Microalgae Polysaccharides: An Overview of Production, Characterization, and Potential Applications
Abstract
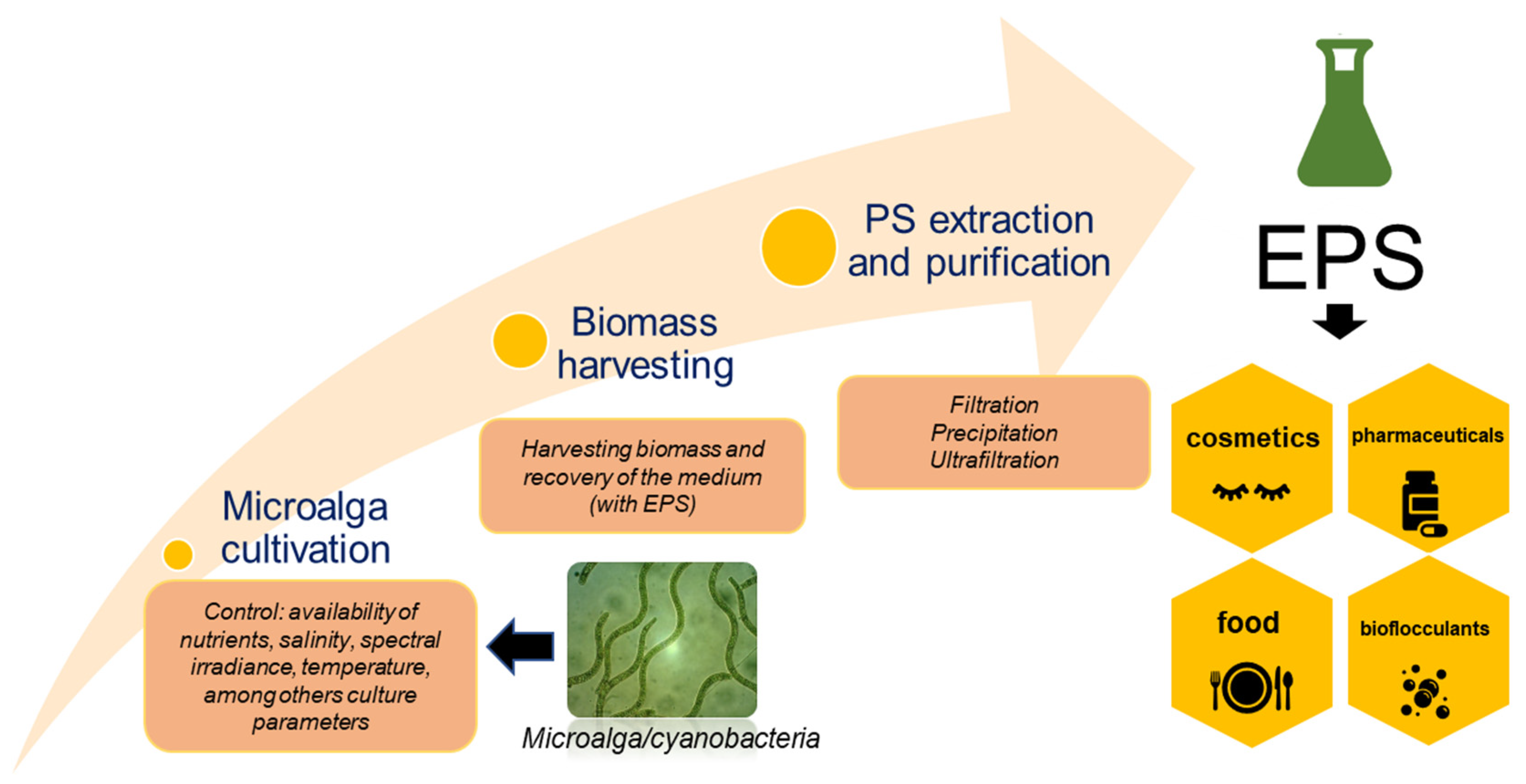
:1. Introduction
2. Polysaccharides from Microalgae
3. Influential Parameters in the Production of Polysaccharides by Microalgae
3.1. Nitrogen Source and Salinity
3.2. Temperature and Light Intensity
4. Extraction and Fractionation/Purification
5. Characterization of Microalgal Polysaccharides
5.1. Chemical Composition
5.2. Functional Characteristics
5.3. Rheological Properties
6. Applications of Polysaccharides
7. Challenges and Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, J.A.V.; Freitas, B.C.B.; Moraes, L.; Zaparoli, M.; Morais, M.G. Progress in the physicochemical treatment of microalgae biomass for value-added product recovery. Bioresour. Technol. 2020, 301, 122727. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-X.; Liu, X.-Y.; Xiao, Z.; Huang, Y.-F.; Liu, B. Antioxidant activities of polysaccharides obtained from Chlorella pyrenoidosa via different ethanol concentrations. Int. J. Biol. Macromol 2016, 91, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Phélippé, M.; Gonçalves, O.; Thouand, G.; Cogne, G.; Laroche, C. Characterization of the polysaccharides chemical diversity of the cyanobacteria Arthrospira Platensis. Algal Res. 2019, 38, 101426. [Google Scholar] [CrossRef]
- Wan, X.; Li, X.; Liu, D.; Gao, X.; Chen, Y.; Chen, Z.; Fu, C.; Lin, L.; Liu, B.; Zhao, C. Physicochemical characterization and antioxidant effects of green microalga Chlorella pyrenoidosa polysaccharide by regulation of microRNAs and gut microbiota in Caenorhabditis elegans. Int. J. Biol. Macromol. 2021, 168, 152–162. [Google Scholar] [CrossRef]
- Gaignard, C.; Gargouch, N.; Dubessay, P.; Delattre, C.; Pierre, G.; Laroche, C.; Fendri, I.; Abdelkafi, S.; Michaud, P. New horizons in culture and valorization of red microalgae. Biotechnol. Adv. 2019, 37, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Su, L.; Chen, S.; Zhao, L.; Wang, H.; Ding, F.; Chen, H.; Shi, R.; Wang, Y.; Huang, Z. Physicochemical characterization and functional analysis of the polysaccharide from the edible microalga Nostoc Sphaeroides. Molecules 2018, 23, 508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.; Zhu, S.; Li, S.; Feng, Y.; Wu, H.; Zeng, M. Microalgae polysaccharides ameliorates obesity in association with modulation of lipid metabolism and gut microbiota in high-fat-diet fed C57BL/6 mice. Int. J. Biol. Macromol. 2021, 182, 1371–1383. [Google Scholar] [CrossRef]
- Liberman, G.N.; Ochbaum, G.; Mejubovsky-Mikhelis, M.; Bitton, R.; Arad, S.M. Physico-chemical characteristics of the sulfated polysaccharides of the red microalgae Dixoniella grisea and Porphyridium aerugineum. Int. J. Biol. Macromol. 2020, 145, 1171–1179. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef]
- Prybylski, N.; Toucheteau, C.; Alaoui, H.E.; Bridiau, N.; Maugard, T.; Abdelkafi, S.; Fendri, I.; Delattre, C.; Dubessay, P.; Pierre, G.; et al. Bioactive polysaccharides from microalgae. In Handbook of Microalgae-Based Processes and Products; Elsevier: Amsterdam, The Netherlands, 2020; pp. 533–571. [Google Scholar] [CrossRef]
- Cruz, D.; Vasconcelos, V.; Pierre, G.; Michaud, P.; Delattre, C. Exopolysaccharides from cyanobacteria: Strategies for bioprocess development. Appl. Sci. 2020, 10, 3763. [Google Scholar] [CrossRef]
- Givaudan. Hydrintense™—A Moisturising Wave for Your Skin. Available online: https://www.givaudan.com/fragrance-beauty/active-beauty/products/hydrintense (accessed on 18 July 2021).
- Chai, W.S.; Tan, W.G.; Munawaroh, H.S.H.; Gupta, V.K.; Ho, S.-H.; Show, P.L. Multifaceted roles of microalgae in the application of wastewater biotreatment: A review. Environ. Pollut. 2021, 269, 116236. [Google Scholar] [CrossRef]
- Morais, M.G.; Morais, E.G.; Cardias, B.B.; Vaz, B.S.; Moreira, J.B.; Mitchell, B.G.; Costa, J.A.V. Microalgae as a source of sustainable biofuels. In Recent Developments in Bioenergy Research; Elsevier: Amsterdam, The Netherlands, 2020; pp. 253–271. [Google Scholar] [CrossRef]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C.W. Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci. Total Environ. 2020, 716, 137116. [Google Scholar] [CrossRef]
- Lucas, B.F.; Morais, M.G.; Santos, T.D.; Costa, J.A.V. Spirulina for snack enrichment: Nutritional, physical and sensory evaluations. LWT 2018, 90, 270–276. [Google Scholar] [CrossRef]
- Chanda, M.-J.; Merghoub, N.; Arroussi, H.E. Microalgae polysaccharides: The new sustainable bioactive products for the development of plant bio-stimulants? World J. Microbiol. Biotechnol. 2019, 35, 177. [Google Scholar] [CrossRef]
- Jesus, C.S.; Assis, D.J.; Rodriguez, M.B.; Filho, J.A.M.; Costa, J.A.V.; Ferreira, E.S.; Druzian, J.I. Pilot-scale isolation and characterization of extracellular polymeric substances (EPS) from cell-free medium of Spirulina sp. LEB-18 cultures under outdoor conditions. Int. J. Biol. Macromol. 2019, 124, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, O.N.; Khangembam, R.; Shamjetshabam, M.; Sharma, A.S.; Oinam, G.; Brand, J.J. Characterization and optmization of bioflocculant exopolyssaccharide production by Cyanobacteria Nostoc sp. BTA97 and Anabaena sp. BTA 990 in culture conditions. Appl. Biochem. Biotechnol. 2015, 176, 1950–1963. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Liu, H.; Su, J.; Lan, C.Q.; Zhong, M.; Hu, X. Production, isolation and bioactive estimation of extracellular polysaccharides of green microalga Neochloris Oleoabundans. Algal Res. 2020, 48, 101883. [Google Scholar] [CrossRef]
- Trabelsi, L.; Ouada, H.B.; Zili, F.; Mazhoud, N.; Ammar, J. Evaluation of Arthrospira platensis extracellular polymeric substances production in photoautotrophic, heterotrophic and mixotrophic conditions. Folia Microbiol. 2013, 58, 39–45. [Google Scholar] [CrossRef]
- Vo, H.N.P.; Ngo, H.H.; Guo, W.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Zhang, X.; Liang, H.; Xue, S. Selective carbon sources and salinities enhance enzymes and extracellular polymeric substances extrusion of Chlorella sp. for potential co-metabolism. Bioresour. Technol. 2020, 303, 122877. [Google Scholar] [CrossRef] [PubMed]
- Deamici, K.M.; Morais, M.G.; Santos, L.O.; Muylaert, K.; Gardarin, C.; Costa, J.A.V.; Laroche, C. Static magnetic fields effects on polysaccharides production by different microalgae strains. J. Appl. Sci. 2021, 11, 5299. [Google Scholar] [CrossRef]
- González-Fernández, C.; Ballesteros, M. Linking microalgae and cyanobacteria culture conditions and key-enzymes for carbohydrate accumulation. Biotechnol. Adv. 2012, 30, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Vargas, M.A.; Olivares, H.R.; Guerrero, M.G. Exopolysaccharide production by the cyanobacterium Anabaena sp. ATCC 33047 in batch and continuous culture. J. Biotechnol. 1998, 60, 175–182. [Google Scholar] [CrossRef]
- Lupi, F.M.; Fernandes, H.M.L.; Tomé, M.M.; Sá-Correia, I.; Novais, J.M. Influence of nitrogen source and photoperiod on exopolysaccharide synthesis by the microalga Botryococcus braunii UC 58. Enzyme Microb. Technol. 1994, 16, 546–550. [Google Scholar] [CrossRef]
- Bafana, A. Characterization and optimization of production of exopolysaccharide from Chlamydomonas reinhardtii. Carbohydr. Polym. 2013, 95, 746–752. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Chen, F. Production and characterization of exopolysaccharides from Chlorella zofingiensis and Chlorella vulgaris with anti-colorectal cancer activity. Int. J. Biol. Macromol. 2019, 134, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Xia, L.; Zhou, X.; Zhang, D.; Hu, C. Effects of Light Intensity on Components and Topographical Structures of Extracellular Polysaccharides from the Cyanobacteria Nostoc sp. J. Microbiol. 2014, 52, 179–183. [Google Scholar] [CrossRef]
- Yu, H.; Jia, S.; Dai, Y. Accumulation of exopolysaccharides in liquid suspension culture of Nostoc flagelliforme cells. Biotechnol. Appl. Biochem. 2010, 160, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Villay, A.; Laroche, C.; Roriz, D.; El Alaoui, H.; Delbac, F.; Michaud, P. Optimisation of culture parameters for exopolysaccharides production by the microalga Rhodella violacea. Bioresour. Technol. 2013, 146, 732–735. [Google Scholar] [CrossRef]
- Chentir, I.; Hamdi, M.; Doumandji, A.; HadjSadok, A.; Ouada, H.B.; Nasri, M.; Jridi, M. Enhancement of extracellular polymeric substances (EPS) production in Spirulina (Arthrospira sp.) by two-step cultivation process and partial characterization of their polysaccharidic moiety. Int. J. Biol. Macromol. 2017, 105, 1412–1420. [Google Scholar] [CrossRef]
- Mota, R.; Guimaraes, R.; Büttel, Z.; Rossi, F.; Colica, G.; Silva, C.J.; Santos, C.; Gales, L.; Zille, A.; Philippis, R.; et al. Production and characterization of extracellular carbohydrate polymer from Cyanothece sp. CCY 0110. Carboydr. Polym. 2013, 93, 1408–1415. [Google Scholar] [CrossRef]
- Jindal, N.; Singh, D.P.; Khattar, J.I.S. Kinetics and physico-chemical characterization of exopolysaccharides produced by the cyanobacterium Oscillatoria formosa. J. Microbiol. Biotechnol. 2011, 27, 2139–2146. [Google Scholar] [CrossRef]
- Khattar, J.I.S.; Singh, D.P.; Jindal, N.; Kaur, N.; Singh, Y.; Rahi, P.; Gulati, A. Isolation and characterization of exopolysaccharides produced by the cyanobacterium Limnothrix redekei PUPCCC 116. Appl. Biochem. Biotechnol. 2010, 162, 1327–1338. [Google Scholar] [CrossRef]
- Han, P.-P.; Sun, Y.; Jia, S.-R.; Zhong, C.; Tan, Z.-L. Effects of light wavelengths on extracellular and capsular polysaccharide production by Nostoc flagelliforme. Carbohydr. Polym. 2014, 105, 145–151. [Google Scholar] [CrossRef]
- Razaghi, A.; Godhe, A.; Albers, E. Effects of nitrogen on growth and carbohydrate formation in Porphyridium cruentum. Cent. Eur. J. Biol. 2014, 9, 156–162. [Google Scholar] [CrossRef]
- Soanen, N.; Silva, E.; Gardarin, C.; Michaud, P.; Laroche, C. Improvement of exopolysaccharide production by Porphyridium marinum. Bioresour. Technol. 2016, 213, 231–238. [Google Scholar] [CrossRef]
- Chen, L.-Z.; Li, D.-H.; Song, L.R.; Hu, C.X.; Wang, G.H.; Liu, Y.D. Effects of salt stress on carbohydrate metabolism in desert soil alga Microcoleus vaginatus Gom. J. Integr. Plant. Biol. 2006, 48, 914–919. [Google Scholar] [CrossRef]
- Chi, Z.; Su, C.D.; Lu, W.D. A new exopolysaccharide produced by marine Cyanothece sp. 113. Bioresour. Technol. 2007, 98, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, G.E.; Ranglová, K.; Manoel, J.C.; Grivalsky, T.; Kopecky, J.; Masojídek, J. Bioethanol production from microalgae polysaccharides. Folia Microbiol. 2019, 64, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Jesus, C.S.; Uebel, L.S.; Costa, S.S.; Miranda, A.L.; Morais, E.G.; Morais, M.G.; Costa, J.A.V.; Nunes, I.L.; Ferreira, E.S.; Druzian, J.I. Outdoor pilot-scale cultivation of Spirulina sp. LEB-18 in different geographic locations for evaluating its growth and chemical composition. Bioresour. Technol. 2018, 256, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, L.; Ouada, H.B.; Bacha, H.; Ghoul, M. Combined effect of temperature and light intensity on growth and extracellular polymeric substance production by the cyanobacterium Arthrospira platensis. J. Appl. Phycol. 2009, 21, 405–412. [Google Scholar] [CrossRef]
- Gaignard, C.; Laroche, C.; Pierre, G.; Dubessay, P.; Delattre, C.; Gardarin, C.; Gourvil, P.; Probert, I.; Dubuffet, A.; Michaud, P. Screening of marine microalgae: Investigation of new exopolysaccharide producers. Algal Res. 2019, 44, 101711. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Liu, H.; Zhou, A.; Cao, Y.; Liu, X. Preliminary characterization of the structure and immunostimulatory and anti-aging properties of the polysaccharide fraction of Haematococcus pluvialis†. RSC Adv. 2018, 8, 9243–9252. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, O.N.; Mondal, A.; Bhunia, B.; Bandyopadhyay, T.K.; Jaladi, P.; Oinam, G.; Indrama, T. Purification, characterization and biotechnological potential of new exopolysaccharide polymers produced by cyanobacterium Anabaena sp. CCC 745. Polymer 2019, 178, 121695. [Google Scholar] [CrossRef]
- Sushytskyi, L.; Lukáč, P.; Synytsya, A.; Bleha, R.; Rajsiglová, L.; Capek, P.; Pohl, R.; Vannucci, L.; Čopíková, J.; Kaštánek, P. Immunoactive polysaccharides produced by heterotrophic mutant of green microalga Parachlorella kessleri HY1 (Chlorellaceae). Carbohydr. Polym. 2020, 246, 116588. [Google Scholar] [CrossRef]
- Corrêa, P.S.; Júnior, W.G.M.; Martins, A.A.; Caetano, N.S.; Mata, T.M. Microalgae biomolecules: Extraction, separation and purification methods. Processes 2021, 9, 10. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Huang, X.; Cheong, K.-T. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs. 2017, 15, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urh, M.; Simpson, D.; Zhao, K. Affinity chromatography: General methods. Methods Enzymol. 2009, 463, 417–438. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, X.; Wu, D.; Ouyang, Y.; Gao, L.; Chen, Z.; El-Seedi, H.R.; Wang, M.-f.; Chen, X.; Zhao, C. Characterization of the structure and analysis of the anti-oxidant effect of microalga Spirulina platensis polysaccharide on Caenorhabditis elegans mediated by modulating microRNAs and gut microbiota. Int. J. Biol. Macromol. 2020, 163, 2295–2305. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.-Z.; Ai, C.; Chen, Y.-H.; Gao, X.-X.; Zhong, R.-T.; Liu, B.; Chen, X.-H.; Zhao, C. Physicochemical characterization of a polysaccharide from green microalga Chlorella pyrenoidosa and its hypolipidemic activity via gut microbiota regulation in rats. J. Agric. Food Chem. 2020, 68, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Reck, L.; Andrade, F.R.N.; Maia, H.D.; Silva, D.C.; Araújo, A.L.A.C.; Abreu, K.V.; Batista, J.L.; Cavancante, K.M.S.P. Extraction of sulfated polysaccharide from microalgae Chlorella vulgaris produced in effluent generated by pisciculture. Braz. J. Develop. 2020, 6, 64379–64387. [Google Scholar] [CrossRef]
- Rachidi, F.; Benhima, R.; Kasmi, Y.; Sbabou, L.; Arroussi, H.E. Evaluation of microalgae polysaccharides as biostimulants of tomato plant defense using metabolomics and biochemical approaches. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Zhu, P.; Ma, S.; Wang, M.; Hu, Y. Purification, characterization and immunomodulatory activity of polysaccharides from stem lettuce. Carbohydr. Polym. 2018, 188, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; De Philippis, R. Exocellular polysaccharides in microalgae and cyanobacteria: Chemical features, role and enzymes and genes involved in their biosynthesis. In The Physiology of Microalgae, 1st ed.; Borowitzka, M.A., Beardall, J., Raven, J.A., Eds.; Springer: Cham, Switzerland, 2016; pp. 565–590. [Google Scholar] [CrossRef]
- Kumar, D.; Kaštánek, P.; Adhikary, S.P. Exopolysaccharides from cyanobacteria and microalgae and their commercial application. Curr. Sci. 2018, 115, 234–241. [Google Scholar] [CrossRef]
- Ahmed, M.; Moerdijk-Poortvliet, T.C.W.; Wijnholds, A.; Stal, L.J.; Hasnain, S. Isolation, characterization and localization of extracellular polymeric substances from the cyanobacterium Arthrospira platensis strain MMG-9. Eur. J. Phycol. 2014, 49, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Yao, C.; Sun, Y.; Chen, W.; Tan, H.; Cao, X.; Xue, S.; Yin, H. Production and structural characterization of a new type of polysaccharide from nitrogen-limited Arthrospira platensis cultivated in outdoor industrial-scale open raceway ponds. Biotechnol. Biofuels 2019, 12, 131. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, extraction and characterization of Chlorella vulgaris soluble polysaccharides and their applications in AgNPs biosynthesis and biostimulation of plant growth. Sci. Rep. 2020, 10, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, F.; Micheletti, E.; Bruno, L.; Adhikary, S.P.; Albertano, P.; De Philippis, R. Characteristics and role of the exocellular polysaccharides produced by fi e cyanobacteria isolated from phototrophic biofilms growing on Indian stone monuments. Biofouling 2012, 28, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Trabelsi, L.; Chaieb, O.; Mnari, A.; Abid-Essafi, S.; Aleya, L. Partial characterization and antioxidant and antiproliferative activities of the aqueous extracellular polysaccharides from the thermophilic microalgae Graesiella sp. Complement. Altern. Med. 2016, 12, 210. [Google Scholar] [CrossRef]
- Hussein, M.; Abou-ElWafa, G.S.; Shaaban-Dessuuki, S.A.; Hassan, N.I. Characterization and antioxidant activity of exopolysaccharide secreted by Nostoc Carneum. Int. J. Pharm. 2015, 11, 432–439. [Google Scholar] [CrossRef] [Green Version]
- De Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Bioactivity and Applications of Polysaccharides from Marine Microalgae. In Polysaccharides; Springer: Cham, Switzerland, 2014; pp. 1–38. [Google Scholar] [CrossRef]
- Shepherd, R.; Rockey, J.; Sutherland, I.W.; Roller, S. Novel bioemulsifiers from microorganisms for use in foods. J. Biotechnol. 1995, 40, 207–217. [Google Scholar] [CrossRef]
- Dwek, R.A. Glycobiology: Toward understanding the function of sugars. Chem. Rev. 1996, 96, 683–720. [Google Scholar] [CrossRef] [PubMed]
- Decamp, A.; Michelo, O.; Rabbat, C.; Laroche, C.; Grizeau, D.; Pruvost, J.; Gonçalves, O. A new, quick, and simple protocol to evaluate microalgae polysaccharide composition. Mar. Drugs 2021, 19, 101. [Google Scholar] [CrossRef]
- Barboríková, J.; Šutovská, M.; Kazimierová, I.; Jošková, M.; Fraňová, S.; Kopecký, J.; Capek, P. Extracellular polysaccharide produced by Chlorella vulgaris—Chemical characterization and anti-asthmatic profile. Int. J. Biol. Macromol. 2019, 135, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kashif, A.S.; Hwang, Y.J.; Park, J.K. Potent biomedical applications of isolated polysaccharides from marine microalgae Tetraselmis species. Bioprocess Biosyst. Eng. 2018, 41, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Tannin-Spitz, T.; Bergman, M.; Grossman, S.; Arad, S. Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J. Appl. Phycol. 2005, 17, 215–222. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Zhou, Y. Immunomodulation and antitumor activities of different molecular-weight polysaccharides from Porphyridium cruentum. Carbohydr. Polym. 2012, 87, 1206–1210. [Google Scholar] [CrossRef]
- Chen, X.; Song, L.; Wang, H.; Liu, S.; Yu, H.; Wang, X.; Li, R.; Liu, T.; Li, P. Partial characterization, the immune modulation and anticancer activities of sulfated polysaccharides from filamentous microalgae Tribonema sp. Molecules 2019, 24, 322. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Wan, H.; Wang, R.; Hao, D. Sulfated polysaccharides from Phaeodactylum tricornutum: Isolation, structural characteristics, and inhibiting HepG2 growth activity in vitro. PeerJ 2019, 7, e6409. [Google Scholar] [CrossRef] [Green Version]
- Liberman, G.N.; Ochbaum, G.; Arad, S.M.; Bitton, R. The sulfated polysaccharide from a marine red microalga as a platform for the incorporation of zinc ions. Carbohydr. Polym. 2016, 152, 658–664. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Kyomugasho, C.; Looveren, N.V.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Loey, A.M.V. Molecular and rheological characterization of different cell wall fractions of Porphyridium cruentum. Carbohydr. Polym. 2018, 195, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Behrouzian, F.; Razavi, S.M.; Karazhiyan, H. Intrinsic viscosity of cress (Lepidium sativum) seed gum: Effect of salts and sugars. Food Hydrocoll. 2014, 35, 100–105. [Google Scholar] [CrossRef]
- Rachidi, F.; Benhima, R.; Sbabou, L.; Arroussi, H.E. Microalgae polysaccharides bio-stimulating effect on tomato plants: Growth and metabolic distribution. Biotechnol. Rep. 2020, 25, e00426. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Silva, L.; Paulo, J.; Faria, M.; Nogueira, N.; Cordeiro, N. Microalgal-based biopolymer for nano- and microplastic removal: A possible biosolution for wastewater treatment. Environ. Pollut. 2020, 263, 114385. [Google Scholar] [CrossRef]
- Arad, S.M.; Levy-Ontman, O. Red microalgal cell-wall polysaccharides: Biotechnological aspects. Curr. Opin. Biotechnol. 2010, 21, 358–364. [Google Scholar] [CrossRef]
- Pierre, G.; Delattre, C.; Dubessay, P.; Jubeau, S.; Vialleix, C.; Cadoret, J.-P.; Probert, I.; Michaud, P. What Is in Store for EPS Microalgae in the Next Decade? Molecules 2019, 24, 4296. [Google Scholar] [CrossRef] [Green Version]
| Microalga/Cyanobacteria | Reactor | Culture Medium Composition | Polysaccharide Production | Growing Condition (Temperature and Light Intensity) | Reference | |
|---|---|---|---|---|---|---|
| g L−1 | g gbiomass−1 | |||||
| Anabaena sp. ATCC 33047 | Flask (1 L) | NaHCO, KCl, K2HPO4, MgSO4, CaCl2, NaCl | 17.2 | - | 460 µE m−2 s−1; 40 °C | [25] |
| Botryococcus braunii UC 58 | Cylindrical flask | Chu13; KNO3; CO(NH2)2; (NH4)2CO3; 1% CO2, 31 d | 2.4 | 2.2 | 250 μE m−2 s−1; 25 °C | [26] |
| Chlamydomonas reinhardtii | Flask (1 L) | CaCl2; NaNO3; K2HPO4; MgSO4 9 d | 0.63 | - | 20 µmol m−2 s−1 | [27] |
| Chlorella sp. | Flask (1 L) | CH3OH; C2H5OH; C12H22O11; C6H12O6; CH3COONa; C2H5NO2; NaHCO3; two salinity conditions (fresh water and sea water) | 0.03 | - | Intense lighting (studied factor); 20 °C | [22] |
| Chlorella zofingiensis | Flask | Mixotrophic cultivation (BG-11; 5.0 g L−1 glucose); 5 d | 0.21 | 0.09 | 40 μE m−2 s−1; 25 °C | [28] |
| Nostoc sp. | Flask (0.25 L) | BG-11 | 0.17 | 0.21 | 80 µE m−2 s−1; 25 °C | [29] |
| Nostoc flageliforme | Flask (500 mL) | BG-11 | 0.22 | - | 60 µmol m−2 s−1; 25 °C | [30] |
| Rhodella violacea | Vertical photobioreactor with flask (700 mL) | F/2 medium supplemented with NaNO3 and NaH2PO4 | 0.59 | - | 420 µE m−2 s−1; 24 °C | [31] |
| Spirulina sp. | Flask (5 L) and flask (0.25 L) | Zarrouk medium; 1 g L−1 NaCl, 5 d; 40 g L−1 NaCl; 3 d | - | 1.0 | 80 µmol m−2 s−1; 10 µmol m−2 s−1; 30 °C | [32] |
| Microalga/Cyanobacteria | Biomass Pretreatment for Polysaccharide Extraction | Main Isolation Processes | Polysaccharides Contents and/or Molecular Weight | Reference |
|---|---|---|---|---|
| Spirulina platensis | Ultrasonic treatment; pH from 11 to 7. | Four volumes of ethanol; Deproteinization (protease addition); Dialysis; Fractionation using ion exchange chromatography (DEAE A-52 column) and gel filtration chromatography (G-100 column) at a flow rate of 0.5 mL min−1. | 16.7% (1996 kDa) | [51] |
| Chlorella pyrenoidosa | Ultrasound assisted extraction. | Four volumes of ethanol; Deproteinization (protease addition); Dialysis; Fractionation using ion exchange chromatography (DEAE-52 column) and gel filtration chromatography (G-100 column) at a flow rate of 0.42 mL min−1. | 5630 kDa | [52] |
| Chlorella vulgaris | Hydration in sodium acetate buffer + cysteine + EDTA (pH 5.0) and incubation with crude papain at 60 °C. | Two volumes of ethanol; Fractionation using DEAE-Sephaore ion exchange column eluted with NaCl solutions at a rate of 1 mL min−1. | 15.0% | [53] |
| Phaeodactylum tricornutum, Porphyridium sp., Dunaliella salina and Arthrospira platensis | Dry biomass suspended in distilled water and incubated at 90 °C under agitation. | Two volumes of ethanol; Recovery by centrifugation + washing (three times with absolute ethanol). | 12.7% (Phaeodactylum tricornutum) 5.5% (Porphyridium sp.) 4.1% (Dunaliella salina) 2.5% (Arthrospaira platensis) | [54] |
| Neochloris oleoabundans | Heating (80 °C) and concentration of the cell-free medium to a quarter of its original volume under reduced pressure and 60 °C. | Addition of 95% ethanol until ethanol content reaches 30%; Deproteinization with Sevag agent (n-butyl alcohol: chloroform: = 1:4); Dialysis in cellulose membrane tube and concentration under reduced pressure; Fractionation over a DEAE-Sephacel column and gel filtration chromatography on Sephadex G200 eluted with 0.1 M NaCl. | 1 g L−1 (crude polysaccharide); 0.73 g g−1 (after purification, 517 kDa). | [20] |
| Haematococcus Pluvialis | Ultrasound assisted extraction | Anhydrous ethanol precipitation; Deproteinization with Sevag agent (n-butanol: chloroform = 1:5); Fractionation using a DEAEcellulose-52 chromatography column (2 mL min−1); Sephacryl S-400 gel permeation chromatography (0.3 mL min−1). | 23413 kDa | [45] |
| Microorganism | Sugar Composition | Uronic Acids | Sulphates | Methylated Sugars | Amino Acids | Reference |
|---|---|---|---|---|---|---|
| Anabaena sp. | Glucose *, xylose *, rhamnose | + | − | − | − | [46] |
| Arthrospira platensis | Fructose, fucose, galactose, glucose *, mannose, rhamnose, ribose, xylose | + | − | − | − | [58] |
| Arthrospira platensis | Glucose *, rhamnose, arabinose, fucose, mannose, galactose, xylose | + | − | − | − | [59] |
| Chlorella pyrenoidosa | Mannose, rhamnose, glucose, fucose, xylose, arabinose | + | − | − | − | [52] |
| Chlorella vulgaris | Fructose, glucose *, maltose, lactose, rhamnose, arabinose | + | + | − | + | [60] |
| Dixoniella grisea | Xylose *, rhamnose | + | + | + | − | [8] |
| Gloeocapsa sp. | Fucose, rhamnose, arabinose *, galactose, glucose *, mannose, fructose, xylose *, ribose | + | − | − | − | [61] |
| Graesiella sp. | Glucose, galactose, mannose, fucose *, rhamnose, xylose, arabinose, ribose | + | + | − | + | [62] |
| Neochloris oleoabundans | Glucose *, mannose *, galactose *, xylose, ribose, arabinose, rhamnose | + | − | − | + | [20] |
| Nostoc carneum | Xylose *, glucose | + | + | − | − | [63] |
| Porphyridium aerugineum | Xylose *, glucose, galactose | + | + | + | − | [8] |
| Spirulina sp. LEB 18 | Glucose, fructose, galactose | + | − | − | + | [18] |
| Strains | Potential application | Reference |
|---|---|---|
| Desmodesmus sp., P. tricornutum, Porphorydium sp., Arthrospira platensis, and Dunaliella salina | Plant (tomato) resistance inducer | [54] |
| Arthrospira platensis, Dunaliella salina, and Porphorydium sp. | Plant (tomato) bio-stimulant | [78] |
| Chlorella vulgaris | Plant (wheat and French bean) bio-stimulant | [60] |
| Nostoc sphaeroides | Food and health industries | [6] |
| Spirulina platensis | Functional food | [51] |
| Porphyridium sp. | Thickening/lubrication agent | [8] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.A.V.; Lucas, B.F.; Alvarenga, A.G.P.; Moreira, J.B.; de Morais, M.G. Microalgae Polysaccharides: An Overview of Production, Characterization, and Potential Applications. Polysaccharides 2021, 2, 759-772. https://doi.org/10.3390/polysaccharides2040046
Costa JAV, Lucas BF, Alvarenga AGP, Moreira JB, de Morais MG. Microalgae Polysaccharides: An Overview of Production, Characterization, and Potential Applications. Polysaccharides. 2021; 2(4):759-772. https://doi.org/10.3390/polysaccharides2040046
Chicago/Turabian StyleCosta, Jorge Alberto Vieira, Bárbara Franco Lucas, Ana Gabrielle Pires Alvarenga, Juliana Botelho Moreira, and Michele Greque de Morais. 2021. "Microalgae Polysaccharides: An Overview of Production, Characterization, and Potential Applications" Polysaccharides 2, no. 4: 759-772. https://doi.org/10.3390/polysaccharides2040046
APA StyleCosta, J. A. V., Lucas, B. F., Alvarenga, A. G. P., Moreira, J. B., & de Morais, M. G. (2021). Microalgae Polysaccharides: An Overview of Production, Characterization, and Potential Applications. Polysaccharides, 2(4), 759-772. https://doi.org/10.3390/polysaccharides2040046







