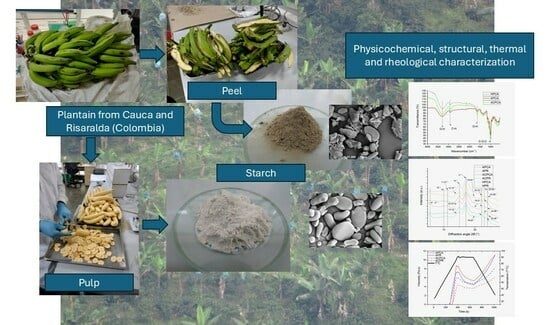
Extraction and Characterization of Starches from the Pulp and Peel of Native Plantain (Musa AAB Simmonds) from Two Colombian Departments
Abstract
1. Introduction
2. Materials
3. Experimental Procedure
3.1. Extraction of Starches from Plantain Pulp and Peel
3.2. Statistical Analysis
3.3. Physicochemical and Morphological Characterization of Starches from Plantain Pulp and Peel
3.3.1. Proximal Analysis
3.3.2. pH Determination
3.3.3. Morphology and Particle Size
3.3.4. Amylose Content
3.4. Structural Properties of Starches in Plantain Pulp and Peel
3.4.1. Fourier Transform Infrared Spectrometry (ATR-FTIR)
3.4.2. X-Ray Diffraction
3.5. Thermal Properties of the Starches in Plantain Pulp and Peels
3.5.1. Differential Scanning Calorimetry (DSC)
3.5.2. Thermogravimetric Analysis (TGA)
3.6. Techno-Functional Properties of Starches from Plantain Pulp and Peel
3.7. Rheological Properties of Plantain Peel and Pulp Starch
3.8. Statistical Design
4. Results and Discussions
4.1. Extraction of Starches from Plantain Pulp and Peels
4.1.1. General Characteristics of Plantain Bunch By-Products
4.1.2. Starch Extraction
4.2. Physicochemical, Thermal, and Structural Characterization of Flours and Starches from Plantain Coproducts
4.2.1. Proximal Analysis
4.2.2. pH Determination
4.2.3. Morphology and Particle Size
4.2.4. Amylose Content
4.3. Structural Properties of Starches in Plantain Pulp and Peel
4.3.1. Fourier Transform Infrared Spectrometry (ATR-FTIR)
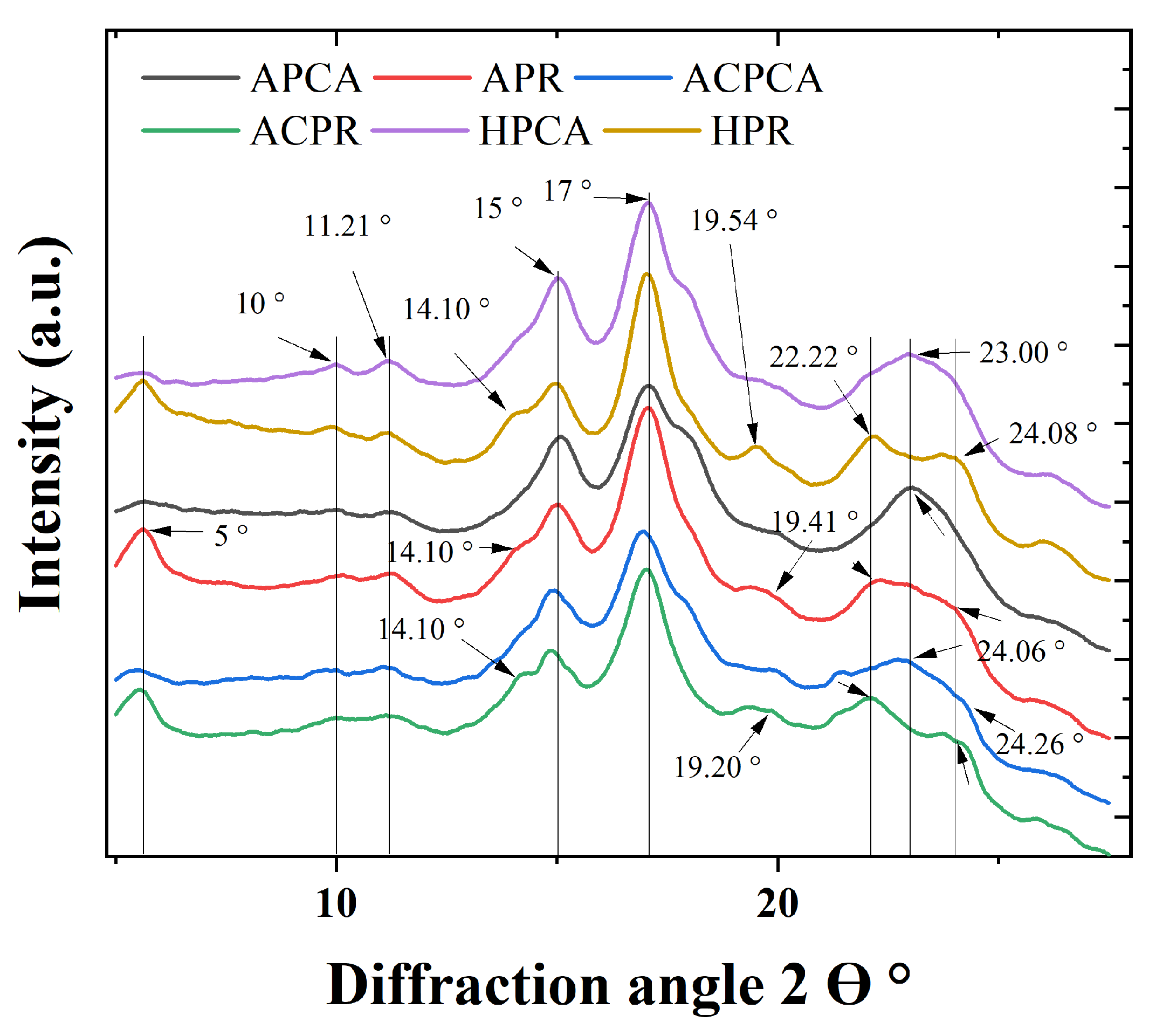
4.3.2. X-Ray Diffraction
4.4. Thermal Properties of the Starches in Plantain Pulp and Peels
4.5. Techno-Functional Properties of Starches from Plantain Pulp and Peel
4.6. Rheological Properties of Plantain Pulp and Peel Starches
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dita, M.; Garming, H.; Den Bergh, I.; Staver, C.; Lescot, T. Banana in Latin America and the Caribbean: Current state challenges and perspectives. Acta Hortic. 2013, 986, 365–380. [Google Scholar] [CrossRef]
- Jaramillo, R. Banana and Plantain Production in Latin America and the Caribbean. In Proceedings of the International Workshop, Cairns, Australia, 13–17 October 1986. Banana and Plantain Breeding Strategies: ACIAR No. 21, 197p. [Google Scholar]
- Minagricultura. CADENA DE PLÁTANO; 2020. Available online: https://sioc.minagricultura.gov.co/Platano/Documentos/2020-03-31%20Cifras%20Sectoriales.pdf (accessed on 24 February 2025).
- CORPOICA. El cultivo de Plátano; 1999. Available online: https://repository.agrosavia.co/handle/20.500.12324/2095 (accessed on 24 February 2025).
- Arizabaleta, C.; Giraldo, A. Diversificación de Productos Agroindustriales Colombianos para Exportación; Colegio de Estudios Superiores de Administración—CESA: Bogota, Colombia, 2018. [Google Scholar]
- Gómez Soto, J.A.; Sánchez Toro, Ó.J.; Matallana Pérez, L.G. Processes of transformation: Perspective of use for the residues of the plantain agro-industry. Prod. Limpia 2021, 16, 6–30. [Google Scholar]
- Mohapatra, D.; Mishra, S.; Meda, V. Plantains and their postharvest uses: An overview. Stewart Postharvest Rev. 2009, 5, 1–11. [Google Scholar]
- Serna-Jiménez, J.A.; Siles López, J.Á.; De Los Ángeles Martín Santos, M.; Chica Pérez, A.F. Exploiting waste derived from Musa spp. processing: Banana and plantain. Biofuels Bioprod. Biorefining 2023, 17, 1046–1067. [Google Scholar] [CrossRef]
- Castañeda-Niño, J.P.; Mina-Hernandez, J.H.; Valadez-González, A. Potential uses of Musaceae wastes: Case of application in the development of bio-based composites. Polymers 2021, 13, 1844. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Carmona, F.; Morales-Matos, Y.; Lambis-Miranda, H.; Pasqualino, J. Starch extraction potential from plantain peel wastes. J. Environ. Chem. Eng. 2017, 5, 4980–4985. [Google Scholar] [CrossRef]
- Mestres, C.; Taylor, M.; McDougall, G.; Arufe, S.; Tran, T.; Nuwamanya, E.; Dufour, D.; Nakitto, M.; Meghar, K.; Rinaldo, D.; et al. Contrasting Effects of Polysaccharide Components on the Cooking Properties of Roots, Tubers and Bananas. J. Sci. Food Agric. 2024, 104, 4652–4661. [Google Scholar] [CrossRef]
- Paramasivam, S.K.; Saravanan, A.; Narayanan, S.; Shiva, K.N.; Ravi, I.; Mayilvaganan, M.; Pushpa, R.; Uma, S. Exploring differences in the physicochemical, functional, structural, and pasting properties of banana starches from dessert, cooking, and plantain cultivars (Musa spp.). Int. J. Biol. Macromol. 2021, 191, 1056–1067. [Google Scholar] [CrossRef]
- Ferreira-Villadiego, J.; García-Echeverri, J.; Vidal, M.V.; Pasqualino, J.; Meza-Castellar, P.; Lambis-Miranda, H.A. Chemical modification and characterization of starch derived from plantain (Musa paradisiaca) peel waste, as a source of biodegradable material. Chem. Eng. Trans. 2018, 65, 763–768. [Google Scholar]
- Nasrin, T.A.A.; Noomhorm, A.; Anal, A.K. Physico-Chemical Characterization of Culled Plantain Pulp Starch, Peel Starch, and Flour. Int. J. Food Prop. 2015, 18, 165–177. [Google Scholar] [CrossRef]
- Torres-Vargas, O.L.; Gaytan-Martinez, M.; Fernanda, C.C.; Millán-Malo, B.M.; Rodriguez-Garcia, M.E. Changes in the Physicochemical Properties of Isolated Starch and Plantain (Musa AAB Simmonds) Flours for Early Maturity Stage. Heliyon 2023, 9, e18939. [Google Scholar] [CrossRef]
- Dufour, D.; Gibert, O.; Giraldo, A.; Sánchez, T.; Reynes, M.; Pain, J.P.; González, A.; Fernández, A.; Díaz, A. Differentiation between cooking bananas and dessert bananas. 2. Thermal and functional characterization of cultivated Colombian Musaceae (Musa sp.). J. Agric. Food Chem. 2009, 57, 7870–7876. [Google Scholar] [CrossRef] [PubMed]
- Megazyme Total Starch Assay Kit (AA/AMG). Available online: https://www.megazyme.com/total-starch-assay-kit (accessed on 24 February 2025).
- De La Torre-Gutierrez, L.; Torruco-Uco, J.G.; Castellanos-Ruelas, A.; Chel-Guerrero, L.A.; Betancur-Ancona, D. Isolation and structure investigations of square banana (Musa balbisiana) starch. Starch-Stärke 2007, 59, 326–333. [Google Scholar] [CrossRef]
- ISO 1871:2009; Food and Feed Products—General Guidelines for the Determination of Nitrogen by the Kjeldahl Method. International Organization for Standardization ISO: Geneva, Switzerland, 2021.
- ISO 2171:2010; Cereals, Pulses and by-Products—Determination of Ash Yield by Incineration. International Organization for Standardization ISO: Geneva, Switzerland, 2023.
- Norma Técnica Colombiana NTC1776:2019; Test Method to Determine the Total Evaporable Moisture Content of Aggregate by Drying. NTC: Bogotá, Colombia, 2019.
- Norma Técnica Colombiana NTC 668:1973; Alimentos y Materias Primas Determinación de los Contenidos de Grasa y Fibra Cruda. NTC: Bogotá, Colombia, 2002.
- Kumar, P.S.; Saravanan, A.; Sheeba, N.; Uma, S. Structural, functional characterization and physicochemical properties of green banana flour from dessert and plantain bananas (Musa spp.). LWT—Food Sci. Technol. 2019, 116, 108524. [Google Scholar] [CrossRef]
- Ramirez-Cortes, R.; Bello-Pérez, L.A.; Gonzalez-Soto, R.A.; Gutierrez-Meraz, F.; Alvarez-Ramirez, J. Isolation of plantain starch on a large laboratory scale. Starch-Starke 2016, 68, 488–495. [Google Scholar] [CrossRef]
- Giraldo Toro, A.; Gibert, O.; Ricci, J.; Dufour, D.; Mestres, C.; Bohuon, P. Digestibility prediction of cooked plantain flour as a function of water content and temperature. Carbohydr. Polym. 2015, 118, 257–265. [Google Scholar] [CrossRef] [PubMed]
- ASTME1252-98; Standard Practice for General Techniques for Obtaining Infrared Spectra for Qualitative Analysis. ASTM: West Conshohocken, PA, USA, 2021.
- Liu, H.; Yu, L.; Chen, L.; Li, L. Retrogradation of corn starch after thermal treatment at different temperatures. Carbohydr. Polym. 2007, 69, 756–762. [Google Scholar] [CrossRef]
- Nara, S.; Komiya, T. Studies on the Relationship Between Water-satured State and Crystallinity by the Diffraction Method for Moistened Potato Starch. Starch-Stärke 1987, 35, 407–410. [Google Scholar] [CrossRef]
- Shittu, R.; Lasekan, O.; Karim, R.; Sulaiman, R. Plantain-starch: Microstructural, physicochemical, and morphological characteristics of two cultivars grown in Malaysia. Starch-Starke 2016, 68, 1187–1195. [Google Scholar] [CrossRef]
- Rodriguez, L. Elaboración de un Material Biocompuesto a Partir de la Fibra de Plátano. Master’s Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2014. [Google Scholar]
- Padhi, S.; Dwivedi, M. Physico-chemical, structural, functional and powder flow properties of unripe green banana flour after the application of Refractance window drying. Future Foods 2022, 5, 100101. [Google Scholar] [CrossRef]
- Chavez-Salazar, A.M.; Castellanos-Galeano, F.J.; Martinez-Hernandez, L.J. Effect of process variables in the production of fried green plantain in vacuum. Vitae 2017, 24, 38–46. [Google Scholar] [CrossRef]
- Montoya, J.; Quintero, V.D.; Lucas, J.C. Thermal and rheological evaluation of flour and starch from banana dominico harton (Musa paradisiaca ABB). Temas Agrar. 2014, 19, 214–233. [Google Scholar] [CrossRef]
- Mejía-Gutiérrez, L.; Giraldo-Gómez, G.; Ramírez-Ramírez, D. Efecto de la edad de cosecha en las características poscosecha del plátano Dominico-Hartón (Musa AAB Simmonds). Acta Agron. 2012, 61, 345–352. [Google Scholar]
- Hoyos-Leyva, J.D.; Jaramillo-Jiménez, P.A.; Giraldo-Toro, A.; Dufour, D.; Sánchez, T.; Lucas-Aguirre, J.C. Physical, morphological characterization and evaluation of pasting curves of Musa spp. Acta Agron. 2012, 61, 214–229. [Google Scholar]
- Flores-Gorosquera, E.; García-Suárez, F.J.; Flores-Huicochea, E.; Núñez-Santiago, M.C.; González-Soto, R.A.; Bello-Pérez, L.A. Yield of starch extraction from plantain (Musa paradisiaca). Pilot plant study. Acta Cient. Venez. 2004, 55, 86–90. [Google Scholar] [PubMed]
- Agama-Acevedo, E.; Bello-Pérez, L.A.; Pacheco-Vargas, G.; Evangelista-Lozano, S. Inner structure of plantain starch granules by surface chemical gelatinization: Morphological, physicochemical and molecular properties. Rev. Mex. Ing. Química 2015, 14, 73–80. [Google Scholar]
- Almanza-Benitez, S.; Osorio-Díaz, P.; Méndez-Montealvo, G.; Islas-Hernández, J.J.; Bello-Perez, L.A. Addition of acid-treated unripe plantain flour modified the starch digestibility, indigestible carbohydrate content and antioxidant capacity of semolina spaghetti. LWT—Food Sci. Technol. 2015, 62, 1127–1133. [Google Scholar] [CrossRef]
- Flores-Silva, P.C.; Rodriguez-Ambriz, S.L.; Bello-Pérez, L.A. Gluten-Free Snacks Using Plantain-Chickpea and Maize Blend: Chemical Composition, Starch Digestibility, and Predicted Glycemic Index. J. Food Sci. 2015, 80, C961–C966. [Google Scholar] [CrossRef]
- García-Solís, S.E.; Bello-Pérez, L.A.; Agama-Acevedo, E.; Flores-Silva, P.C. Plantain flour: A potential nutraceutical ingredient to increase fiber and reduce starch digestibility of gluten-free cookies. Starch-Starke 2018, 70, 1700107. [Google Scholar] [CrossRef]
- Fuentes-Zaragoza, E.; Riquelme-Navarrete, M.J.; Sánchez-Zapata, E.; Pérez-Álvarez, J.A. Resistant starch as functional ingredient: A review. Food Res. Int. 2010, 43, 931–942. [Google Scholar] [CrossRef]
- Saura Calixto, P.F.; Abla, R. Resistant starch: An indigestible fraction of foods. Grasas Aceites 1991, 42, 239–242. [Google Scholar] [CrossRef]
- Li, M.; Tian, X.; Jin, R.; Li, D. Preparation and characterization of nanocomposite films containing starch and cellulose nanofibers. Ind. Crops Prod. 2018, 123, 654–660. [Google Scholar] [CrossRef]
- Otegbayo, B.; Lana, O.; Ibitoye, W. Isolation And Physicochemical Characterization Of Starches Isolated From Plantain (Musa Paradisiaca) And Cooking Banana (Musa Sapientum). J. Food Biochem. 2010, 34, 1303–1318. [Google Scholar] [CrossRef]
- Nwokocha, L.M.; Williams, P.A. Some properties of white and yellow plantain (Musa paradisiaca, Normalis) starches. Carbohydr. Polym. 2009, 76, 133–138. [Google Scholar] [CrossRef]
- Bertolini, A. Starches Characterization, Properties and Applications; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2010. [Google Scholar]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant starch: Promise for improving human health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Janssen, L.; Moscicki, L. Thermoplastic Starch A Green Material for Various Industries; Wiley-VCH Verlag: Weinheim, Germany, 2009. [Google Scholar]
- Pelissari, F.M.; Andrade-Mahecha, M.M.; Sobral, P.J.D.A.; Menegalli, F.C. Isolation and characterization of the flour and starch of plantain bananas (Musa paradisiaca). Starch-Starke 2012, 64, 382–391. [Google Scholar] [CrossRef]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the crystallinity degree of starch having different types of crystal structure after mechanical pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Flanagan, B.M.; Gilbert, E.P.; Gidley, M.J. A novel approach for calculating starch crystallinity and its correlation with double helix content: A combined XRD and NMR study. Biopolymers 2008, 89, 761–768. [Google Scholar] [CrossRef]
- Bi, Y.; Zhang, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C.; Hong, Y. Effect of ripening on in vitro digestibility and structural characteristics of plantain (Musa ABB) starch. Food Hydrocoll. 2019, 93, 235–241. [Google Scholar] [CrossRef]
- Soares, C.A.; Peroni-Okita, F.H.G.; Cardoso, M.B.; Shitakubo, R.; Lajolo, F.M.; Cordenunsi, B.R. Plantain and banana starches: Granule structural characteristics explain the differences in their starch degradation patterns. J. Agric. Food Chem. 2011, 59, 6672–6681. [Google Scholar] [CrossRef]
- Waliszewski, K.N.; Aparicio, M.A.; Bello, L.A.; Monroy, J.A. Changes of banana starch by chemical and physical modification. Carbohydr. Polym. 2003, 52, 237–242. [Google Scholar] [CrossRef]
- Ekafitri, R.; Kumalasari, R.; Suryani, Y.; Acahyadi, N.S.; Desnilasari, D.; Mayasti, N.K.I.; Surahman, D.N. Characteristics of flour plantain: Use of fruit peels and ripening fruit stages. Emir. J. Food Agric. 2021, 33, 980–991. [Google Scholar] [CrossRef]
- Monroy, Y.; Rivero, S.; García, M.A. Microstructural and techno-functional properties of cassava starch modified by ultrasound. Ultrason. Sonochemistry 2018, 42, 795–804. [Google Scholar] [CrossRef]
- Cheetham, N.W.H.; Tao, L. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydr. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- Chung, H.J.; Liu, Q.; Lee, L.; Wei, D. Relationship between the structure, physicochemical properties and in vitro digestibility of rice starches with different amylose contents. Food Hydrocoll. 2011, 25, 968–975. [Google Scholar] [CrossRef]
- Fekete, E.; Bella, É.; Csiszár, E.; Móczó, J. Improving physical properties and retrogradation of thermoplastic starch by incorporating agar. Int. J. Biol. Macromol. 2019, 136, 1026–1033. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, P.; Sui, Z.; Bao, J. Physicochemical properties of starches from diverse rice cultivars varying in apparent amylose content and gelatinisation temperature combinations. Food Chem. 2015, 172, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Composition, structure, physicochemical properties, and modifications of cassava starch. Carbohydr. Polym. 2015, 122, 456–480. [Google Scholar] [CrossRef]
- Agarwal, S. Major factors affecting the characteristics of starch based biopolymer films. Eur. Polym. J. 2021, 160, 110788. [Google Scholar] [CrossRef]
- Liu, X.; Yu, L.; Xie, F.; Li, M.; Chen, L.; Li, X. Kinetics and mechanism of thermal decomposition of corn starches with different amylose/amylopectin ratios. Starch-Starke 2010, 62, 139–146. [Google Scholar] [CrossRef]
- Olatunde, G.O.; Arogundade, L.K.; Orija, O.I. Chemical, functional and pasting properties of banana and plantain starches modified by pre-gelatinization, oxidation and acetylation. Cogent Food Agric. 2017, 3, 1283079. [Google Scholar] [CrossRef]
- Srichuwong, S.; Sunarti, T.C.; Mishima, T.; Isono, N.; Hisamatsu, M. Starches from different botanical sources II: Contribution of starch structure to swelling and pasting properties. Carbohydr. Polym. 2005, 62, 25–34. [Google Scholar] [CrossRef]
- Yuan, T.Z.; Ai, Y. Pasting and gelation behaviors and in vitro digestibility of high-amylose maize starch blended with wheat or potato starch evaluated at different heating temperatures. Food Hydrocoll. 2022, 131, 107783. [Google Scholar] [CrossRef]
- Chaudhary, A.L.; Torley, P.J.; Halley, P.J.; McCaffery, N.; Chaudhary, D.S. Amylose Content and Chemical Modification Effects on Thermoplastic Starch from Maize—Processing and Characterisation Using Conventional Polymer Equipment. Carbohydr Polym 2009, 78, 917–925. [Google Scholar] [CrossRef]
- Castañeda Niño, J.P. Estudio de La Retrogradación En Películas Flexibles Obtenidas a Partir de Mezclas de Almidón Nativo de Yuca, Ácido Poli-Láctico (PLA) y Policaprolactona (PCL). Master’s Thesis, Universidad del Valle, Cali, Colombia, 2012. [Google Scholar]



| Code | Origin | Plantain By-Product |
|---|---|---|
| HPCA | Cauca | Pulp flour |
| APCA | Pulp starch | |
| HCPCA | Peel flour | |
| ACPCA | Peel starch | |
| HPR | Risaralda | Pulp flour |
| APR | Pulp starch | |
| HCPR | Peel flour | |
| ACPR | Peel starch |
| Species | Source | By-Product | Dry Matter (%) | Pulp/Peel Ratio | Average Finger Weight (g) | Average Diameter (cm) |
|---|---|---|---|---|---|---|
| Plantain (finger) | Risaralda | Pulp | 24.81 ± 1.71 a | 56.71 ± 3.95 a | 302.49 ± 27.23 a | 4.79 ± 0.47 a |
| Peel | 4.25 ± 2.96 b | 43.28 ± 3.95 b | ||||
| Cauca | Pulp | 26.19 ± 3.03 a | 58.69 ± 2.37 a | 331.22 ± 33.37 a | 4.46 ± 0.33 a | |
| Peel | 5.96 ± 2.01 b | 41.31 ± 2.37 b |
| Treatment | Sodium Metabisulfite Content (%) | Solution pH | Starch Extraction on Dry Basis (%) | Starch Extraction on Wet Basis (%) |
|---|---|---|---|---|
| 1 | 0 | 6.00 ± 0.04 | 74.24 ± 5.89 a,b | 17.07 ± 1.35 a,b |
| 2 | 0.1 | 5.94 ± 0.04 | 72.47 ± 3.86 a | 16.67 ± 0.89 a |
| 3 | 0.3 | 5.80 ± 0.02 | 81.40 ± 1.28 b | 18.72 ± 0.29 b |
| 4 | 0.6 | 5.71 ± 0.06 | 78.05 ± 0.84 a,b | 17.95 ± 0.19 a,b |
| 5 | 0.9 | 5.54 ± 0.07 | 75.32 ± 2.52 a,b | 17.32 ± 0.58 a,b |
| 6 | 1.2 | 5.46 ± 0.04 | 80.21 ± 1.61 a,b | 18.45 ± 0.37 a,b |
| Origin | Sodium Metabisulfite Concentration (%) | Starch Extraction on Dry Basis (%) | Starch Extraction on Wet Basis (%) |
|---|---|---|---|
| Cauca (APCA) | 0.3 | 76.70 ± 3.43 a | 19.03 ± 1.20 a |
| 1.2 | 85.57 ± 2.51 b | 21.23 ± 0.62 b | |
| Risaralda (APR) | 0.3 | 83.67 ± 0.12 b | 20.76 ± 0.03 b |
| 1.2 | 89.81 ± 3.09 b | 22.28 ± 0.77 b |
| Properties | Plantain Origin | |||||||
|---|---|---|---|---|---|---|---|---|
| Cauca | Risaralda | |||||||
| Pulp | Peel | Pulp | Peel | |||||
| Flour | Starch | Flour | Starch | Flour | Starch | Flour | Starch | |
| Code | HPCA | APCA | HCPCA | ACPCA | HPR | APR | HCPR | ACPR |
| Moisture (%) | 7.27 | 7.07 | - | 8.21 | 7.95 | 8.19 | - | 8.22 |
| Ashes (%) | 12.59 | <2.0 | - | 12.02 | 13.59 | <2.0 | - | 15.18 |
| Crude fiber (% d.b.) | 49.65 | 50.69 | - | 48.20 | 43.82 | 37.29 | - | 46.36 |
| Protein (% d.b.) | 2.57 | <2.0 | - | <2.0 | 2.60 | <2.0 | - | <2.0 |
| Lipids (% d.b.) | <5.0 | <5.0 | - | <5.0 | <5.0 | <5.0 | - | <5.0 |
| pH | 6.01 ± 0.05 d | 5.36 ± 0.09 c | 5.03 ± 0.03 b | 5.83 ± 0.06 d | 5.85 ± 0.13 d | 4.62 ± 0.02 a | 4.91 ± 0.01 b | 4.85 ± 0.05 b |
| Average diameter (µm) | 42.20 | 31.42 | - | 32.58 | 53.16 | 33.40 | - | 49.06 |
| Diameter–Wide (µm) | - | 13.52 ± 5.21 c | - | 9.80 ± 4.19 a | - | 14.13 ± 4.66 c | - | 12.00 ± 4.92 b |
| Diameter–Large (µm) | - | 22.90 ± 8.16 b | - | 18.26 ± 8.65 a | - | 23.96 ± 8.26 b | - | 22.88 ± 10.21 b |
| Amylose (%) | - | 28.37 ± 0.28 c | - | 18.6 ± 0.73 a | - | 22.66 ± 1.59 b | - | 17.76 ± 0.19 a |
| Crystallinity pattern | C | C | - | B | B | B | - | B |
| Relative crystallinity (%) | 43.67 | 45.78 | - | 37.02 | 50.77 | 46.09 | - | 33.39 |
| Tm (°C) | 127.60 | 125.51 | - | 125.93 | 124.51 | 122.67 | - | 122.82 |
| ΔHm (J/g) | 92.10 | 97.49 | - | 94.71 | 89.00 | 93.76 | - | 91.77 |
| Td (°C) | 286.64 | 311.03 | - | 290.60 | 294.46 | 316.92 | - | 303.71 |
| WAI (g/g) | - | 240.0 ± 1.0 a | - | 328.0 ± 7.0 b | - | 497.0 ± 56.0 c | - | 444.0 ± 16.0 c |
| WSI (g/g) | - | 91.0 ± 8.0 a | - | 370.0 ± 10.0 d | - | 122.0 ± 9.0 b | - | 282.0 ± 17.0 c |
| SWP (g/g) | - | 241.0 ± 1.0 a | - | 334.0 ± 5.0 b | - | 500 ± 46.0 c | - | 450.0 ± 5.0 c |
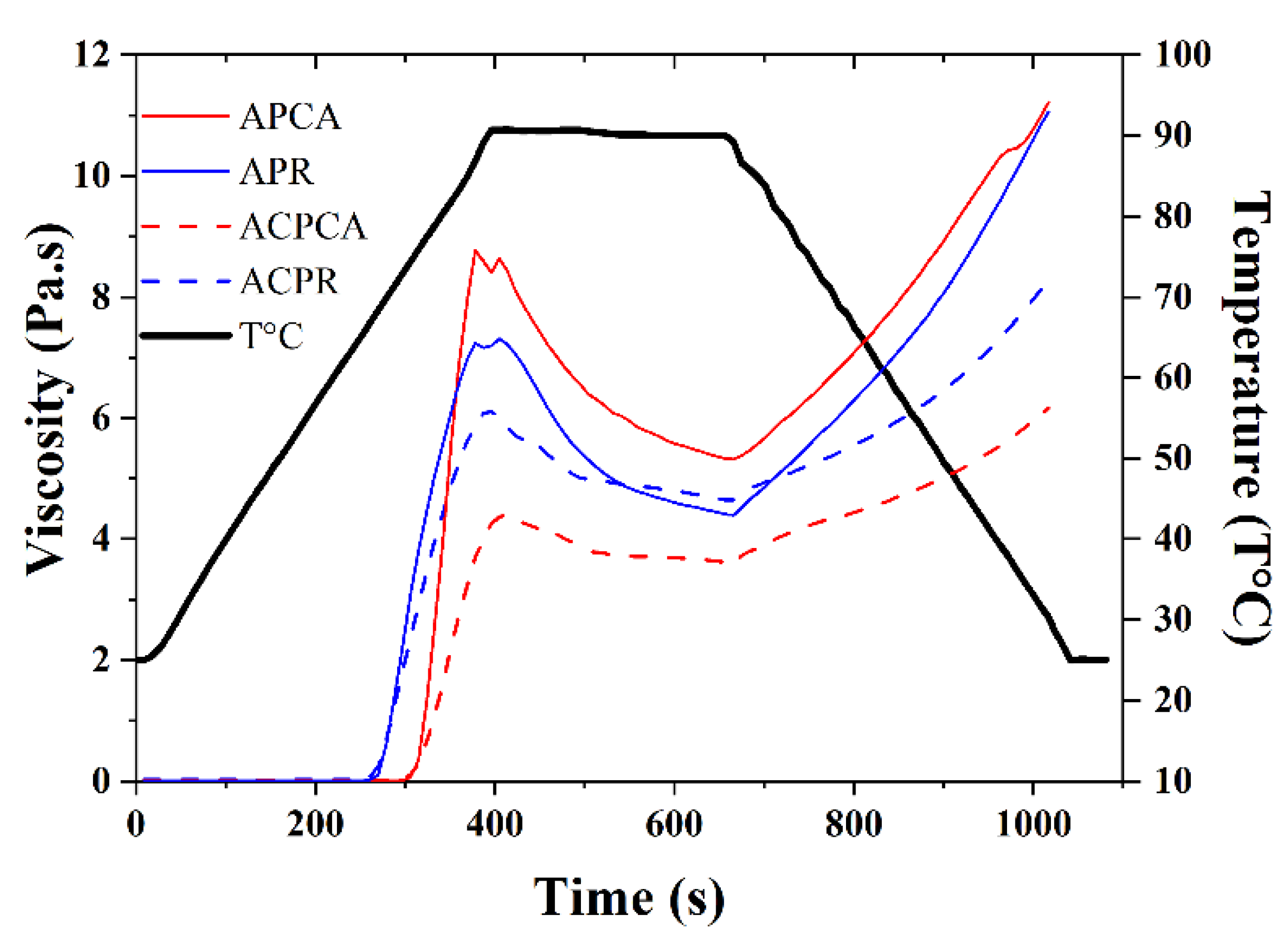
| Onset temperature (°C) | - | 73.00 | - | 71.60 | - | 66.80 | - | 63.90 |
| Temperature pasting (°C) | - | 86.50 | - | 91.60 | - | 90.90 | - | 90.60 |
| Peak viscosity (Pa.s) | - | 8.76 | - | 4.39 | - | 7.31 | - | 6.11 |
| Breakdown viscosity (Pa.s) | - | 5.32 | - | 3.62 | - | 4.40 | - | 4.64 |
| Setback viscosity (Pa.s) | - | 11.22 | - | 6.17 | - | 11.06 | - | 8.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda-Niño, J.P.; Mina-Hernández, J.H.; Solanilla-Duque, J.F. Extraction and Characterization of Starches from the Pulp and Peel of Native Plantain (Musa AAB Simmonds) from Two Colombian Departments. Polysaccharides 2025, 6, 34. https://doi.org/10.3390/polysaccharides6020034
Castañeda-Niño JP, Mina-Hernández JH, Solanilla-Duque JF. Extraction and Characterization of Starches from the Pulp and Peel of Native Plantain (Musa AAB Simmonds) from Two Colombian Departments. Polysaccharides. 2025; 6(2):34. https://doi.org/10.3390/polysaccharides6020034
Chicago/Turabian StyleCastañeda-Niño, Juan Pablo, José Herminsul Mina-Hernández, and José Fernando Solanilla-Duque. 2025. "Extraction and Characterization of Starches from the Pulp and Peel of Native Plantain (Musa AAB Simmonds) from Two Colombian Departments" Polysaccharides 6, no. 2: 34. https://doi.org/10.3390/polysaccharides6020034
APA StyleCastañeda-Niño, J. P., Mina-Hernández, J. H., & Solanilla-Duque, J. F. (2025). Extraction and Characterization of Starches from the Pulp and Peel of Native Plantain (Musa AAB Simmonds) from Two Colombian Departments. Polysaccharides, 6(2), 34. https://doi.org/10.3390/polysaccharides6020034