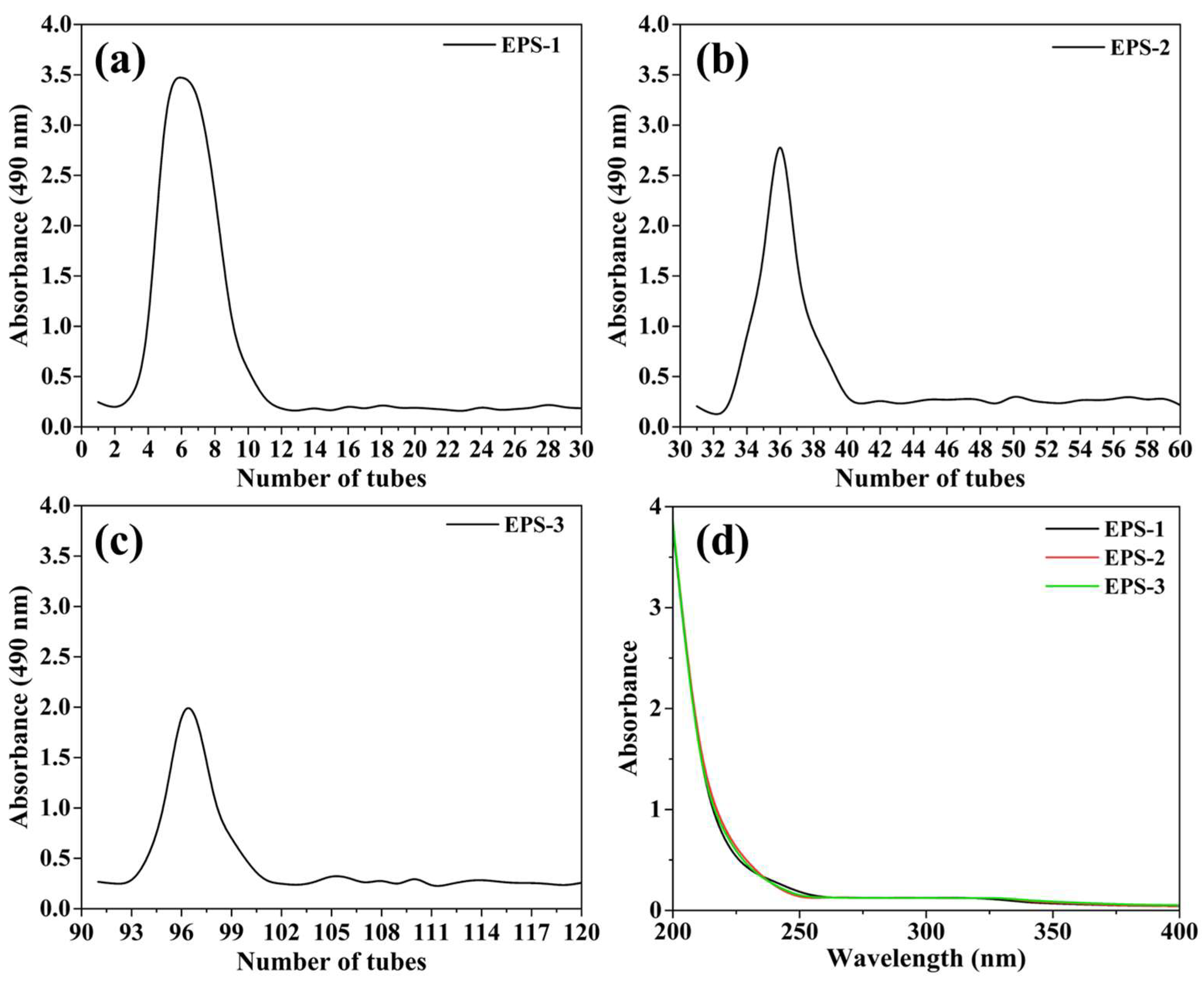
2.3. Purification and Fractionation of EPS
2.4. Biochemical Assays and Characterization Studies
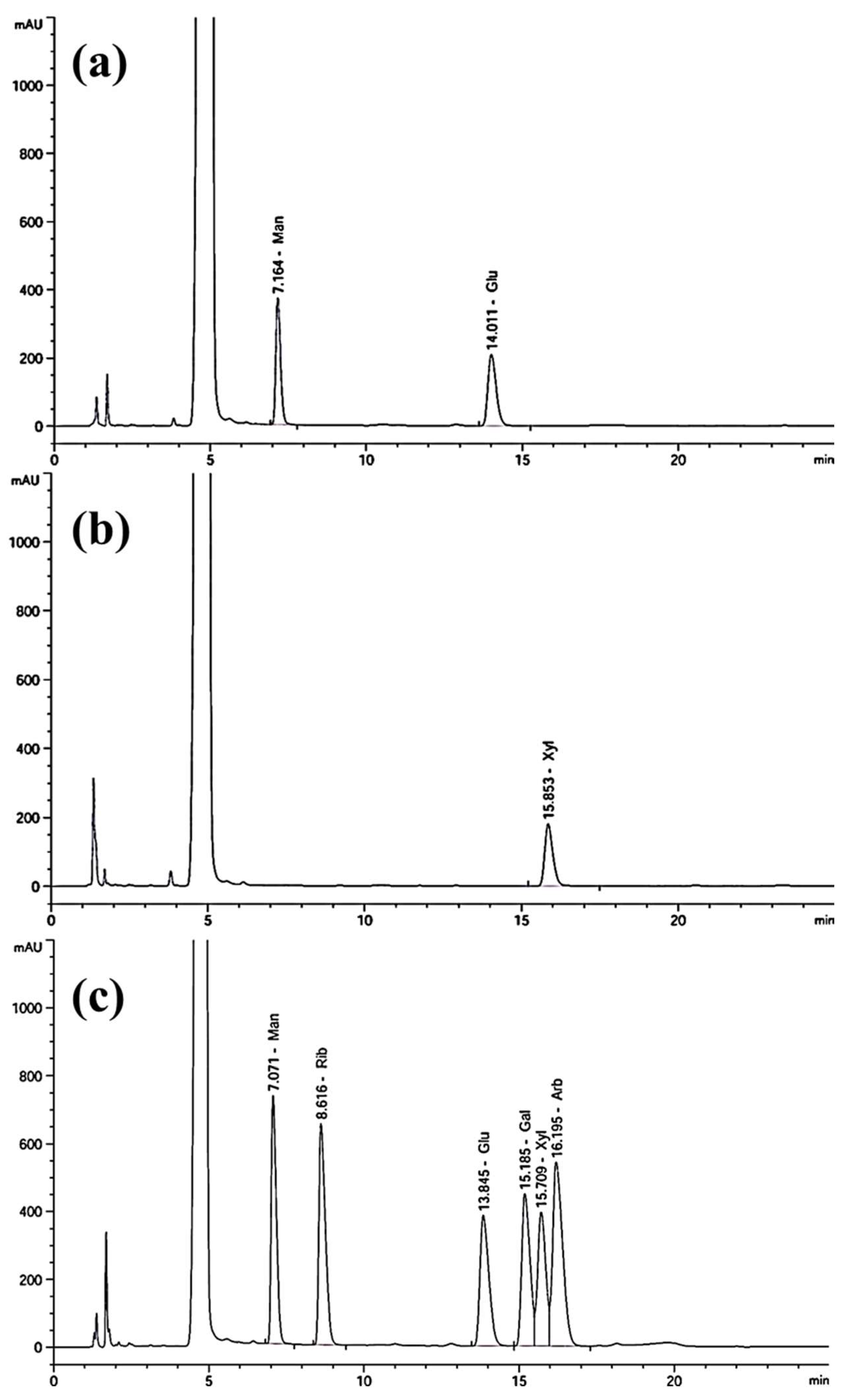
2.5. Monosaccharide Composition Analysis
2.6. Free Radical Scavenging Assay
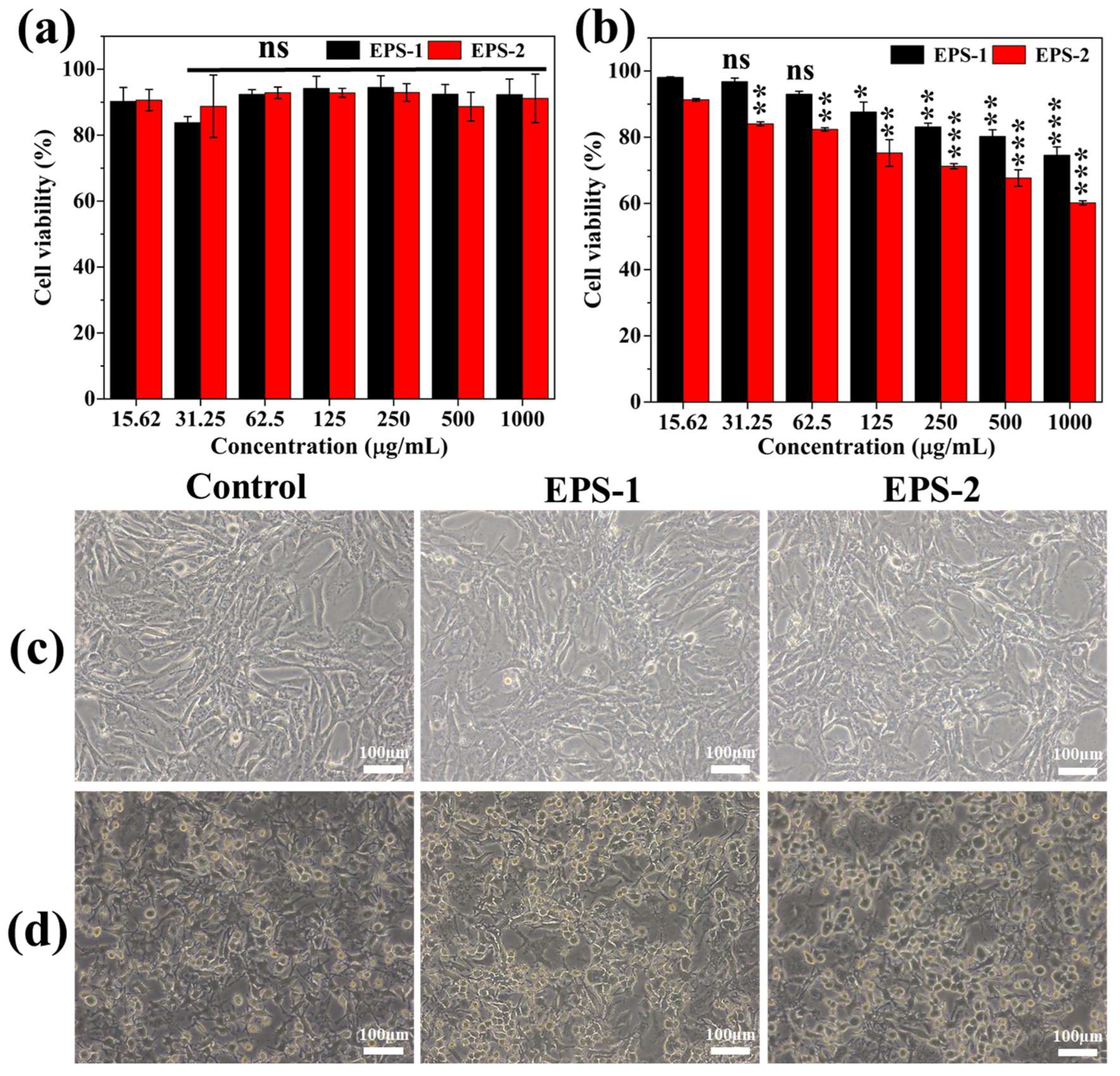
2.7. Cytotoxicity Assay
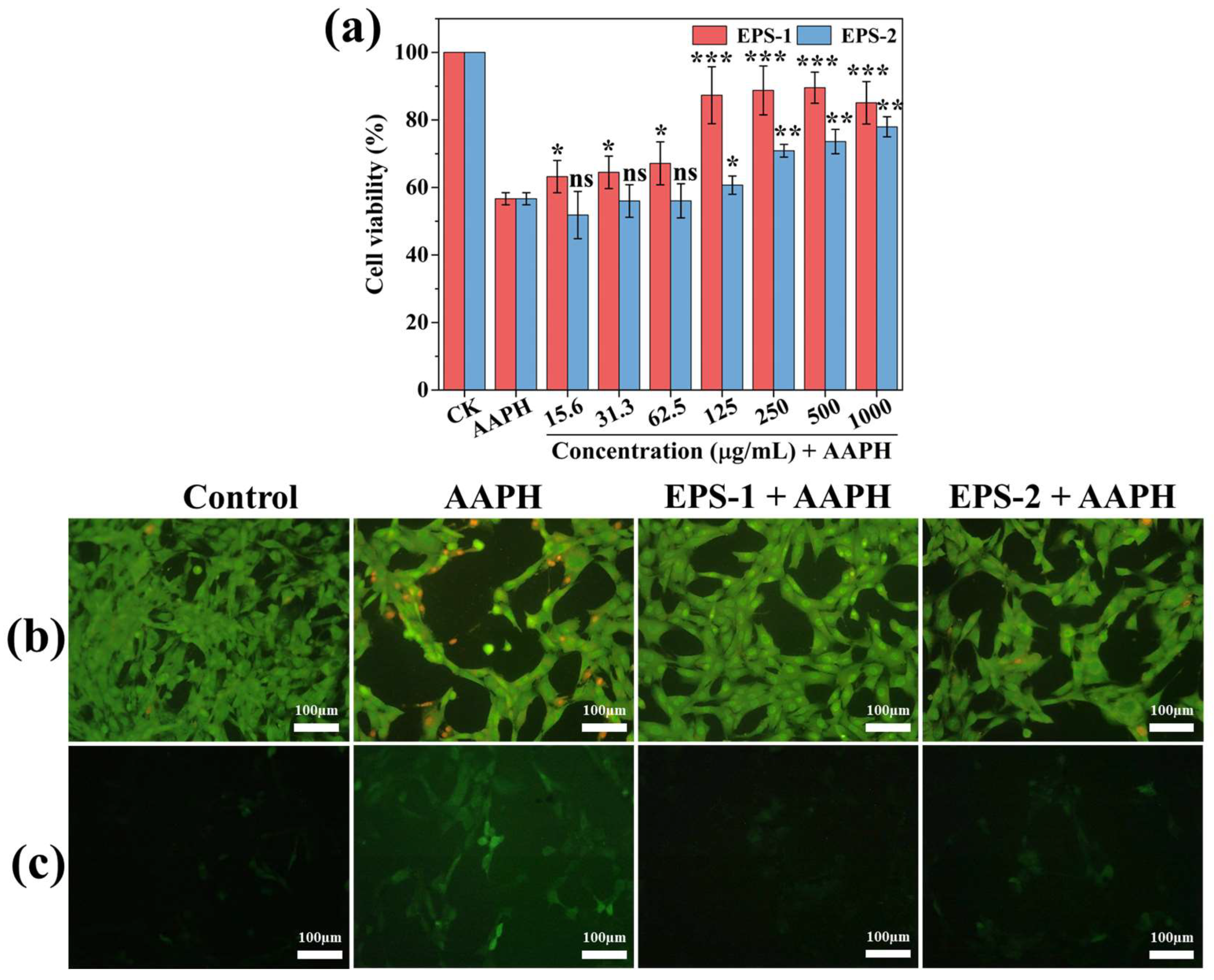
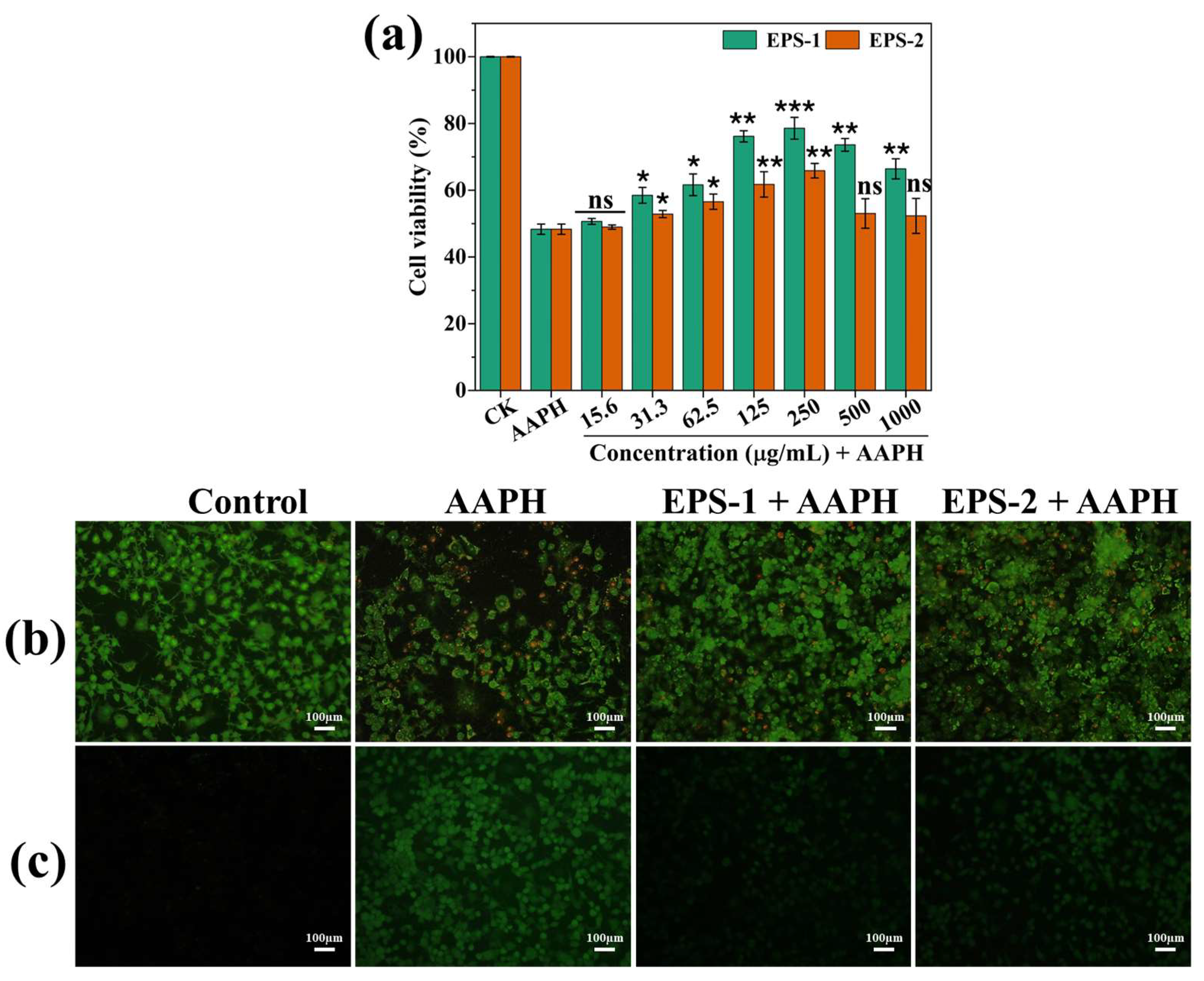
2.8. Cellular Antioxidant Assay
2.9. AAPH-Induced ROS Inhibition Assay
2.10. Hemolysis Assay
2.11. Statistical Analysis
3. Results and Discussion
3.1. EPS Production by L. graminis in MRS Broth
3.2. Isolation, Purification, and Biochemical Composition of EPS
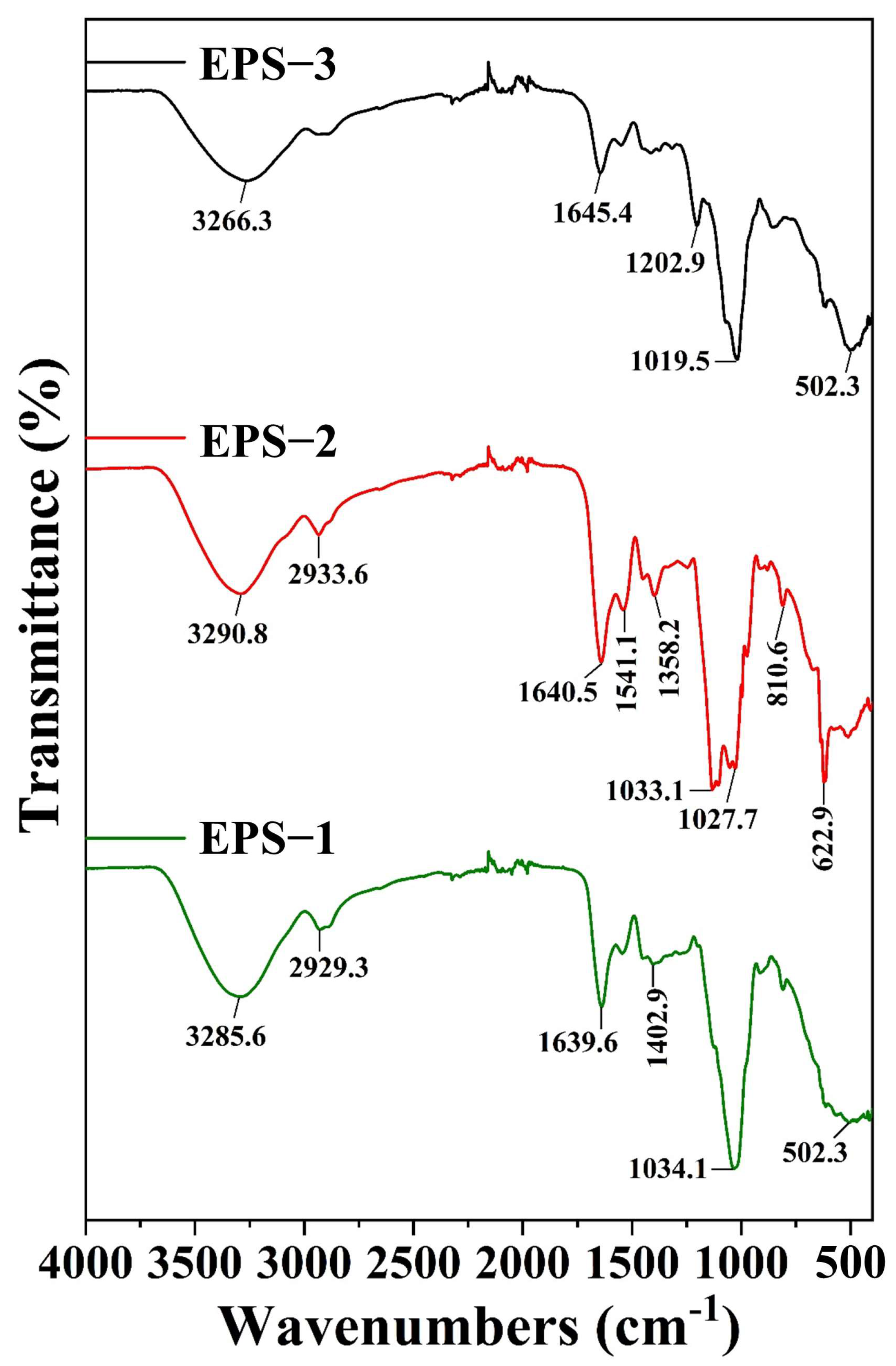
3.3. FT-IR Analysis
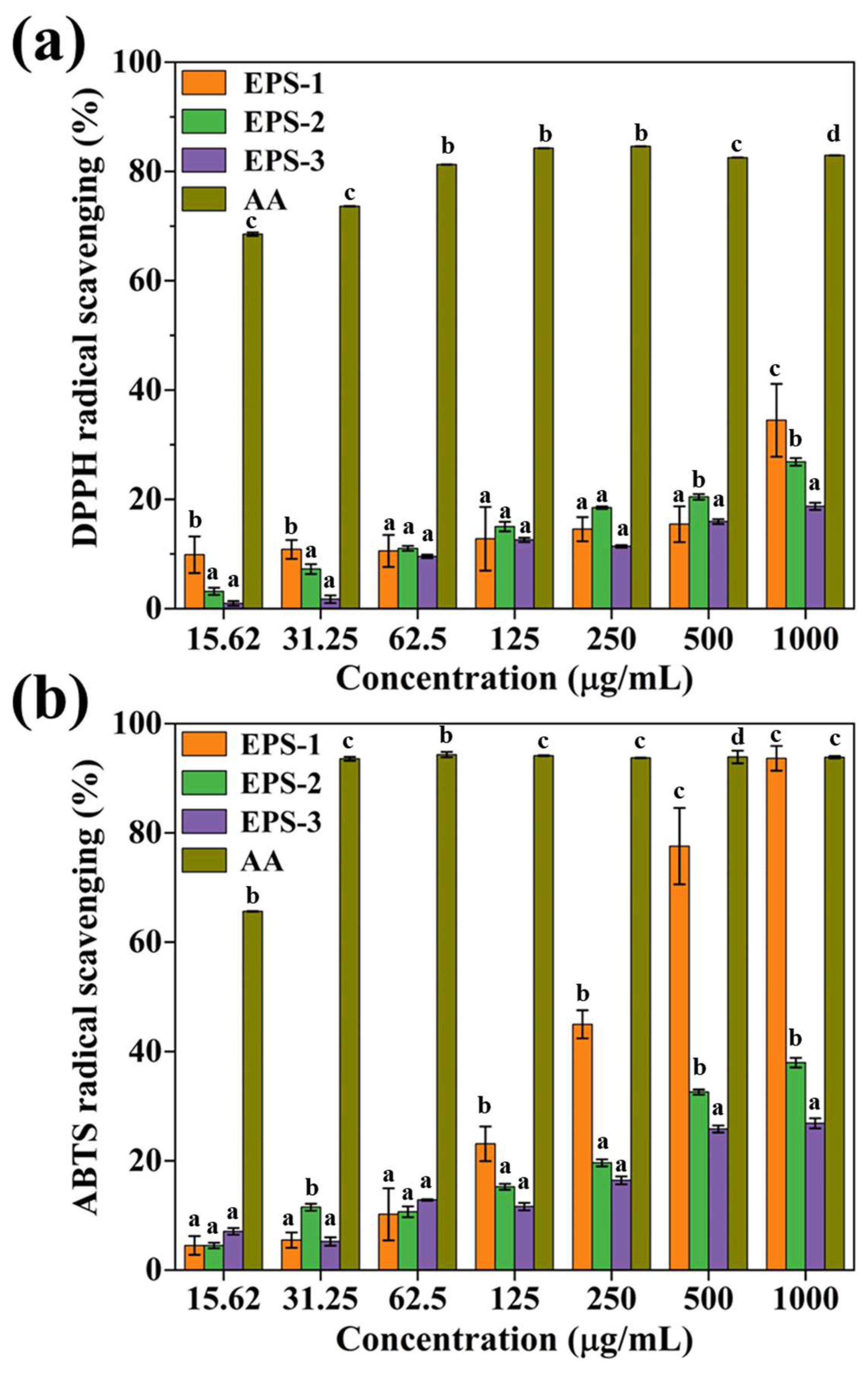
3.4. Free Radical Scavenging Activity
3.5. Monosaccharide Composition of EPS
3.6. Cytotoxic Activity
3.7. Cellular Antioxidant Activity
3.8. AAPH-Induced ROS Inhibition Assay
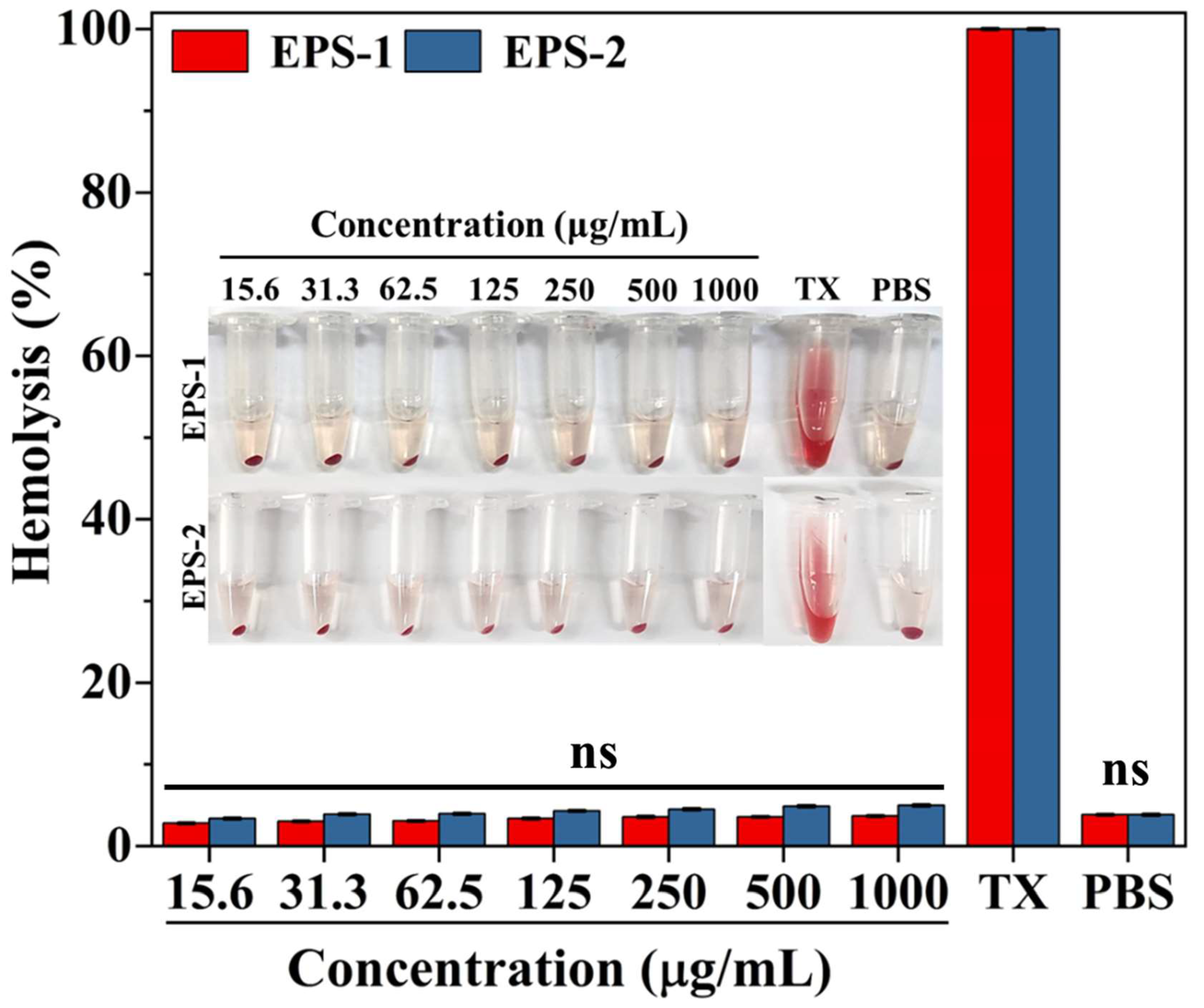
3.9. Hemolytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, E.; Lee, J.; Seo, S.; Uddin, S.; Lee, S.; Han, S.B.; Cho, S. Regulation of anti-inflammatory and antioxidant responses by methanol extract of Mikania cordata (Burm. f.) BL Rob. leaves via the inactivation of NF-κB and MAPK signaling pathways and activation of Nrf2 in LPS-induced RAW 264.7 macrophages. Biomed. Pharmacother. 2023, 168, 115746. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, Z.; Zhang, J.; Yang, Q.; Ren, Z.; Zhang, C.; Liu, M.; Gao, Z.; Zhao, H.; Jia, L. Anti-inflammatory and hepatoprotective effects of exopolysaccharides isolated from Pleurotus geesteranus on alcohol-induced liver injury. Sci. Rep. 2018, 8, 10493. [Google Scholar] [CrossRef]
- Ghavidel, F.; Amiri, H.; Tabrizi, M.H.; Hosseini, H.; Sahebkar, A. The combinational effect of inulin and resveratrol on the oxidative stress and inflammation level in a rat model of diabetic nephropathy. Curr. Dev. Nutr. 2023, 8, 102059. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-J.; Nan, G.-X. Oxidative stress-induced angiogenesis. J. Clin. Neurosci. 2019, 63, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Shoura, S.M.S.; Naghsh, N.; Moslemi, E.; Kavyani, Z.; Moridpour, A.H.; Musazadeh, V.; Dehghan, P. Can resveratrol supplementation affect biomarkers of inflammation and oxidative stress? An umbrella meta-analysis. J. Funct. Foods 2022, 99, 105360. [Google Scholar] [CrossRef]
- Gómez, P.I.; Mayorga, J.; Flaig, D.; Castro-Varela, P.; Jaupi, A.; Ulloa, P.A.; Soto-Bartierra, J.; Henríquez, V.; Rojas, V. Looking beyond Arthrospira: Comparison of antioxidant and anti-inflammatory properties of ten cyanobacteria strains. Algal Res. 2023, 74, 103182. [Google Scholar] [CrossRef]
- Allu, I.; Kumar Sahi, A.; Kumari, P.; Sakhile, K.; Sionkowska, A.; Gundu, S. A brief review on cerium oxide (CeO2NPs)-based scaffolds: Recent advances in wound healing applications. Micromachines 2023, 14, 865. [Google Scholar] [CrossRef]
- Shen, M.; Wang, Y.; Bing, T.; Tang, Y.; Liu, X.; Yu, Y. Alendronate triggered dual-cascade targeting prodrug nanoparticles for enhanced tumor penetration and sting activation of osteosarcoma. Adv. Funct. Mater. 2023, 33, 2307013. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Li, D.; Zhao, Y.; Zhang, X.; Zeng, X.; Yang, Z.; Li, S. Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int. J. Biol. Macromol. 2013, 54, 270–275. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.A.; F. Abd El-Kareem, H.; Alzamami, A.; A. Fahmy, C.; H. Elesawy, B.; Mostafa Mahmoud, M.; Ghareeb, A.; El Askary, A.; H. Abo Nahas, H.; G. M. Attallah, N.; et al. Novel Exopolysaccharide from Marine Bacillus subtilis with Broad Potential Biological Activities: Insights into Antioxidant, Anti-Inflammatory, Cytotoxicity, and Anti-Alzheimer Activity. Metabolites 2022, 12, 715. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Zamora-Pineda, J.; Kalinina, O.; Sperling, A.I.; Knight, K.L. Mechanism of TLR4-Mediated Anti-Inflammatory Response Induced by Exopolysaccharide from the Probiotic Bacillus subtilis. J. Immunol. 2023, 211, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.G.; Mohamed, S.S.; Ibrahim, A.Y.; El Awady, M.E.; Youness, E.R. Exopolysaccharide produced by paenibacillus lactes NRC1: Its characterization and anti-inflammatory activity via cyclooxygenases inhibitory activity and modulation of inflammation related cytokines. Der Pharma Chem. 2016, 8, 16–26. [Google Scholar]
- Mgomi, F.C.; Yang, Y.-R.; Cheng, G.; Yang, Z.-Q. Lactic acid bacteria biofilms and their antimicrobial potential against pathogenic microorganisms. Biofilm 2023, 5, 100118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, C.; Jia, S.; Wang, B.; Zhou, K.; Chen, S.; Yang, Y.; Liu, S. Purification, characterization and antioxidant activity of the exopolysaccharide from Weissella cibaria SJ14 isolated from Sichuan paocai. Int. J. Biol. Macromol. 2018, 115, 820–828. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Han, K.; Naveen, K.V.; Wang, M.-H. Antioxidant and Antibacterial Effects of Potential Probiotics Isolated from Korean Fermented Foods. Int. J. Mol. Sci. 2022, 23, 10062. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kashyap, P.K.; Chauhan, S.; Negi, Y.S.; Goel, N.K.; Rattan, S. Biocompatible carboxymethyl chitosan-modified glass ionomer cement with enhanced mechanical and anti-bacterial properties. Int. J. Biol. Macromol. 2022, 223, 1506–1520. [Google Scholar] [CrossRef]
- Naveen, K.V.; Park, S.; Saravanakumar, K.; Sathiyaseelan, A.; Wang, M.-H. Ultraperformance liquid chromatography-based metabolite profiling and cytotoxic activity of the ethyl acetate extract of two endophytic Penicillium sp. Process Biochem. 2023, 130, 366–378. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Wang, M.-H. Cerium oxide decorated 5-fluorouracil loaded chitosan nanoparticles for treatment of hepatocellular carcinoma. Int. J. Biol. Macromol. 2022, 216, 52–64. [Google Scholar] [CrossRef]
- Islam, S.T.; Lam, J.S. Synthesis of bacterial polysaccharides via the Wzx/Wzy-dependent pathway. Can. J. Microbiol. 2014, 60, 697–716. [Google Scholar] [CrossRef] [PubMed]
- Becker, A. Challenges and perspectives in combinatorial assembly of novel exopolysaccharide biosynthesis pathways. Front. Microbiol. 2015, 6, 687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fan, C.; Liu, S.; Zang, Z.; Jiao, L.; Zhang, L. Chemical composition and antitumor activity of polysaccharide from Inonotus obliquus. J. Med. Plants Res. 2011, 5, 1251–1260. [Google Scholar]
- Greenfield, L.K.; Whitfield, C. Synthesis of lipopolysaccharide O-antigens by ABC transporter-dependent pathways. Carbohydr. Res. 2012, 356, 12–24. [Google Scholar] [CrossRef]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef]
- Liang, S.; Wang, X.; Li, C.; Liu, L. Biological activity of lactic acid bacteria exopolysaccharides and their applications in the food and pharmaceutical industries. Foods 2024, 13, 1621. [Google Scholar] [CrossRef]
- Vandana; Das, S. Genetic regulation, biosynthesis and applications of extracellular polysaccharides of the biofilm matrix of bacteria. Carbohydr. Polym. 2022, 291, 119536. [Google Scholar] [CrossRef]
- Nguyen, P.-T.; Nguyen, T.-T.; Bui, D.-C.; Hong, P.-T.; Hoang, Q.-K.; Nguyen, H.-T. Exopolysaccharide production by lactic acid bacteria: The manipulation of environmental stresses for industrial applications. AIMS Microbiol. 2020, 6, 451. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, H.M.; Rochfort, K.D.; Maye, S.; MacLeod, G.; Brabazon, D.; Loscher, C.; Freeland, B. Exopolysaccharides of lactic acid bacteria: Production, purification and health benefits towards functional food. Nutrients 2022, 14, 2938. [Google Scholar] [CrossRef]
- Vázquez-Vargas, C.C.; Cordero-Soto, I.N.; Flores-Maciel, H.A.; Lara-Ceniceros, T.E.; Gallegos-Infante, A.; González-Herrera, S.M.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, O.M. Bioproduction of exopolysaccharides by lactic acid bacteria using agave by-products. Process Biochem. 2024, 146, 234–240. [Google Scholar] [CrossRef]
- Ai, L.; Guo, Q.; Ding, H.; Guo, B.; Chen, W.; Cui, S.W. Structure characterization of exopolysaccharides from Lactobacillus casei LC2W from skim milk. Food Hydrocoll. 2016, 56, 134–143. [Google Scholar] [CrossRef]
- Park, S.; Saravanakumar, K.; Sathiyaseelan, A.; Park, S.; Hu, X.; Wang, M.-H. Cellular antioxidant properties of nontoxic exopolysaccharide extracted from Lactobacillales (Weissella cibaria) isolated from Korean kimchi. LWT 2022, 154, 112727. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Yang, Y.; Zhao, A.; Yang, Z. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int. J. Biol. Macromol. 2015, 74, 119–126. [Google Scholar] [CrossRef]
- Rani, R.P.; Anandharaj, M.; Ravindran, A.D. Characterization of a novel exopolysaccharide produced by Lactobacillus gasseri FR4 and demonstration of its in vitro biological properties. Int. J. Biol. Macromol. 2018, 109, 772–783. [Google Scholar] [CrossRef]
- Ibarburu, I.; Puertas, A.I.; Berregi, I.; Rodríguez-Carvajal, M.A.; Prieto, A.; Dueñas, M.T. Production and partial characterization of exopolysaccharides produced by two Lactobacillus suebicus strains isolated from cider. Int. J. Food Microbiol. 2015, 214, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Mokarram, R.R.; Khiabani, M.S.; Bari, M.R.; Khaledabad, M.A. Exopolysaccharides production by Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp. lactis BB12: Optimization of fermentation variables and characterization of structure and bioactivities. Int. J. Biol. Macromol. 2019, 123, 752–765. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.Y.M.; Reehana, N.; Jayaraj, K.A.; Ahamed, A.A.P.; Dhanasekaran, D.; Thajuddin, N.; Alharbi, N.S.; Muralitharan, G. Statistical optimization of exopolysaccharide production by Lactobacillus plantarum NTMI05 and NTMI20. Int. J. Biol. Macromol. 2016, 93, 731–745. [Google Scholar] [CrossRef]
- Li, W.; Ji, J.; Chen, X.; Jiang, M.; Rui, X.; Dong, M. Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2-1. Carbohydr. Polym. 2014, 102, 351–359. [Google Scholar] [CrossRef]
- Di, W.; Zhang, L.; Wang, S.; Yi, H.; Han, X.; Fan, R.; Zhang, Y. Physicochemical characterization and antitumour activity of exopolysaccharides produced by Lactobacillus casei SB27 from yak milk. Carbohydr. Polym. 2017, 171, 307–315. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Tian, Z.; Yang, Y.; Yang, Z. Characterization of an exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibet Kefir. Carbohydr. Polym. 2015, 125, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Chen, Y.; Wang, C.; Yang, R.; Bin, X. Purification and characterization of exopolysaccharide produced by Weissella cibaria YB-1 from pickle Chinese cabbage. Int. J. Biol. Macromol. 2018, 120, 1315–1321. [Google Scholar] [CrossRef]
- Yang, X.; Ren, Y.; Zhang, L.; Wang, Z.; Li, L. Structural characteristics and antioxidant properties of exopolysaccharides isolated from soybean protein gel induced by lactic acid bacteria. LWT 2021, 150, 111811. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, L.; Zhu, M.; Wu, S.; Wang, X.; Li, D.; Liu, C.; Feng, Z.; Tian, B. Separation, structural characteristics and biological activity of lactic acid bacteria exopolysaccharides separated by aqueous two-phase system. LWT 2021, 147, 111617. [Google Scholar] [CrossRef]
- Min, W.-H.; Fang, X.-B.; Wu, T.; Fang, L.; Liu, C.-L.; Wang, J. Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J. Biosci. Bioeng. 2019, 127, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, W.; Sun, L.; Sadiq, F.A.; Yang, Y.; Gao, J.; Sang, Y. Preparation screening, production optimization and characterization of exopolysaccharides produced by Lactobacillus sanfranciscensis Ls-1001 isolated from Chinese traditional sourdough. Int. J. Biol. Macromol. 2019, 139, 1295–1303. [Google Scholar] [CrossRef]
- Ng, I.S.; Xue, C. Enhanced exopolysaccharide production and biological activity of Lactobacillus rhamnosus ZY with calcium and hydrogen peroxide. Process Biochem. 2017, 52, 295–304. [Google Scholar] [CrossRef]
- Wen, Z.-S.; Liu, L.-J.; OuYang, X.-K.; Qu, Y.-L.; Chen, Y.; Ding, G.-F. Protective effect of polysaccharides from Sargassum horneri against oxidative stress in RAW264. 7 cells. Int. J. Biol. Macromol. 2014, 68, 98–106. [Google Scholar] [CrossRef]
- Yang, H.-L.; Korivi, M.; Lin, M.-K.; Chang, H.C.-W.; Wu, C.-R.; Lee, M.-S.; Chen, W.T.-L.; Hseu, Y.-C. Antihemolytic and antioxidant properties of pearl powder against 2, 2′-azobis (2-amidinopropane) dihydrochloride-induced hemolysis and oxidative damage to erythrocyte membrane lipids and proteins. J. Food Drug Anal. 2017, 25, 898–907. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Hwang, J.; Ko, J.Y.; Jeon, Y.-J.; Ryu, B. In vitro and in vivo antioxidant activities of polysaccharides isolated from celluclast-assisted extract of an edible brown seaweed, Sargassum fulvellum. Antioxidants 2019, 8, 493. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, Y.; Yue, F.; Liu, L.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Exopolysaccharides produced by lactic acid bacteria and Bifidobacteria: Structures, physiochemical functions and applications in the food industry. Food Hydrocoll. 2019, 94, 475–499. [Google Scholar] [CrossRef]
- Gao, G.; Zhou, J.; Zhou, J.; Wang, H.; Ke, L.; Ding, Y.; Zhang, S.; Ding, W.; Rao, P.; Li, J. Divalent cations of magnesium, iron and copper regulate oxidative responses and inflammatory cytokines in RAW 264.7 macrophages. Food Control 2022, 141, 109212. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.; Wu, T.; Fang, L.; Liu, C.; Min, W. In vitro immunomodulatory effects of acidic exopolysaccharide produced by Lactobacillus planetarium JLAU103 on RAW264.7 macrophages. Int. J. Biol. Macromol. 2020, 156, 1308–1315. [Google Scholar] [CrossRef]
- Kim, K.; Lee, G.; Thanh, H.D.; Kim, J.-H.; Konkit, M.; Yoon, S.; Park, M.; Yang, S.; Park, E.; Kim, W. Exopolysaccharide from Lactobacillus plantarum LRCC5310 offers protection against rotavirus-induced diarrhea and regulates inflammatory response. J. Dairy Sci. 2018, 101, 5702–5712. [Google Scholar] [CrossRef] [PubMed]
- Adesulu-Dahunsi, A.; Sanni, A.; Jeyaram, K. Production, characterization and in vitro antioxidant activities of exopolysaccharide from Weissella cibaria GA44. LWT 2018, 87, 432–442. [Google Scholar] [CrossRef]
- Li, J.-Y.; Jin, M.-M.; Meng, J.; Gao, S.-M.; Lu, R.-R. Exopolysaccharide from Lactobacillus planterum LP6: Antioxidation and the effect on oxidative stress. Carbohydr. Polym. 2013, 98, 1147–1152. [Google Scholar] [CrossRef]
- Xu, X.; Qiao, Y.; Peng, Q.; Dia, V.P.; Shi, B. Probiotic activity of ropy Lactiplantibacillus plantarum NA isolated from Chinese northeast sauerkraut and comparative evaluation of its live and heat-killed cells on antioxidant activity and RAW 264.7 macrophage stimulation. Food & Function 2023, 14, 2481–2495. [Google Scholar]
- Campos-Sánchez, J.C.; Guardiola, F.A.; Esteban, M.Á. In vitro effects of a natural marine algae polysaccharide (λ-carrageenan) on seabream erythrocytes, tumour cell lines and marine bacterial pathogens. J. Appl. Phycol. 2023, 36, 399–409. [Google Scholar] [CrossRef]
- Han, K.; Sathiyaseelan, A.; Saravanakumar, K.; Park, S.-Y.; Shin, S.; Choi, H.B.; Naveen, K.V.; Wang, M.-H. Biomimetic hydroxyapatite-chitosan nanoparticles deliver the erythromycin for improved antibacterial activity. J. Drug Deliv. Sci. Technol. 2022, 72, 103374. [Google Scholar] [CrossRef]
| Source | EPS-1 | EPS-2 | EPS-3 |
|---|---|---|---|
| Crude weight (g) | 1.46 | ||
| Yield (%) | 14.38 | 9.24 | 1.78 |
| Total phenol (mg of GAE/g) | 0.21 ± 0.02 | 0.16 ± 0.01 | 0.09 ± 0.01 |
| Total flavonoid (mg of QE/g) | 0.07 ± 0.008 | 0.04 ± 0.002 | - |
| Nucleic acid (%) | - | - | - |
| Protein (%) | - | - | 0.03 ± 0.001 |
| Source of EPS | Monosaccharide Composition | Free Radical Scavenging (Activity; Concentration Used) | Cellular Antioxidant Activity | Cytotoxicity Analysis (Viability) | Ref. |
|---|---|---|---|---|---|
| L. casei | Glucose (16.03%), mannose (6.94%), arabinose (12.16%), galactose (19.14%), rhamnose (7.93%), fucose (5.30%), ribose (2.95%), Gulcuronic acid (12.37%), Galacturonic acid (6.45%), xylose (10.72%) | DPPH (IC50: 3.24 mg/mL), hydroxyl radical (IC50: 1.03 mg/mL), and ABTS (IC50: 2.42 mg/mL). | - | - | [43] |
| L. sanfranciscensis | Glucose | ABTS (93.43%; 1 mg/mL). | - | - | [46] |
| LAB strain GA44 | Glucose and rhamnose | DPPH (48.9%; 4 mg/mL) superoxide anion radical (77.1%; 4 mg/mL) hydroxyl radical (88%; 4 mg/mL). | - | - | [55] |
| L. plantarum LP6 | Not evaluated | DPPH (64.85%; 1 mg/mL), Linoleic acid peroxidation (66.5%; 1 mg/mL). | - | HepG2 (100%; 256–512 μg/mL), Artemia nauplii (95–100%; 1 mg/mL). | [56] |
| L. planetarium JLAU103 | Approximate molar ratio arabinose (4.05), rhamnose (6.04), fucose (6.29), xylose (5.22), mannose (1.47), fructose (5.21), galactose (2.24), and glucose (1.83) | - | RAW264.7 (promoted IL-6, TNF-α and NO release), (inhibited COX-2 and iNOS expression), (inhibited NF-κB activation) | RAW264.7 (>100%; 0.1 mg/mL) | [45,57] |
| L. graminis strain KNUAS018 | EPS-1; mannose and glucose | DPPH (34.5 ± 6.6%; 1 mg/mL), ABTS (93.6 ± 2.3%; 1 mg/mL) | AAPH-stressed NIH3T3 viability (>85%; 0.25 mg/mL) | AAPH-induced RAW264.7 viability (~80%; 0.25 mg/mL) and reduced ROS level | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, K.; Naveen, K.V.; Zhang, X.; Sathiyaseelan, A.; Kim, H.-Y. Cellular Antioxidant Potential and Cytotoxic Activities of Extracellular Polysaccharides Isolated from Lactobacillus graminis Strain KNUAS018. Polysaccharides 2025, 6, 33. https://doi.org/10.3390/polysaccharides6020033
Han K, Naveen KV, Zhang X, Sathiyaseelan A, Kim H-Y. Cellular Antioxidant Potential and Cytotoxic Activities of Extracellular Polysaccharides Isolated from Lactobacillus graminis Strain KNUAS018. Polysaccharides. 2025; 6(2):33. https://doi.org/10.3390/polysaccharides6020033
Chicago/Turabian StyleHan, Kiseok, Kumar Vishven Naveen, Xin Zhang, Anbazhagan Sathiyaseelan, and Hye-Yong Kim. 2025. "Cellular Antioxidant Potential and Cytotoxic Activities of Extracellular Polysaccharides Isolated from Lactobacillus graminis Strain KNUAS018" Polysaccharides 6, no. 2: 33. https://doi.org/10.3390/polysaccharides6020033
APA StyleHan, K., Naveen, K. V., Zhang, X., Sathiyaseelan, A., & Kim, H.-Y. (2025). Cellular Antioxidant Potential and Cytotoxic Activities of Extracellular Polysaccharides Isolated from Lactobacillus graminis Strain KNUAS018. Polysaccharides, 6(2), 33. https://doi.org/10.3390/polysaccharides6020033







