Chitin as a Sorbent Superior to Other Biopolymers: Features and Applications in Environmental Research, Energy Conversion, and Understanding Evolution of Animals
Abstract
1. Introduction
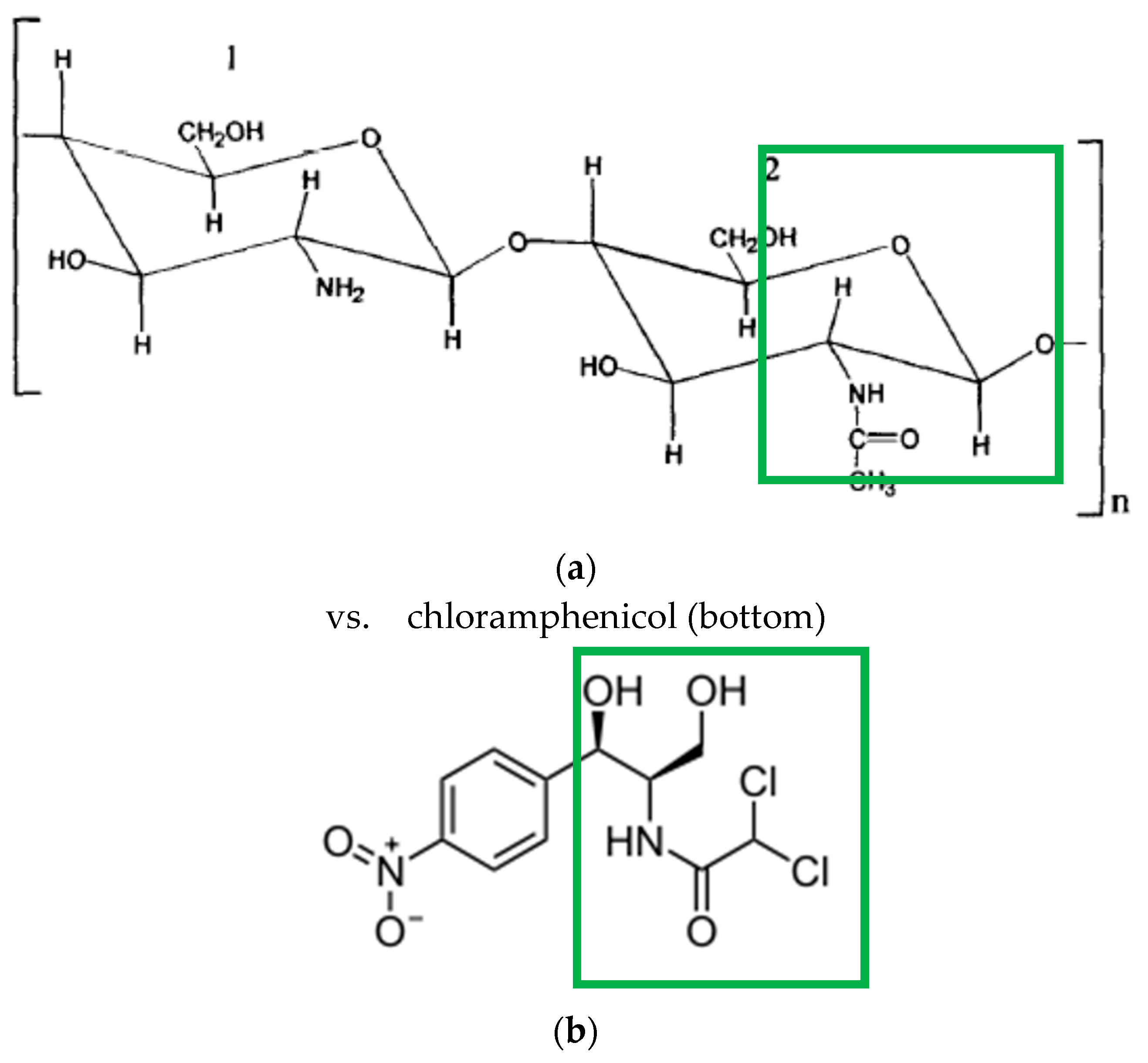
1.1. Chitin and Chitosan—A Comparison of Chemical and Sorptive Properties
- (a)
- does not occur in natural conditions and
- (b)
- is much less robust against thermal or oxidative stress than the native chitin.
1.2. Chemical Peculiarities Which Turn up at Ecotones and Similar Interfaces
2. Materials and Methods
2.1. Technique of Sampling
- (a)
- protection of possibly endangered species and
- (b)
- full control of exposition times while
2.2. Processing of Data
| Substance | Supplier | Purpose | Remarks |
|---|---|---|---|
| chitin | Sigma-Aldrich, Taufkirchen, Germany | sorbent | purified, background levels see text. No electrochemical signal unless metal salts were added |
| Lithium perchlorate | Sigma-Aldrich, Taufkirchen, Germany | Dissolution of chitin, conducting salt in CV | “battery-grade” purity |
| Cation exchanger resin | Amberlite H-120, Supelco, Bellefonte, PA, USA | Fixation and transfer of analytes | Checked for trace metal background, nothing detected. Can be re-used several times after leaching ions bound from chitin solution. Retrieval rate about 70% [18] |
| Nitric acid | Merck Suprapur, Darmstadt, Germany | ||
| Crayfish Orconectes limosus | Caught in local waters, also exuvia were studied | ||
| Eu(III) trifluoromethanesulfonate | Sigma-Aldrich, Taufkirchen, Germany | Photooxidation reagent | |
| Solacor glue | Boldt & Co., Wermelskirchen, Germany | Fixation of chitin flakes | Photo-hardening, metal-free glue, rather resistant towards organic solvents including DMF |
3. Results
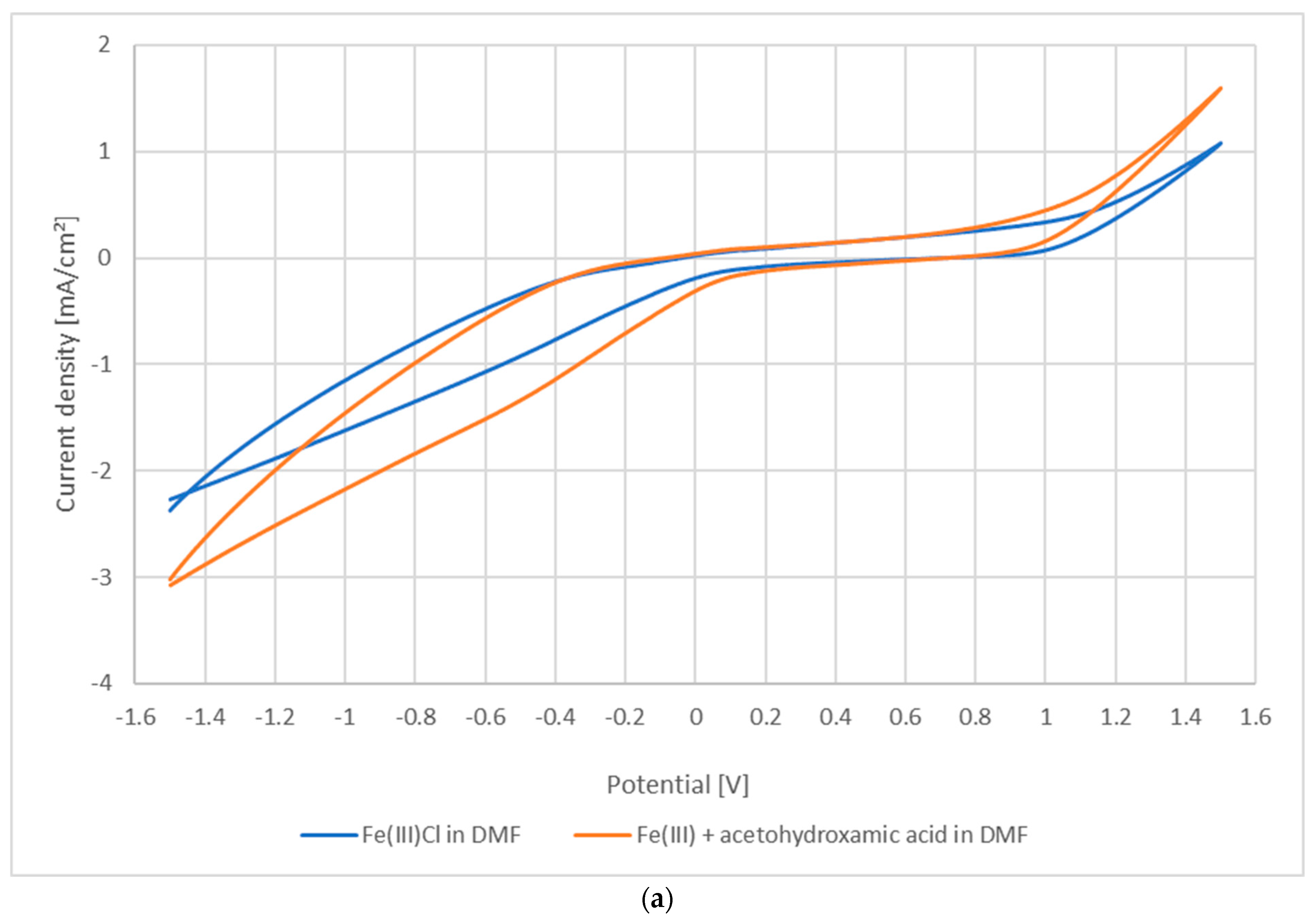
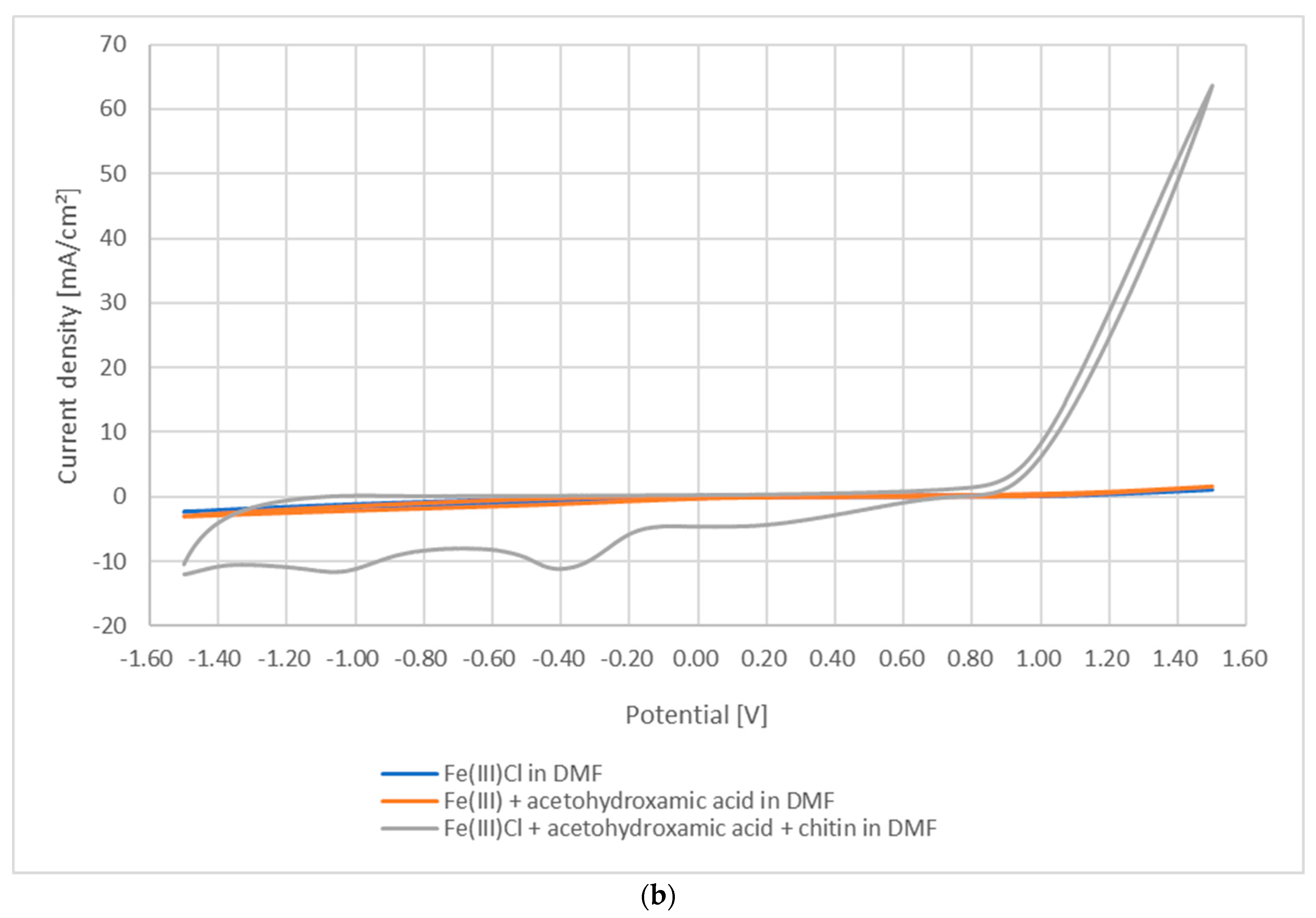
3.1. Photoredox Processes and Retention
3.2. Relationship between Donor Groups and Log σpara or Log P Constants for Metal Coordination to Chitin
3.3. Effects of Boiling Water on M Retention
3.4. Effects of Ligands on M Retention
3.5. Comparison to Chitin on Living Benthic Animals and Their Exuvia
4. Discussion
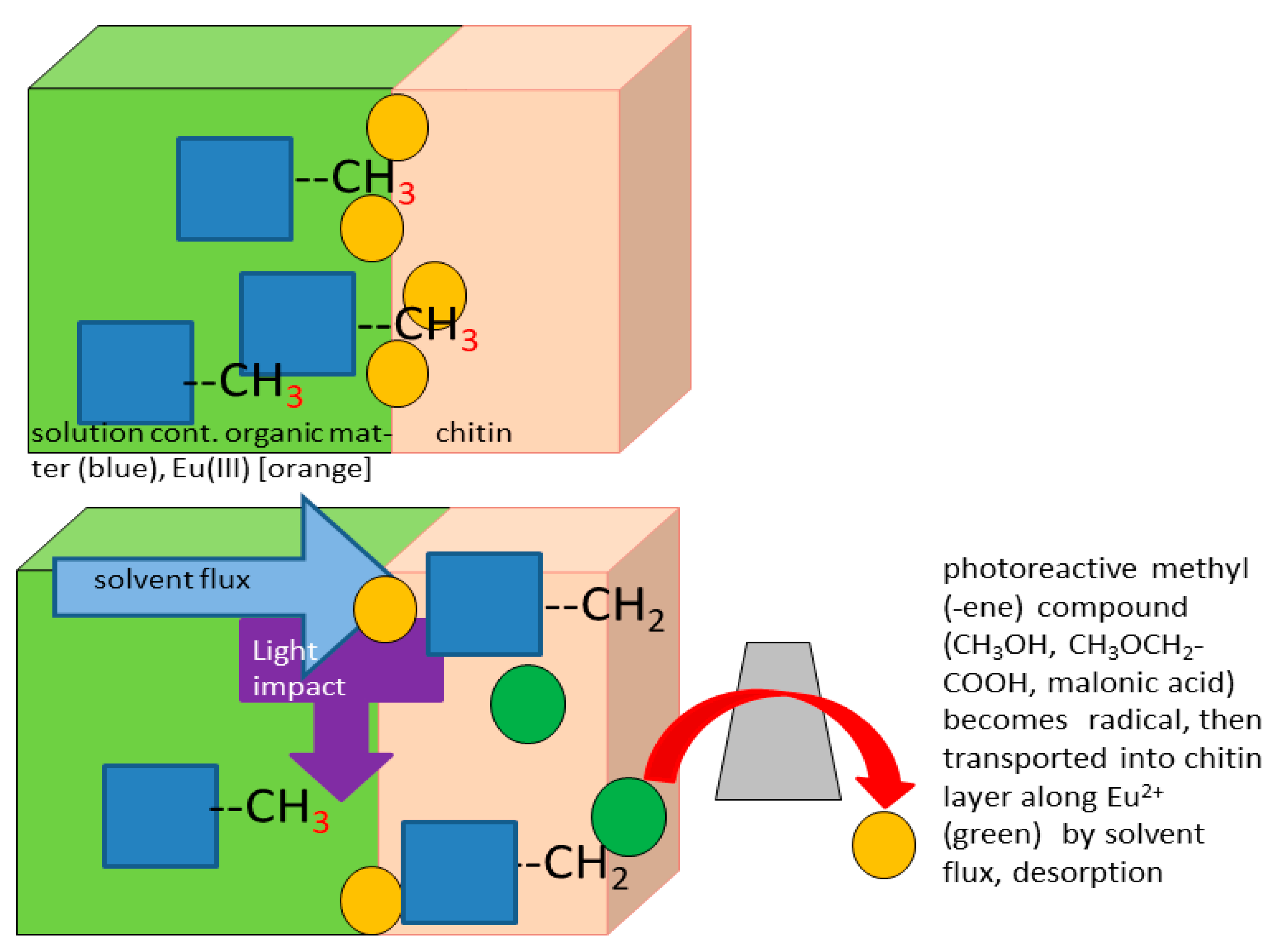
4.1. Photoassisted Redox Reactions Occurring When Adsorbed to Chitin
- (1)
- heterogeneous-catalytic electrotransformations of organic compounds (to be anticipated on Pt) and
- (2)
- electrode corrosion (sizable with Cu).
4.2. Change of Chitin Functions during Evolution
5. Conclusions
6. Outlook: Additional Studies in Lab and Open-Field
- (a)
- Studying Salinic Conditions in Water Bodies
- (b)
- Adsorption vs. Transport around and Inside Wells, Springs, and Seepage Sites
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aq. | aqueous, aquatic (refers to living animals) |
| DMF | N,N-dimethylformamide (solvent) |
| DMSO | dimethyl sulfoxide (solvent) |
| EL(L) | electrochemical ligand parameter; denotes shift of redox potential by replacing member(s) of ligand sphere in RuII/III system |
| FUV | far ultraviolet (λ < 300 nm) |
| H0 | “effective” pH measured in low-water or nonaqueous solvents. Range about–21 (“magic acid”) to > + 30 (strong base solutions in DMSO or liquid ammonia) |
| HFIP | hexafluoroisopropanol (solvent, does dissolve chitin, extremely transparent in FUV, radiation passes down to some 170 nm) |
| HREE | heavy rare-earth elements (Z = 66–71 [Dy…Lu]) |
| ICP-MS | mass spectrometry using an inductively coupled plasma for sample ionization |
| LREE | light rare earth elements (Y and Z = 57–63 [La…Eu]) |
| M | metal |
| M* | molecular mass |
| Mx+ | metal ion in an unspecified oxidation state x |
| NRB | nitrate-reducing bacteria |
| OX− | oxoanion, e.g., nitrate (X = NO2) or acetate (X = CH3CO) |
| REE | rare earth elements (Z = 39; 57–71) |
| Σσ | sum of Hammett constants for a chemical entity (ligand) interacting with some other via two or more functional groups described by their respective Hammett parameters σ. |
References
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Chen, T.; Kumar, G.; Vesnovsky, O.; Topoleski, L.D.T.; Payne, G.F. Chitosan Based Water-Resistant Adhesive. Analogy to Mussel Glue. Biomacromolecules 2000, 1, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Anastopoulos, I.; Bhatnagar, A.; Bikiaris, D.N.; Kyzas, G.Z. Chitin Adsorbents for Toxic Metals: A Review. Int. J. Mol. Sci. 2017, 18, 114. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.C.; Vasconcellos, L.C.G.; Carvalho, T.V.; do Nascimento, R.F. Adsorption of organics to chitin: Removal of Petroleum Spill in Water by Chitin and Chitosan. Orbital-Electron. J. Chem. 2014, 6, 70–74. [Google Scholar] [CrossRef]
- Pinto, P.X.; Al-Abed, S.R.; McKernan, J. Comparison of the efficiency of chitinous and ligneous substrates in metal and sulfate removal from mining-influenced water. J. Environ. Manag. 2018, 227, 321–328. [Google Scholar] [CrossRef]
- Pearlmutter, N.L.; A Lembi, C. Localization of chitin in algal and fungal cell walls by light and electron microscopy. J. Histochem. Cytochem. 1978, 26, 782–791. [Google Scholar] [CrossRef]
- Zitko, V.; Bishop, C.T. Oxidation of polysaccharides by lead tetraacetate in dimethyl sulfoxide. Canad. J. Chem. 1966, 44, 1749–1756. [Google Scholar] [CrossRef]
- Liu, P.; Liu, H.; Schäfer, T.; Gutmann, T.; Gibhardt, H.; Qi, H.; Tian, L.; Zhang, X.C.; Buntkowsky, G.; Zhang, K. Unexpected selective alkaline periodate oxidation of chitin for the isolation of chitin nanocrystals. Green Chem. 2020, 23, 745–751. [Google Scholar] [CrossRef]
- Machałowski, T.; Amemiya, C.; Jesionowski, T. Chitin of Araneae origin: Structural features and biomimetic applications: A review. Appl. Phys. A 2020, 126, 1–17. [Google Scholar] [CrossRef]
- Loppi, S.; Vannini, A.; Monaci, F.; Dagodzo, D.; Blind, F.; Erler, M.; Fränzle, S. Can Chitin and Chitosan Replace the Lichen Evernia prunastri for Environmental Biomonitoring of Cu and Zn Air Contamination? Biology 2020, 9, 301. [Google Scholar] [CrossRef]
- Arthur Stankiewicz, B.; van Bergen, P.F.; Duncan, I.J.; Carter, J.F.; Briggs, D.E.; Evershed, R.P. Recognition of chitin and proteins in invertebrate cuticles using analytical pyrolysis/gas chromatography and pyrolysis/gas chromatography/mass spectrometry. Rapid Comm. Mass Spectrom. 1996, 10, 1747–1757. [Google Scholar] [CrossRef]
- Ehrlich, H.; Rigby, J.K.; Botting, J.P.; Tsurkan, M.V.; Werner, C.; Schwille, P.; Geisler, T. Discovery of 505-million-year old chitin in the basal demosponge Vauxia Gracilenta. Nat. Sci. Rep. 2013, 3, 3497. [Google Scholar] [CrossRef]
- Gupta, N.S.; Michels, R.; Briggs, D.E.G.; Evershed, R.P.; Pancost, R.P. The organic preservation of fossil arthropods: An experimental study. Proc. Biol. Sci. 2006, 273, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Fränzle, S.; Erler, M.; Blind, F.; Ariuntsetseg, L.; Narangarvuu, D. Chitin adsorption in environmental monitoring: Not an alternative to moss monitoring but a method providing (lots of) bonus information. J. Sci. Arts. Univ. Valahia Chem. 2019, 19, 659–674. [Google Scholar]
- Hassanzadeh, P.; Sun, W.; De Silva, J.P.; Jin, J.; Makhnejia, K.; Cross, G.; Rolandi, M. Mechanical properties of self-assembled chitin nanofiber networks. J. Mater. Chem. B 2013, 2, 2461–2466. [Google Scholar] [CrossRef] [PubMed]
- Bell, H.L.; Nebeker, A.V. Preliminary studies on the tolerance of aquatic insects to low pH. J. Kans. Entomol. Soc. 1969, 42, 230–236. [Google Scholar]
- Budelmann, P. Verbreitung der Flusskrebse (Decapoda) in der südlichen Oberlausitz und die Eignung des invasiven Kamberkrebses (Orconectes limosus) für Chitin-Basiertes Monitoring von Schwermetallen in Limnischen Ökosystemen. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2021. [Google Scholar]
- Gebauer, T. Methodische Optimierung des Übertrags von Metallionen aus Umweltprobenmodellen auf Chitinoberflächen und von Diesen zu Zwecken Analytischen Biomonitorings Sowie Untersuchungen zur Diffusion/Ausbreitung von Analyten in Chitinproben. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2016. [Google Scholar]
- Retschke, D. Orientierende Untersuchungen zur Adsorption von Schwermetallen (Nickel) unter dem Einfluss Ausgewählter Komplexliganden Sowie in Arealen Potenzieller und Manifester Methanogenese. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2017. [Google Scholar]
- Kim, Y.J.; Park, C.R. Analysis of problematic complexing behavior of ferric chloride with N,N-Dimethylformamide using combined techniques of FT-IR, XPS, and TGA/DTG. Inorg. Chem. 2002, 41, 6211–6216. [Google Scholar] [CrossRef] [PubMed]
- Masui, M.; Sayo, H.; Tsuda, Y. Anodic Oxidation of Amines. Part 1. Cyclic Voltammetry of Aliphatic Amines at a Stationary Glassy-carbon Electrode. J. Chem. Soc. B 1968, 973–976. [Google Scholar] [CrossRef]
- Erler, M. Untersuchung des Bindungsverhaltens Ausgewählter Elemente und Ihrer Bodenrelevanten Komplexe an Chitin. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2020. [Google Scholar]
- Fränzle, S.; Bauer, A. This observation was not published in print but reported in conference lectures. 2014. [Google Scholar]
- Bauer, A. Orientierende Untersuchungen zur Bindung von Metallionen an Chitin und zur Davon Abhängigen Eignung von Arthropoden zur Bestimmung von Metallionenkonzentrationen in der Umwelt. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2014. [Google Scholar]
- Chen, K.; Bocknek, L.; Manning, B. Oxidation of Cr(III) to Cr(VI) and Production of Mn(II) by Synthetic Manganese(IV) Oxide. Crystals 2021, 11, 443. [Google Scholar] [CrossRef]
- Nico, P.S.; Zasoski, R.J. Mn(III) Center Availability as a Rate Controlling Factor in the Oxidation of Phenol and Sulfide on δ-MnO2. Environ. Sci. Technol. 2001, 35, 3338–3343. [Google Scholar] [CrossRef]
- Sharma, S.; Rai, O.P. Evaluation of stability constants of metal complexes with chloramphenicol. Intern. J. Adv. Sci. Res. 2017, 6. [Google Scholar] [CrossRef][Green Version]
- Fränzle, S. Chemical Elements in Plants and Soil, Habilitation thesis of SF, extended and translated version, originally submittted to Vechta Univ. (Vechta, Germany) in 2007 which gave venia legendi; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Fränzle, S. Estimating and predicting chemical potentials, distributions, speciation modes and mobilities of radiometals in soil, water and biomass. J. Environ. Radioact. 2013, 102, 109–116. [Google Scholar] [CrossRef]
- Masui, H.; Lever, A.B.P. Correlations between the ligand electrochemical parameter, EL(L), and the Hammett substituent parameter, sigma. Inorg. Chem. 1993, 32, 2199–2201. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. Rev. 1991, 97, 165–195. [Google Scholar] [CrossRef]
- Lever, A.B.P. Electrochemical parametrization of metal complex redox potentials, using the ruthenium(III)/ruthenium(II) couple to generate a ligand electrochemical series. Inorg. Chem. 1990, 29, 1271–1285. [Google Scholar] [CrossRef]
- Pol, A.; Barends, T.R.M.; Dietl, A.; Khadem, A.F.; Eygensteyn, J.; Jetten, M.S.M.; den Camp, H.J.M.O. Rare earth metals are essential for methanotrophic life in volcanic mudpots. Environ. Microbiol. 2013, 16, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Picone, N.; den Camp, H.J.M.O. Role of rare earth elements in methanol oxidation. Curr. Opin. Chem. Biol. 2019, 4, 39–44. [Google Scholar] [CrossRef]
- Featherston, E.R.; Cotruvo, J.A., Jr. The biochemistry of lanthanide acquisition, trafficking, and utilization. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021, 1868, 118864. [Google Scholar] [CrossRef] [PubMed]
- Fränzle, S. Hitherto unpublished work. Correlations between (sum of) Hammett constant(-s) of ligand donor groups and the electrochemical ligand parameter (which is defined by dividing total potential effect by denticity of ligand). 2020. [Google Scholar]
- Vijayaraghavan, K.; Balasubramanian, R. Single and binary biosorption of cerium and europium onto crab shell particles. Chem. Eng. J. 2010, 163, 337–343. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Rocchetti, R.; Marangio, G. Separation of zirconium, niobium, cerium and ruthenium on chitin and chitosan columns for the determination of cesium in nuclear fuel solutions. J. Radioanal. Chem. 1972, 10, 17–25. [Google Scholar] [CrossRef]
- Karraker, R.H. Stability Constants of Some Rare-Earth-Metal Chelates. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1961. [Google Scholar]
- Dmytryk, A.; Tuhy, L.; Samoraj, M.; Chojnacka, K.W. Biological Functions of Cadmium, Nickel, Vanadium, and Tungsten. In Recent Advances in Trace Elements; Chojnacka, K.W., Saeid, A., Eds.; Wiley: Hoboken, NY, USA, 2018; pp. 219–234. [Google Scholar] [CrossRef]
- Gonzalez-Davila, M.; Santana-Casiano, M.; Millero, F.J. The Adsorption of Cd(II) and Pb(II) to Chitin in Seawater. J. Colloid Interf. Sci. 2009, 137, 102–107. [Google Scholar] [CrossRef]
- Ramirez-Wong, D.; Ramirez-Cardona, M.; Sánchez-Leija, R.J.; Rugerio, A.; Mauricio-Sánchez, R.A.; Hernández-Landaverde, M.A.; Carranza, A.; Pojman, J.A.; Garay-Tapia, A.M.; Prokhorov, E.; et al. Sustainable-solvent-induced polymorphism in chitin films. Green Chem. 2016, 18, 4303–4311. [Google Scholar] [CrossRef]
- Kolthoff, I.M.; Chantooni, M.K.; Smagowski, H. Acid-base strengths in N,N-dimethylformamide. Anal. Chem. 1970, 42, 1622–1628. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.M.; Hrdina, R.; Abdel-Mohsen, A.-M.; Fouda, M.; Soliman, A.; Mohamed, F.; Mohsin, K.; Pinto, T.D. Chitin and chitosan from Brazilian Atlantic Coast: Isolation, characterization and antibacterial activity. Int. J. Biol. Macromol. 2015, 80, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Bernabé, P.; Becherán, L.; Cabrera, G.; Nesic, A.; Alburquenque, C.; Tapia, C.V.; Taboada, E.; Alderete, J.B.; Ríos, P.D.L. Chilean crab (Aegla cholchol) as a new source of chitin and chitosan with antifungal properties against Candida spp. Int. J. Biol. Macromol. 2020, 149, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A. The aqueous geochemistry of the rare-earth elements: Critical stability constants for complexes with simple carboxylic acids at 25 °C and 1 bar and their application to nuclear waste management. Eng. Geol. 1993, 34, 229–259. [Google Scholar] [CrossRef]
- Couffin, F. Potentiels d´oxydo-reduction des elements lanthanides et actinides dans les solvants organiques; CEA (Internal report (data collection) for CEA (Centre d´Energie Atomique), France. Does cover all lanthanides, many sol-vents including DMF, and actinides Th, Pa, U…Cm, Cf in DMF, DMSO, ethylene diamine, or tributyl phosphate; CEA: Saclay, France, 1980. [Google Scholar]
- Holleck, L. Zur Komplexchemie der Seltenen Erden. Die polarographischen Strompotentialkurven des Europiums als Nachweismittel komplexer Bindung. Z. für Naturf. B 1947, 2, 81–89. [Google Scholar] [CrossRef]
- Blind, F. Orientierende Untersuchungen zur Platinmetall freien Aktivierung von CH-Bindungen für Europium basierte Brennstoffzellenanwendungen. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2018. [Google Scholar]
- Blind, F.; Kluge, M.; Fränzle. Unpublished GC/MS observation using silylation of photoredox products in DMF and alkylation of ions like cyanide.
- Cody, G.D.; Gupta, N.S.; Briggs, D.E.; Kilcoyne, A.; Summons, R.E.; Kenig, F.; Plotnick, R.E.; Scott, A. Molecular signature of chitin-protein complex in Paleozoic arthropods. Geology 2011, 39, 255–258. [Google Scholar] [CrossRef]
- Keleşoglu, S. Comparative Adsorption Studies of Heavy Metal Ions on Chitin and Chitosan Biopolymers. Master’s Thesis, Izmir School of Technology, Izmir, Turkey, 2007. [Google Scholar]
- Jaafarzadeh, N.; Mengelizadeh, N.; Takdastan, A.; Farsani, M.H.; Niknam, N.; Aalipour, M.; Hadei, M.; Bahrami, P. Biosorption of heavy metals from aqueous solutions onto chitin. Int. J. Environ. Health Eng. 2015, 4, 7. [Google Scholar] [CrossRef]
- Sannasi, A.; Hermann, H.R. Chitin in the cephalochordata, Branchisotoma floridae. Cell. Mol. Life Sci. 1970, 26, 351–352. [Google Scholar] [CrossRef]
- Ishay, J.; Benshalom-Shimony, T.; Ben-Shalom, A.; Kristianpoller, N. Photovoltaic effects in the Oriental hornet, Vespa orientalis. J. Insect Physiol. 1992, 38, 37–48. [Google Scholar] [CrossRef]
- Shi, M.; Cui, S. Perfluorinated rare earth metals catalyzed nitration of aromatic compounds. J. Fluor. Chem. 2002, 39, 551–556. [Google Scholar] [CrossRef]
- Pulsfort, J. Biopolymere als Potenzielle Substrate der Photooxidation Durch f-Block-Metalle. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2021. [Google Scholar]


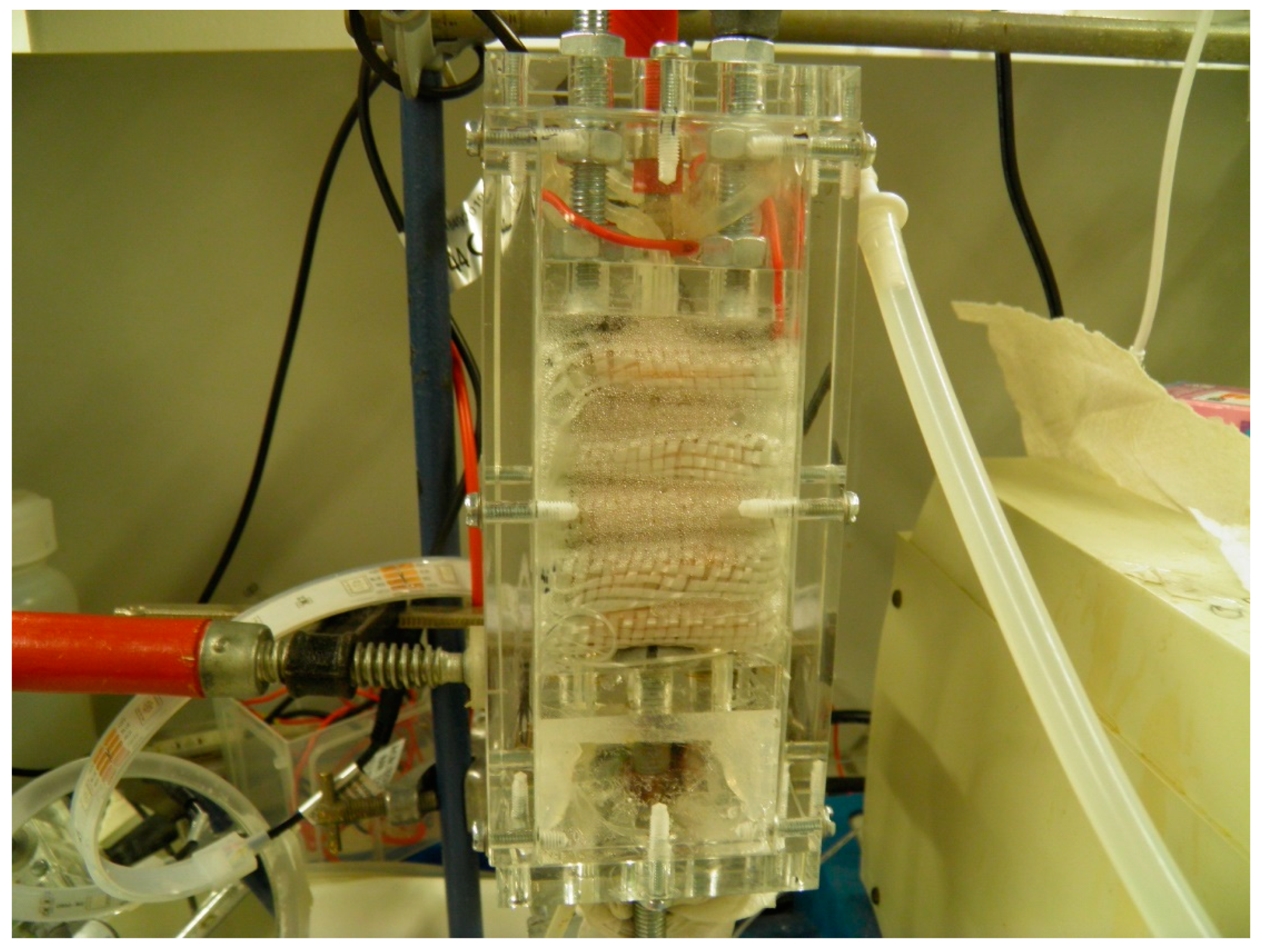
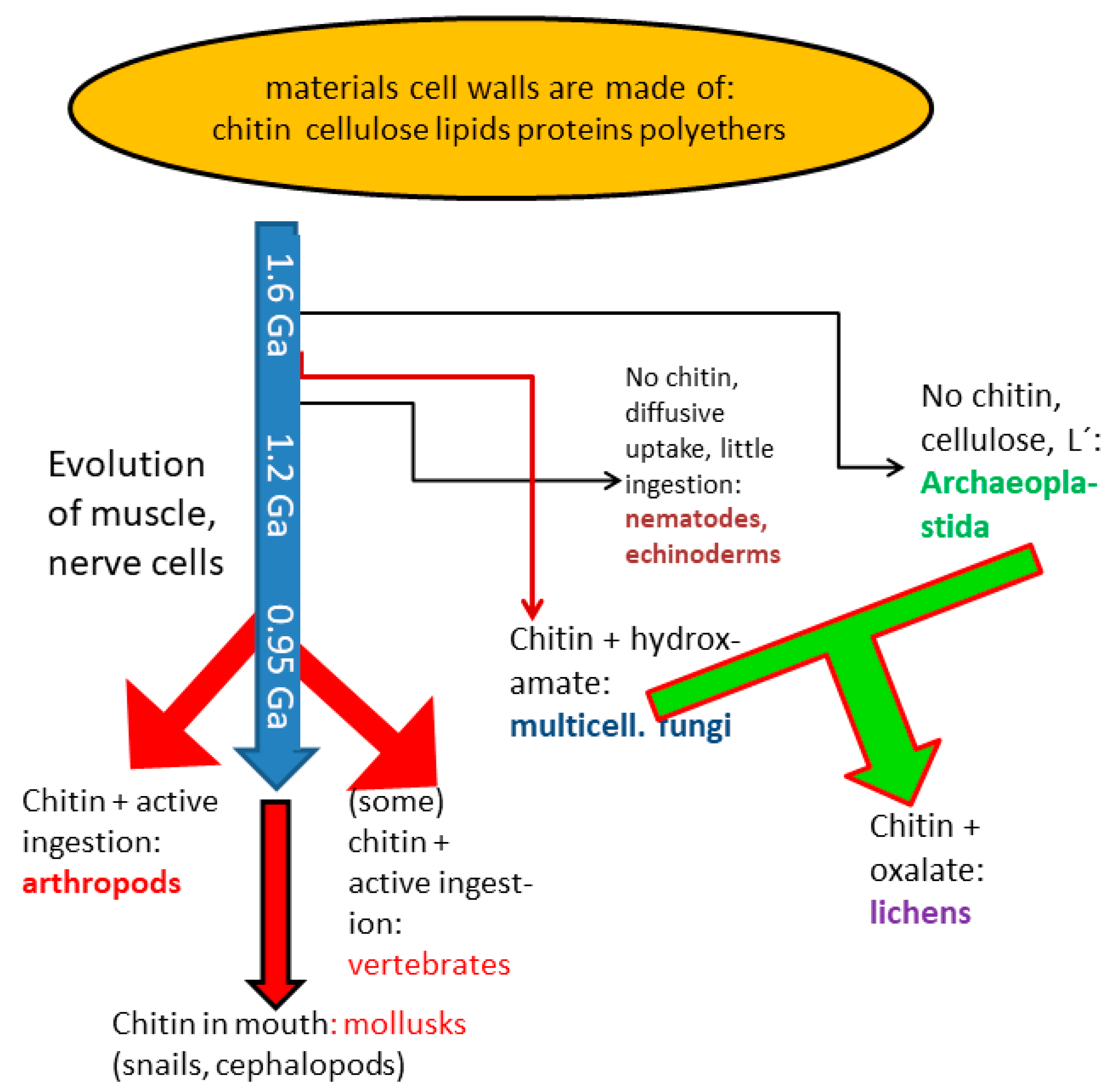
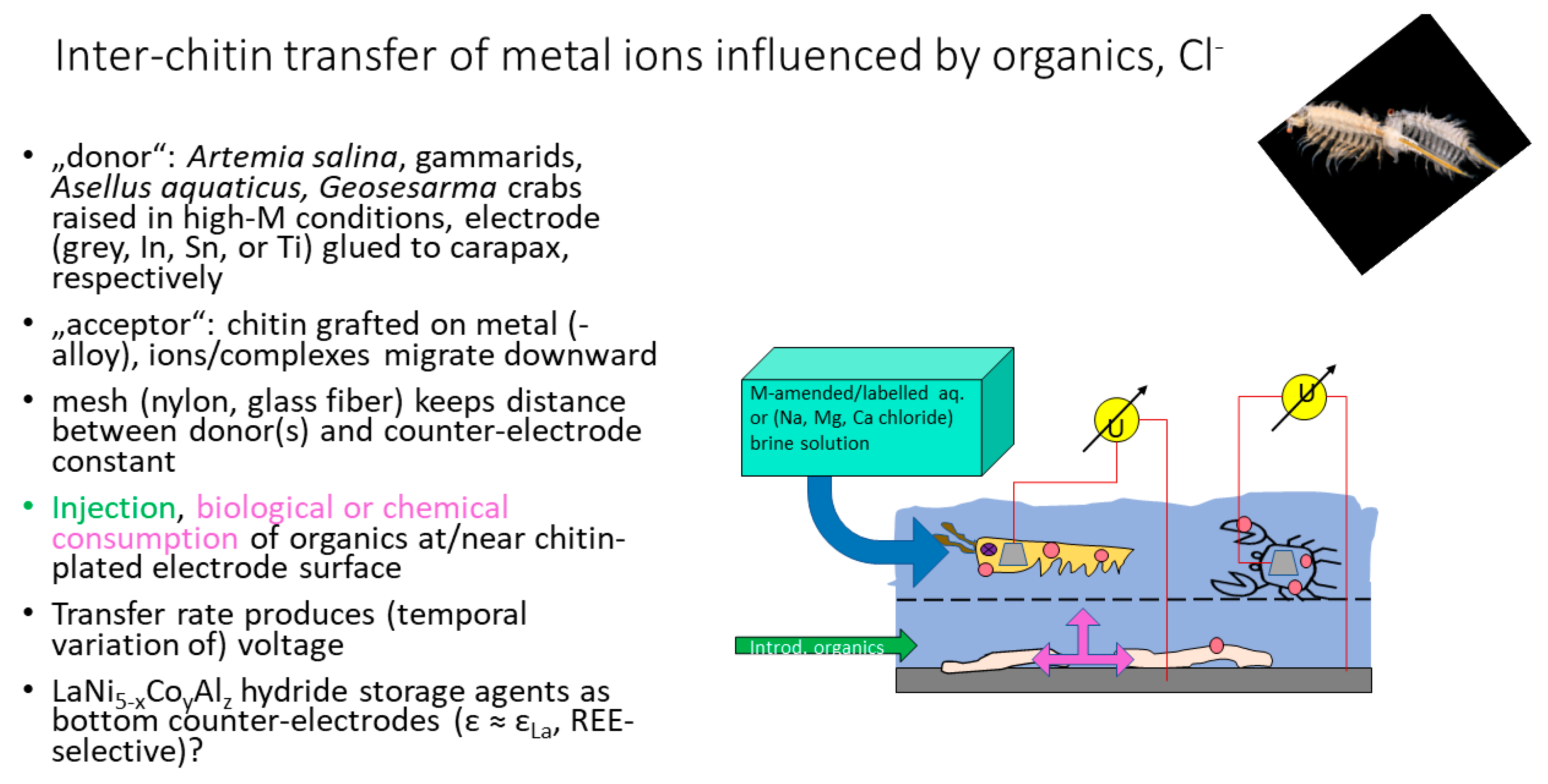
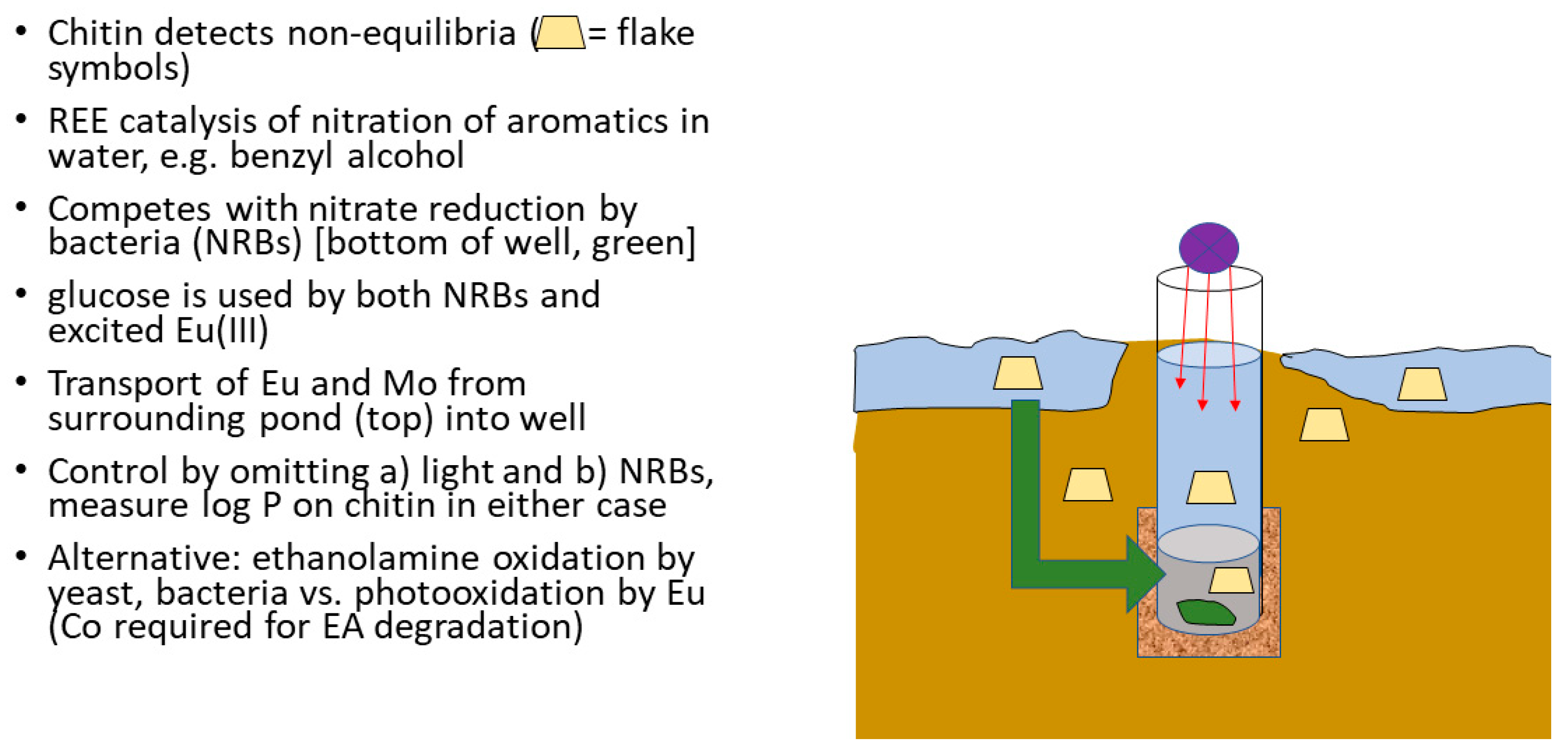
| M2+ | Log k | Remarks |
|---|---|---|
| Pb | 3.26 | Pb does accumulate on chitin more than other metals [9,13] |
| Cd | 3.93 | background on chitin essentially zero |
| Mn | 3.54 | |
| Ni | 3.33 | Detection of methanogenesis |
| Zn | 3.16 | |
| Fe(III) | 4.13 | different oxidation state |
| Kind of Ligands | No. of Ligands in Regression | e [Σσ] | f [V] | Remarks |
|---|---|---|---|---|
| Monodentate, neutral | 7 | 0.278 | 0.124 | N-, P-, As-, S- and C- (isocyanides) donors, which all are π-bonding (NH3, aliphatic amines do not correlate properly) |
| 7 | 0.265 | 0.133 | omitting pyridine, plus AsPh2(CH3) | |
| Monodentate anions | 15 | 0.593 | −0.402 | Halides, pseudohalides including CN−, OH−, carboxylates, etc., not H− |
| Bidentate, different charges | 6 | −0.185 | −0.176 | Negative slope, also includes neutral L en (ethylene diamine) |
| Ligand (Bidentate) | Σσ | σ′ | Σ of “Normal” Ligands | |
|---|---|---|---|---|
| o-phthalate | −0.36 | −0.18 | Carboxylate 0, OH −0.37 | |
| salicylate | −0.40 | OH (aromatic) = −0.22 | ||
| maleate | −0.30 | Acrylic carboxylate −0.15 | Cannot be broken up as definition applies to bidentate ligands |
| Site | Description of Site | Σσ | Σσ′ | Remarks | La Partitions (P) [–] |
|---|---|---|---|---|---|
| I | Pond, former lignite pit (12 m max. depth), exuvia | −0.574 | −0.532 | LA 1.27 FA 0.39 | |
| II | Small river | −0.522 | −0.475 | LA 1.45 FA 6.00 | |
| III | Small lake inhabited by tench (strongly digging fishes, introducing O2 and NO3− into sediment); exuvia | −0.566 | −0.412 | highest value of Σσ′ | LA 0.09 FA 1.32 |
| IV | Shallow pond, very rich in SO42−; many exuvia could be sampled | −0.621 | −0.508 | REEs form sulfatocomplexes; lowest value of Σσ | LA 0.63 FA 10.93 |
| V | Small river | −0.401 | −0.441 | pH > 8; highest value of Σσ, hard sediment | LA 2.19 FA 9.83 |
| VI | Lake, former lignite pit (41 m deep) | −0.470 | −0.573 | pH > 8 | LA 2.60 FA 0.42 |
| VIIa | Flooded former basalt quarry, exuvia | −0.611 | −0.446 | LA 0.36 FA 0.295 | |
| VIIb | Flooded former basalt quarry, living crayfish | −0.582 | −0.459 | Either value of Σσ is very similar to those of VIIa | LA 0.29 FA 4.47 |
| M(III) | a1d | b1d | a2d | b2d | a3d | b3d |
|---|---|---|---|---|---|---|
| Al | 12.48 | 6.92 | ||||
| Cr | −8.50 | 4.52 | ||||
| Y | 2.57 | 5.67 | ||||
| La | [−10.08] | [6.90] | 8.32 | 6.21 | −2.52 | 3.09 |
| Ce | −8.00 [−11.50] | 2.89 [7.58] | 3.70 | 2.56 | −2.58 | 3.31 |
| Pr | 3.95 | 2.57 | −2.74 | 3.35 | ||
| Nd | 4.02 | 2.60 | −2.77 | 3.43 | ||
| Sm | [−11.07] | [7.54] | 8.08 | 6.02 | ||
| Eu | −8.98 [−11.92] | 3.44 [7.97] | 9.45 | 7.02 | ||
| Gd | [−11.55] | [7.71] | 9.91 | 7.05 | ||
| Dy | [−14.55] | [8.90] | 9.65 | 7.04 | ||
| Tm | [−12.58] | [8.02] | ||||
| Yb | [−13.22] | [8.29] | ||||
| Lu | [−12.23] | [7.86] | 10.59 | 7.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blind, F.; Fränzle, S. Chitin as a Sorbent Superior to Other Biopolymers: Features and Applications in Environmental Research, Energy Conversion, and Understanding Evolution of Animals. Polysaccharides 2021, 2, 773-794. https://doi.org/10.3390/polysaccharides2040047
Blind F, Fränzle S. Chitin as a Sorbent Superior to Other Biopolymers: Features and Applications in Environmental Research, Energy Conversion, and Understanding Evolution of Animals. Polysaccharides. 2021; 2(4):773-794. https://doi.org/10.3390/polysaccharides2040047
Chicago/Turabian StyleBlind, Felix, and Stefan Fränzle. 2021. "Chitin as a Sorbent Superior to Other Biopolymers: Features and Applications in Environmental Research, Energy Conversion, and Understanding Evolution of Animals" Polysaccharides 2, no. 4: 773-794. https://doi.org/10.3390/polysaccharides2040047
APA StyleBlind, F., & Fränzle, S. (2021). Chitin as a Sorbent Superior to Other Biopolymers: Features and Applications in Environmental Research, Energy Conversion, and Understanding Evolution of Animals. Polysaccharides, 2(4), 773-794. https://doi.org/10.3390/polysaccharides2040047





