Methods to Improve the First Hydrogenation of the Vanadium-Rich BCC Alloy Ti16V60Cr24
Abstract
1. Introduction
2. Materials and Methods
3. Results
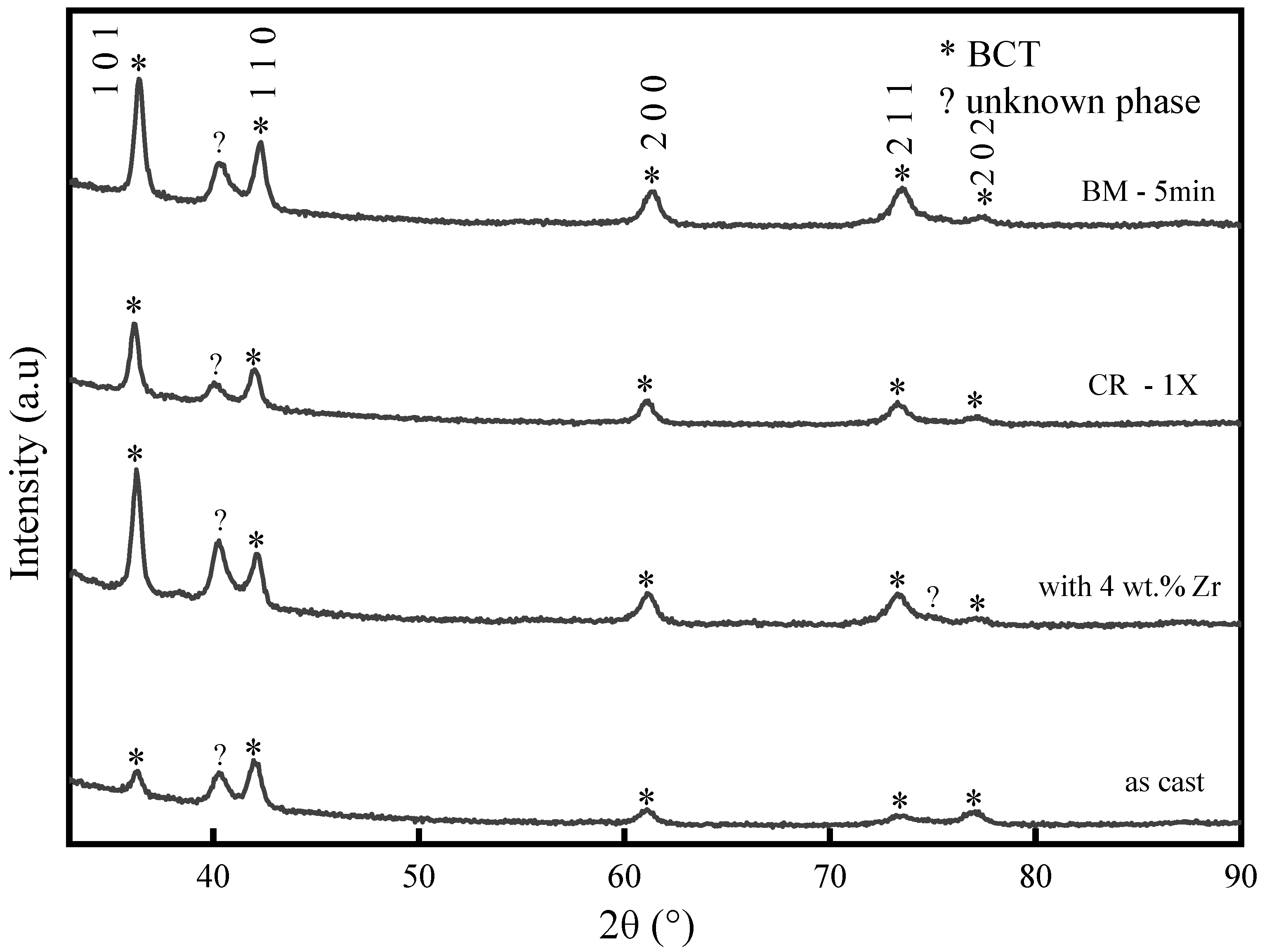
3.1. Microstructure
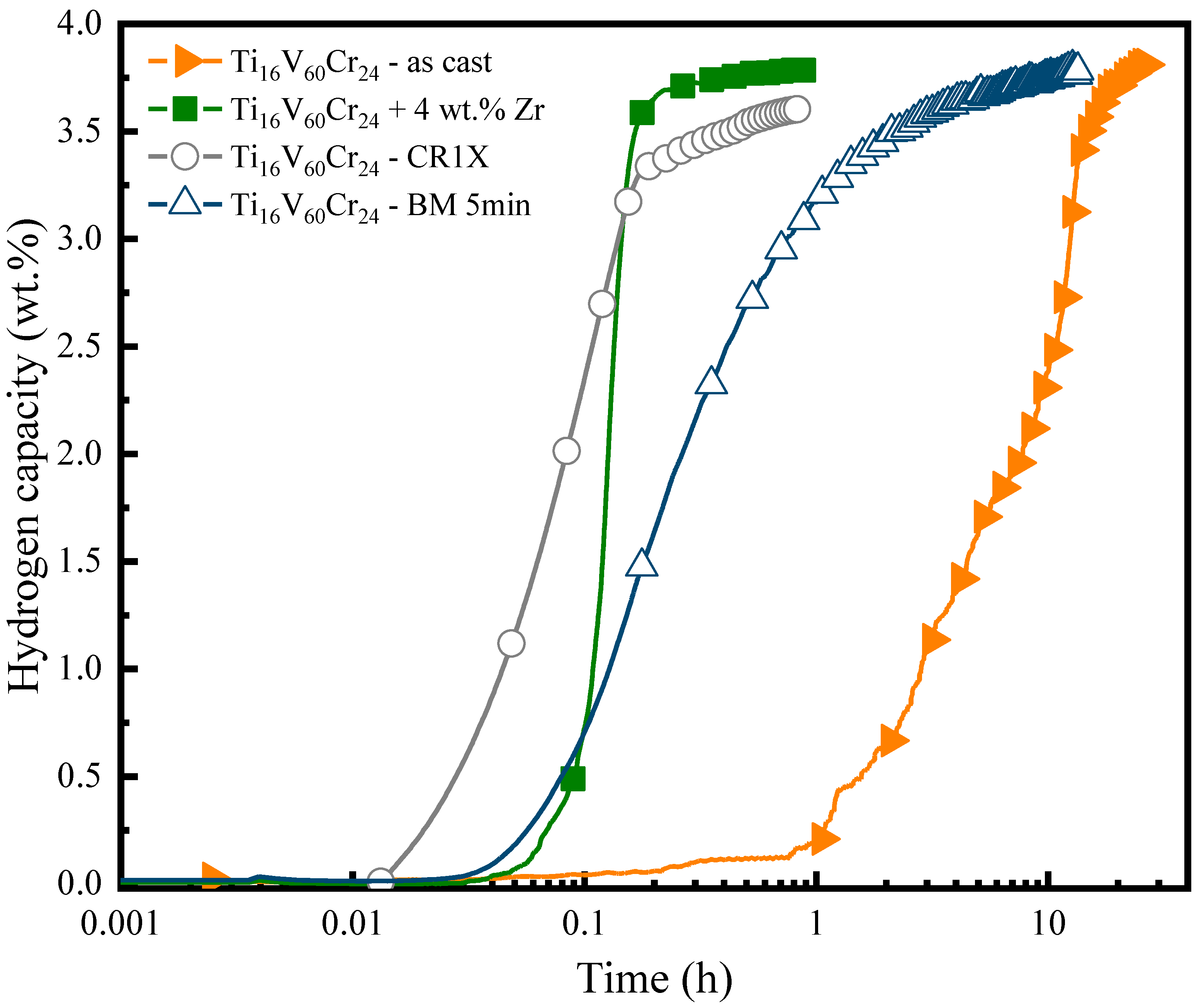
3.2. First Hydrogenation
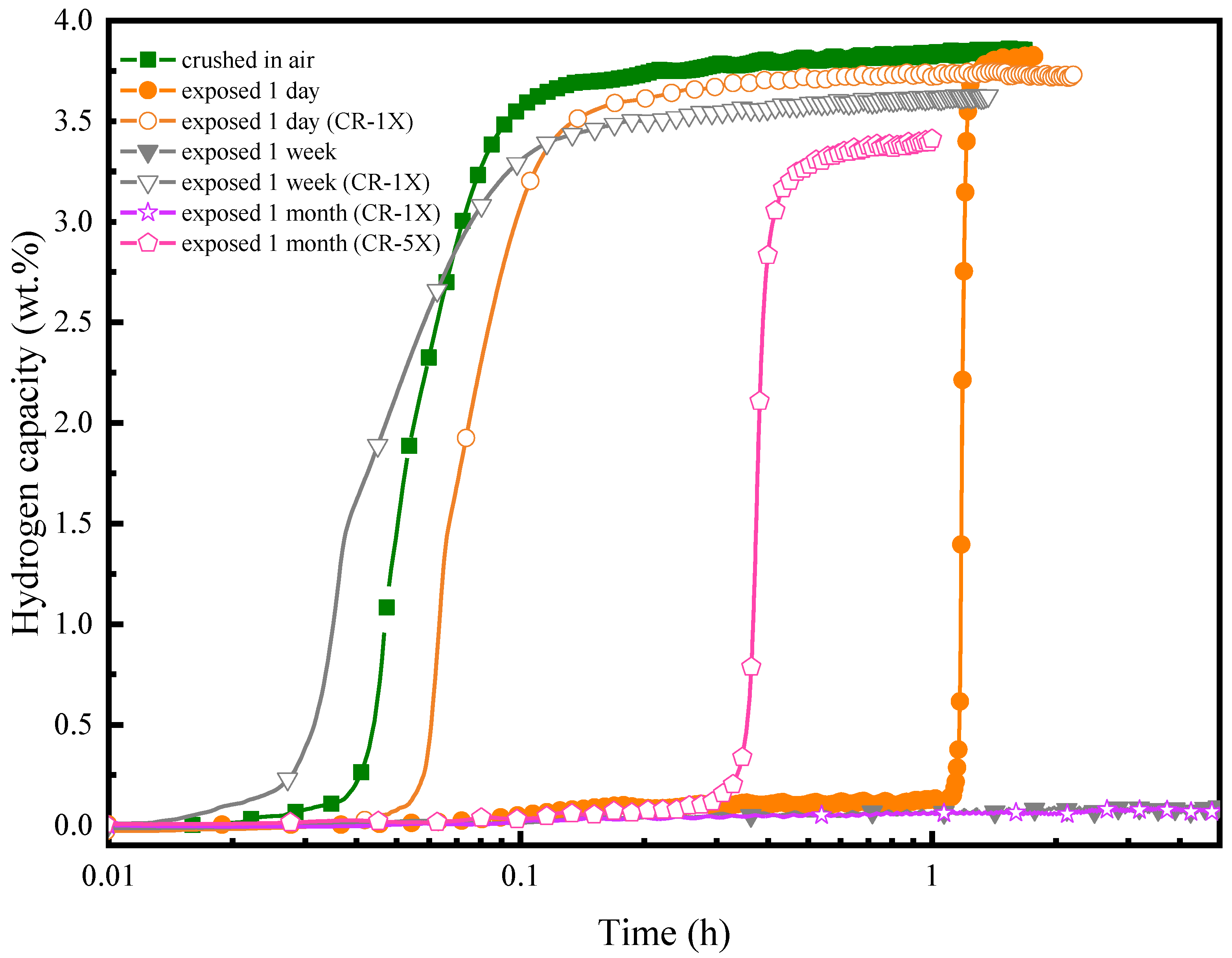
3.3. Air Exposure Effect
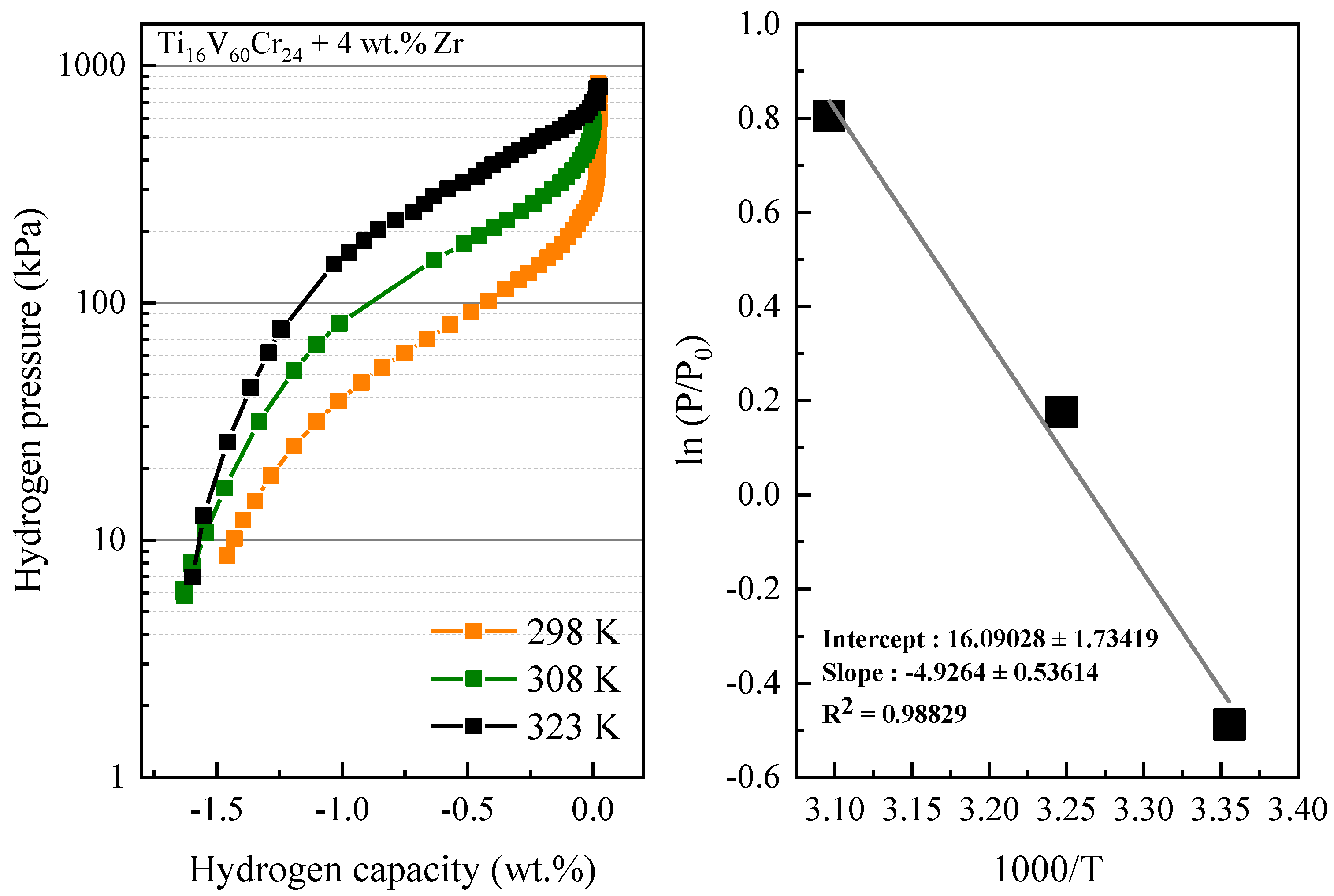
3.4. PCI Curves of Ti16V60Cr20 + 4 wt.% Zr
4. Conclusions
- -
- The addition of 4 wt.% Zr is effective in improving the kinetics of the first hydrogenation of the alloy. It results in a fast absorption kinetic and a maximum hydrogen capacity of 3.8 wt.%.
- -
- Air exposure results in an incubation time which increases with the air exposure time. Cold rolling helps regenerate the alloy by decreasing the incubation time, but it leads to a reduction of capacity.
- -
- Enthalpy and entropy of hydride formation are −41 ± 5 kJ/mol −134 ± 14 J/mol/K, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Kumar, S.; Krishnamurthy, N. Synthesis of V-Ti-Cr Alloys by Aluminothermy Co-Reduction of Its Oxides. Process. Appl. Ceram. 2011, 5, 181–186. [Google Scholar] [CrossRef]
- Kazumi, T.; Tamura, T.; Kamegawa, A.; Takamura, H.; Okada, M. Effect of Absorption-Desorption Cycles on Structure and Stability of Protides in Ti-Cr-V Alloys. Mater. Trans. 2002, 43, 2748–2752. [Google Scholar] [CrossRef]
- Dekhtyarenko, V.A.; Pryadko, T.V.; Savvakin, D.G.; Bondarchuk, V.I.; Mogylnyy, G.S. Hydrogenation Process in Multiphase Alloys of Ti-Zr-Mn-V System on the Example of Ti42.75Zr27Mn20.25V10 Alloy. Int. J. Hydrogen Energy 2021, 46, 8040–8047. [Google Scholar] [CrossRef]
- Mazzolai, G.; Coluzzi, B.; Biscarini, A.; Mazzolai, F.M.; Tuissi, A.; Agresti, F.; Lo Russo, S.; Maddalena, A.; Palade, P.; Principi, G. Hydrogen-Storage Capacities and H Diffusion in Bcc TiVCr Alloys. J. Alloys Compd. 2008, 466, 133–139. [Google Scholar] [CrossRef]
- Xiping, S.; Pei, P.; Peilong, Z.; Guoliang, C. Effect of Vanadium Content on Hydrogen Storage Property in Ti-V-Cr Alloys. Rare Met. 2006, 25, 374–377. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, A.; Ichikawa, T.; Kojima, Y.; Dey, G.K. Development of Vanadium Based Hydrogen Storage Material: A Review. Renew. Sustain. Energy Rev. 2017, 72, 791–800. [Google Scholar] [CrossRef]
- Tsukahara, M.; Takahashi, K.; Mishima, T.; Isomura, A.; Sakai, T. Heat-Treatment Effects of V-Based Solid Solution Alloy with TiNi-Based Network Structure on Hydrogen Storage and Electrode Properties. J. Alloys Compd. 1996, 243, 133–138. [Google Scholar] [CrossRef]
- Cho, S.W.; Yoo, J.H.; Chang, H.K.; Kim, W.B.; Kil, D.S.; Ahn, J.G. Changes in the Microstructure and Hydrogen Storage Properties of Ti-Cr-V Alloys by Ball Milling and Heat Treatment. J. Alloys Compd. 2011, 509, 5545–5550. [Google Scholar] [CrossRef]
- Seo, C.Y.; Kim, J.H.; Lee, P.S.; Lee, J.Y. Hydrogen Storage Properties of Vanadium-Based b.c.c. Solid Solution Metal Hydrides. J. Alloys Compd. 2003, 348, 252–257. [Google Scholar] [CrossRef]
- Miraglia, S.; De Rango, P.; Rivoirard, S.; Fruchart, D.; Charbonnier, J.; Skryabina, N. Hydrogen Sorption Properties of Compounds Based on BCC Ti 1-XV 1-YCr 1+x+y Alloys. J. Alloys Compd. 2012, 536, 1–6. [Google Scholar] [CrossRef]
- Cho, S.W.; Han, C.S.; Park, C.N.; Akiba, E. Hydrogen Storage Characteristics of Ti-Cr-V Alloys. J. Alloys Compd. 1999, 288, 294–298. [Google Scholar] [CrossRef]
- Yoo, J.H.; Shim, G.; Park, C.N.; Kim, W.B.; Cho, S.W. Influence of Mn or Mn plus Fe on the Hydrogen Storage Properties of the Ti-Cr-V Alloy. Int. J. Hydrogen Energy 2009, 34, 9116–9121. [Google Scholar] [CrossRef]
- Huot, J.; Tousignant, M. Effect of Cold Rolling on Metal Hydrides. Mater. Trans. 2019, 60, 1571–1576. [Google Scholar] [CrossRef]
- Sleiman, S.; Aliouat, A.; Huot, J. Enhancement of First Hydrogenation of Ti 1 V 0.9 Cr 1.1 BCC Alloy by Cold Rolling and Ball Milling. Materials 2020, 13, 3106. [Google Scholar] [CrossRef]
- Lyu, P. Effect of Mechanical Deformation on Hydrogen Storage Properties of TiFe-Based Alloys. Ph.D. Thesis, Université du Québec à Trois, Rivières, QC, Canada, 2018. [Google Scholar]
- Khajavi, S.; Rajabi, M.; Huot, J. Effect of Cold Rolling and Ball Milling on First Hydrogenation of Ti0.5Zr0.5 (Mn1-XFex) Cr1, X = 0, 0.2, 0.4. J. Alloys Compd. 2019, 775, 912–920. [Google Scholar] [CrossRef]
- Shashikala, K.; Kumar, A.; Betty, C.A.; Banerjee, S.; Sengupta, P.; Pillai, C.G.S. Improvement of the Hydrogen Storage Properties and Electrochemical Characteristics of Ti 0.85 VFe 0.15 Alloy by Ce Substitution. J. Alloys Compd. 2011, 509, 9079–9083. [Google Scholar] [CrossRef]
- Bibienne, T.; Razafindramanana, V.; Bobet, J.L.; Huot, J. Synthesis, Characterization and Hydrogen Sorption Properties of a Body Centered Cubic 42Ti-21V-37Cr Alloy Doped with Zr7Ni10. J. Alloys Compd. 2015, 620, 101–108. [Google Scholar] [CrossRef]
- Dixit, V.; Huot, J. Investigation of the Microstructure, Crystal Structure and Hydrogenation Kinetics of Ti-V-Cr Alloy with Zr Addition. J. Alloys Compd. 2019, 785, 1115–1120. [Google Scholar] [CrossRef]
- Sleiman, S.; Huot, J. Microstructure and Hydrogen Storage Properties of Ti1V0.9Cr1.1 Alloy with Addition of x Wt % Zr (x = 0, 2, 4, 8, and 12). Inorganics 2017, 5, 86. [Google Scholar] [CrossRef]
- Bruker, A.X.S. TOPAS V3: General profile and structure analysis software for powder diffraction data. In User’s Manual; Bruker AXS: Karlsruhe, Germany, 2005. [Google Scholar]
- Yau, T.-L.; Annamalai, V.E. Corrosion of Zirconium and Its Alloys. Ref. Modul. Mater. Sci. Mater. Eng. 2010, 3, 2094–2134. [Google Scholar] [CrossRef]
- Kamble, A.; Huot, J.; Sharma, P. Effect of Addition of Zr, Ni, and Zr-Ni Alloy on the Hydrogen Absorption of Body Centred Cubic 52Ti-12V-36Cr Alloy. Int. J. Hydrogen Energy 2018, 43, 7424–7429. [Google Scholar] [CrossRef]
- Kamble, A. Effect of Additives, Heat Treatment and Mechanical Deformations on Hydrogen Storage Properties of BCC Alloys. Ph.D. Thesis, Université du Québec à Trois, Rivières, QC, Canada, 2018. [Google Scholar]
- Tamura, T.; Kazumi, T.; Kamegawa, A.; Takamura, H.; Okada, M. Effects of Protide Structures on Hysteresis in Ti-Cr-V Protium Absorption Alloys. Mater. Trans. 2002, 43, 2753–2756. [Google Scholar] [CrossRef]
- Reilly, J.J.; Wiswall, R.H. The Higher Hydrides of Vanadium and Niobium1. Inorg. Chem. 1970, 9, 1678–1682. [Google Scholar] [CrossRef]
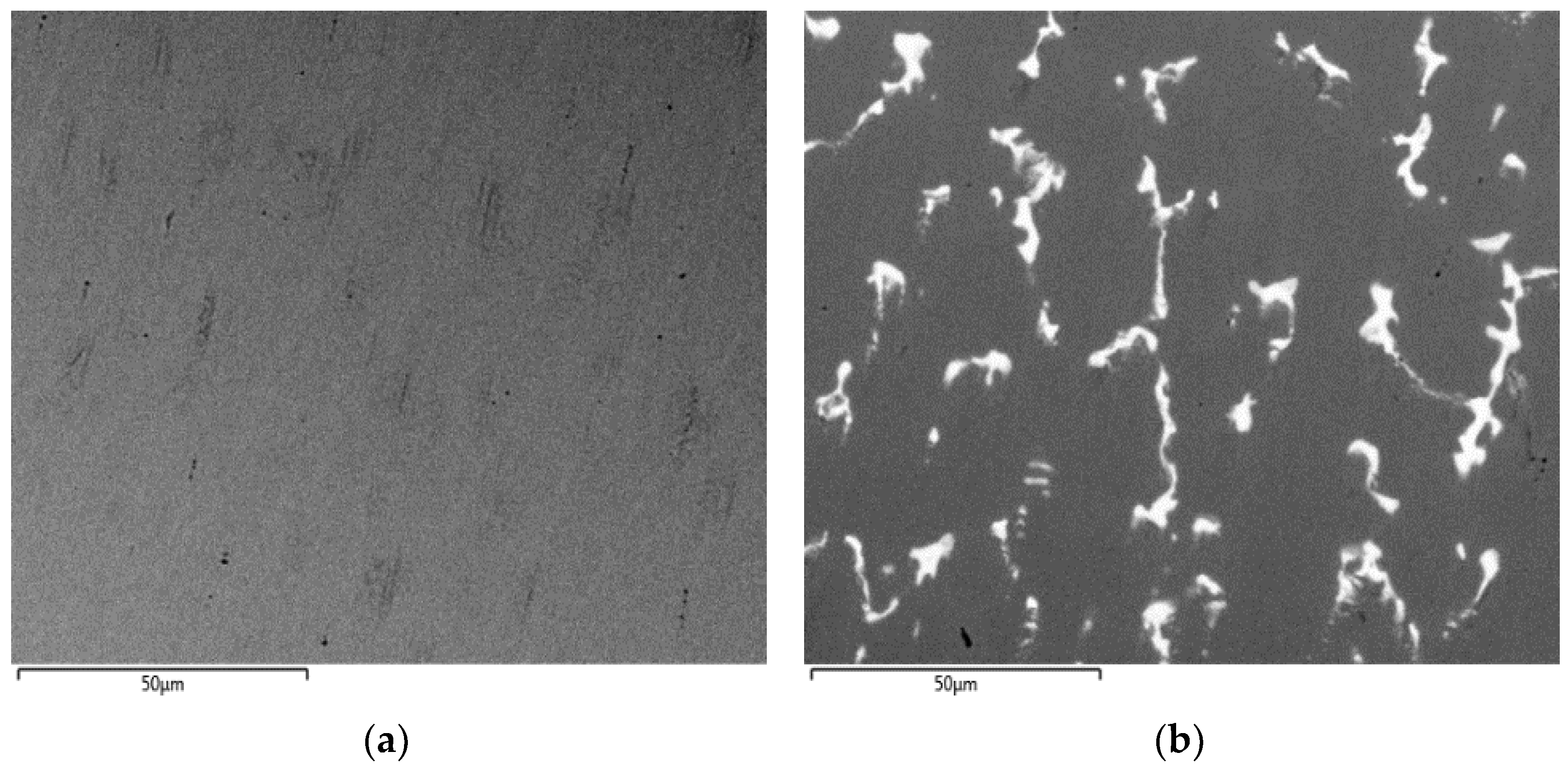
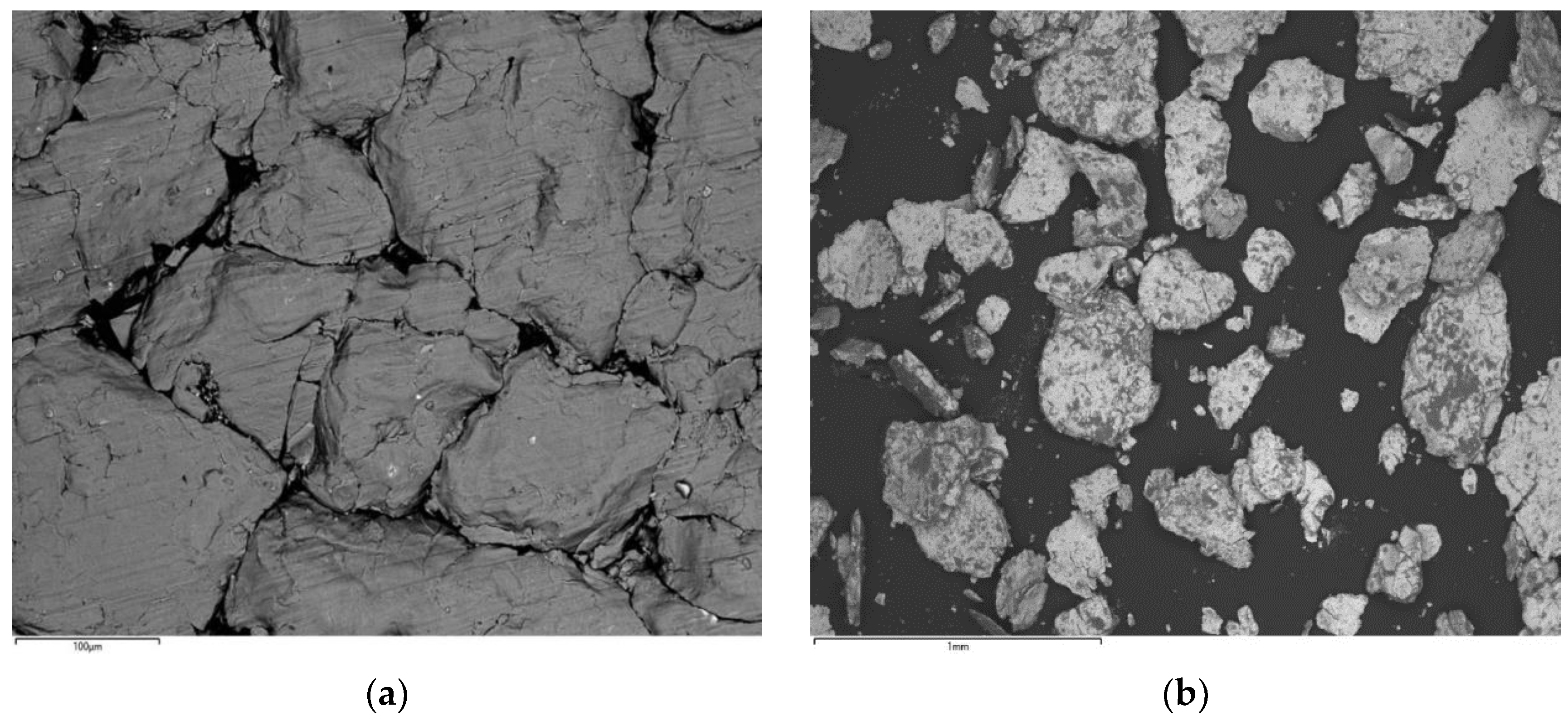

| Element | Matrix Phase | Bright Phase |
|---|---|---|
| Ti | 14.2 | 33.8 |
| V | 62.4 | 5.1 |
| Cr | 22.9 | 1.8 |
| Zr | 0.5 | 59.3 |
| Sample | Lattice Parameter (Å) | Crystallite Size (nm) | Microstrain (%) |
|---|---|---|---|
| as-cast | 3.0295 (4) | 36.1 (2) | 1.08 (2) |
| with 4 wt.% Zr | 3.0331 (6) | 35.0 (2) | 1.36 (2) |
| CR-1X | 3.0325 (4) | 26.2 (1) | 1.02 (2) |
| BM-5 min | 3.0310 (4) | 28.1 (1) | 1.05 (2) |
| Lattice Parameters (Å) | Crystallite Size (nm) | Microstrain (%) | |
|---|---|---|---|
| as-cast | a = 3.0220 (3) c = 4.2408 (9) | 12 (2) | --- |
| +4 wt.% Zr | a = 3.0350 (2) c = 4.2640 (5) | 40 (1) | 1.09 (1) |
| CR-1X | a = 3.0258 (2) c = 4.2586 (4) | 39 (5) | 1.01 (2) |
| BM 5 min | a = 3.0136 (1) c = 4.2912 (2) | 28 (3) | 1.00 (1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravalison, F.; Rabkin, E.; Huot, J. Methods to Improve the First Hydrogenation of the Vanadium-Rich BCC Alloy Ti16V60Cr24. Hydrogen 2022, 3, 303-311. https://doi.org/10.3390/hydrogen3030018
Ravalison F, Rabkin E, Huot J. Methods to Improve the First Hydrogenation of the Vanadium-Rich BCC Alloy Ti16V60Cr24. Hydrogen. 2022; 3(3):303-311. https://doi.org/10.3390/hydrogen3030018
Chicago/Turabian StyleRavalison, Francia, Eugen Rabkin, and Jacques Huot. 2022. "Methods to Improve the First Hydrogenation of the Vanadium-Rich BCC Alloy Ti16V60Cr24" Hydrogen 3, no. 3: 303-311. https://doi.org/10.3390/hydrogen3030018
APA StyleRavalison, F., Rabkin, E., & Huot, J. (2022). Methods to Improve the First Hydrogenation of the Vanadium-Rich BCC Alloy Ti16V60Cr24. Hydrogen, 3(3), 303-311. https://doi.org/10.3390/hydrogen3030018







