Hydrogen Diffusion on, into and in Magnesium Probed by DFT: A Review
Abstract
:1. Introduction
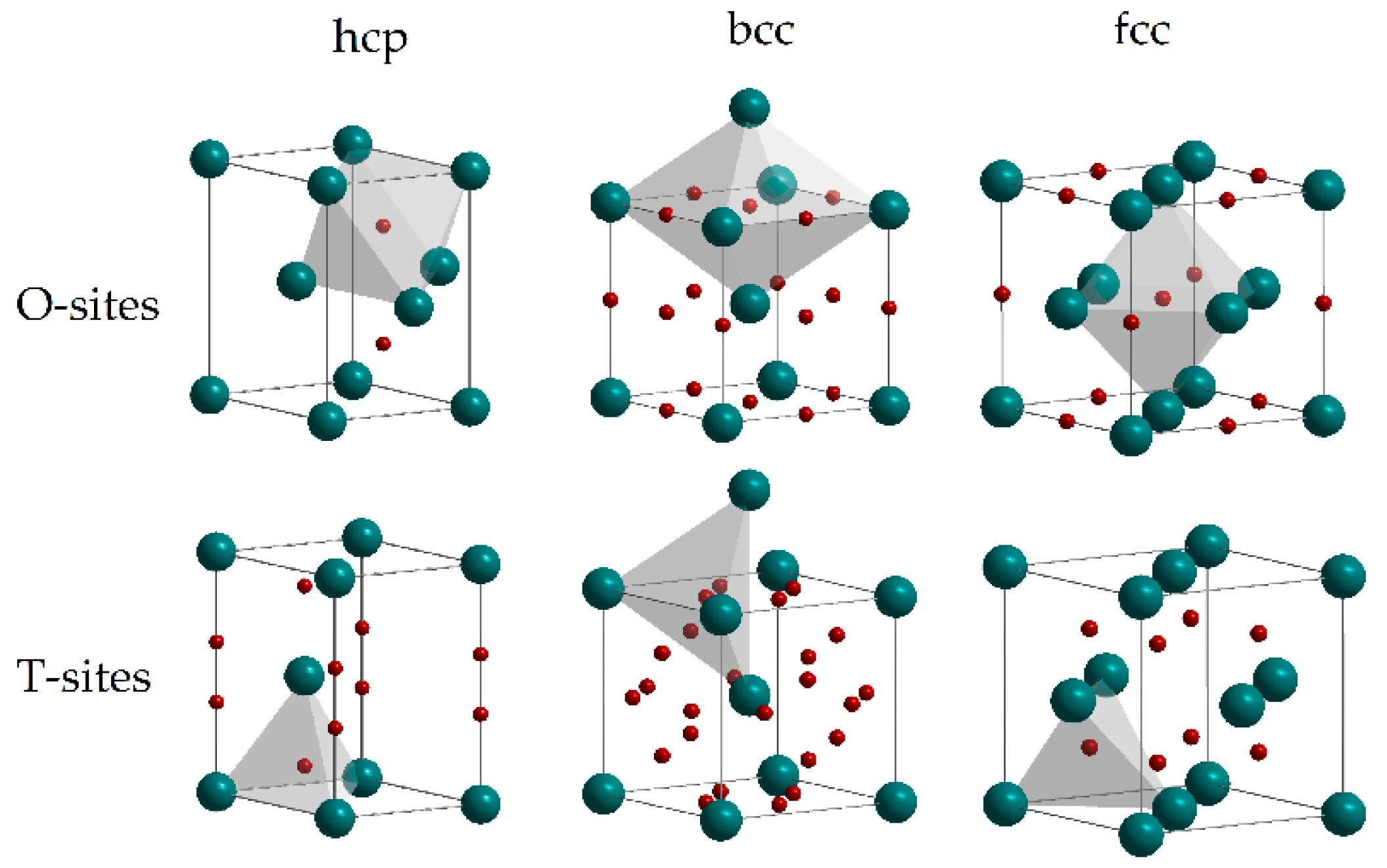
2. Interaction of Hydrogen with Metals
3. DFT Method to Study Hydrogen Diffusion
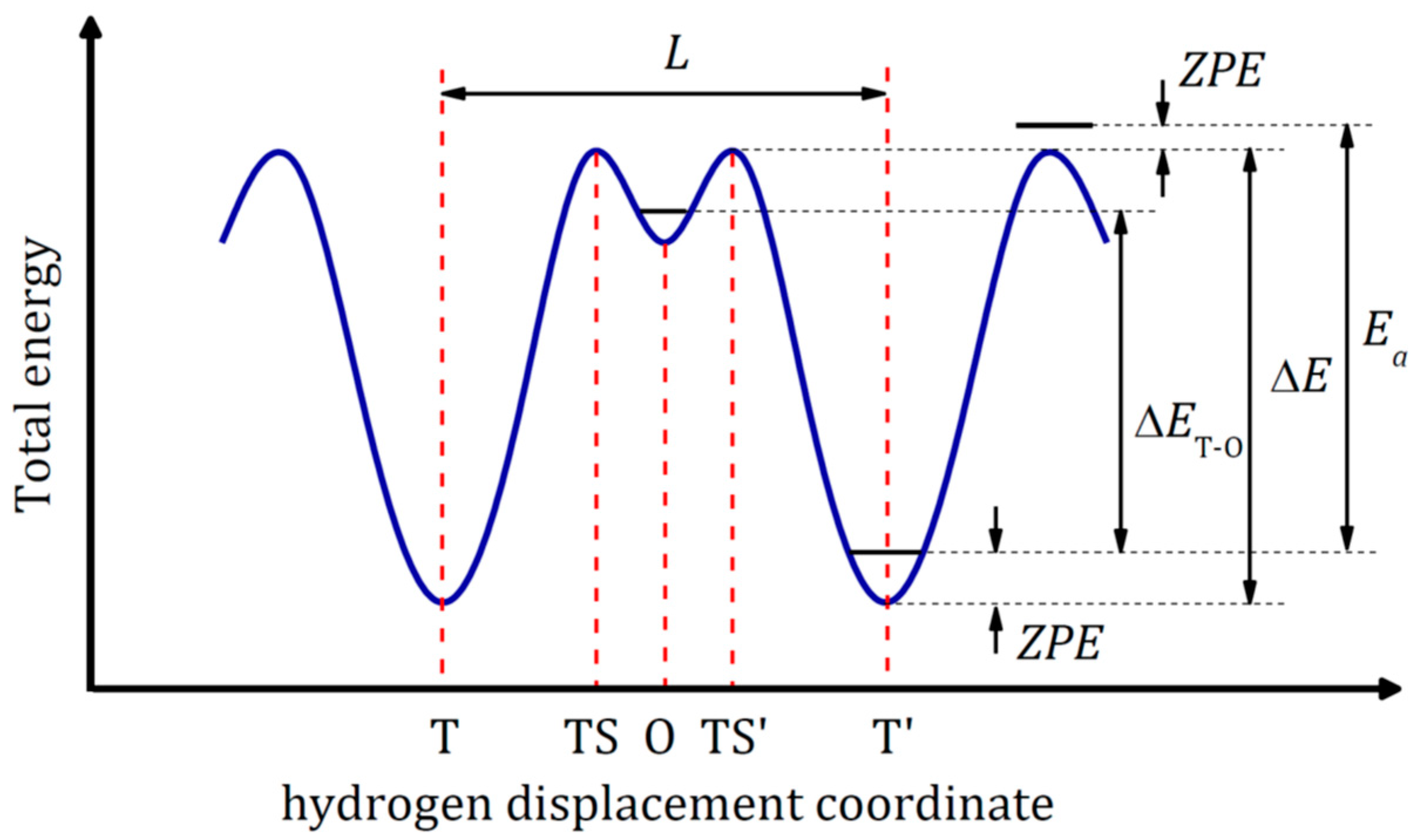
3.1. Potential Energy Surface and Activation Energy
3.2. Hydrogen Jump Rate and Diffusion Coefficient
3.3. Hydrogen Solubility and Hydrogen Vacancy Energies
3.4. Zero-Point Energy
4. DFT Modelling of Mg-H Systems
4.1. Mg-H Bonding and Effect of TM Substitution
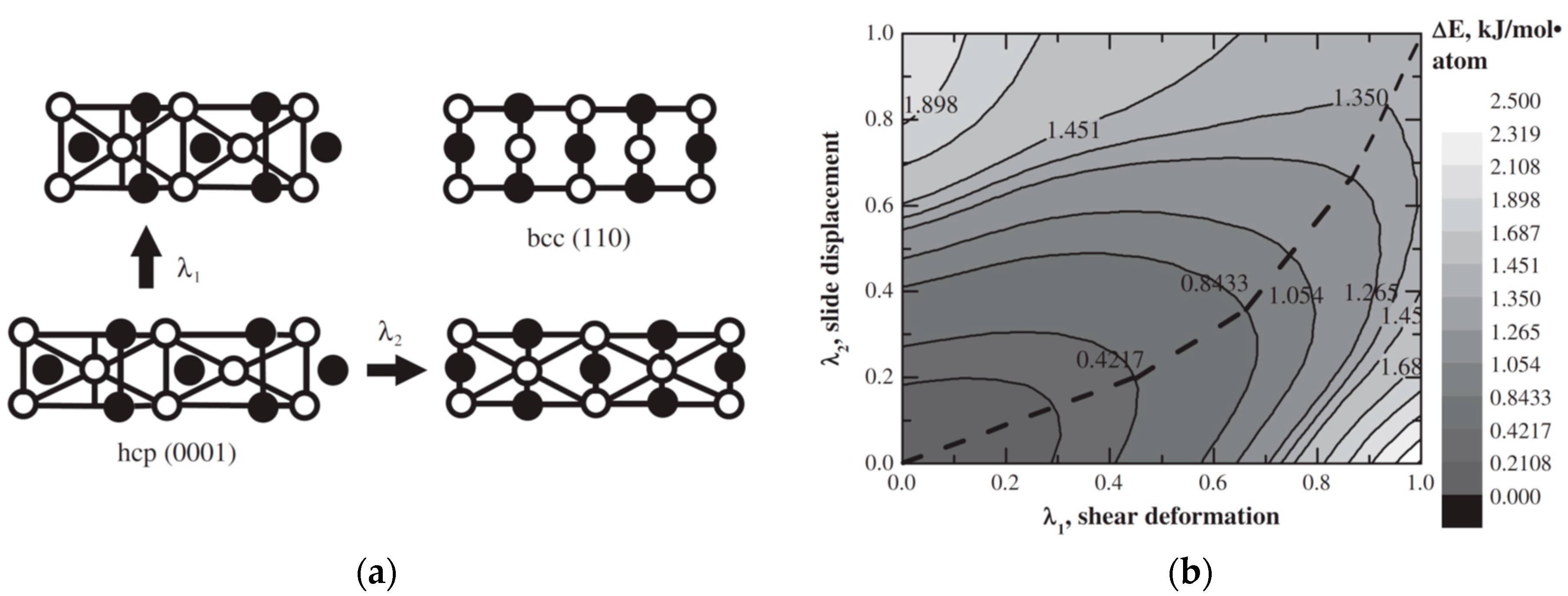
4.2. Mg/TM Thin Films and bcc Mg Phase Stability
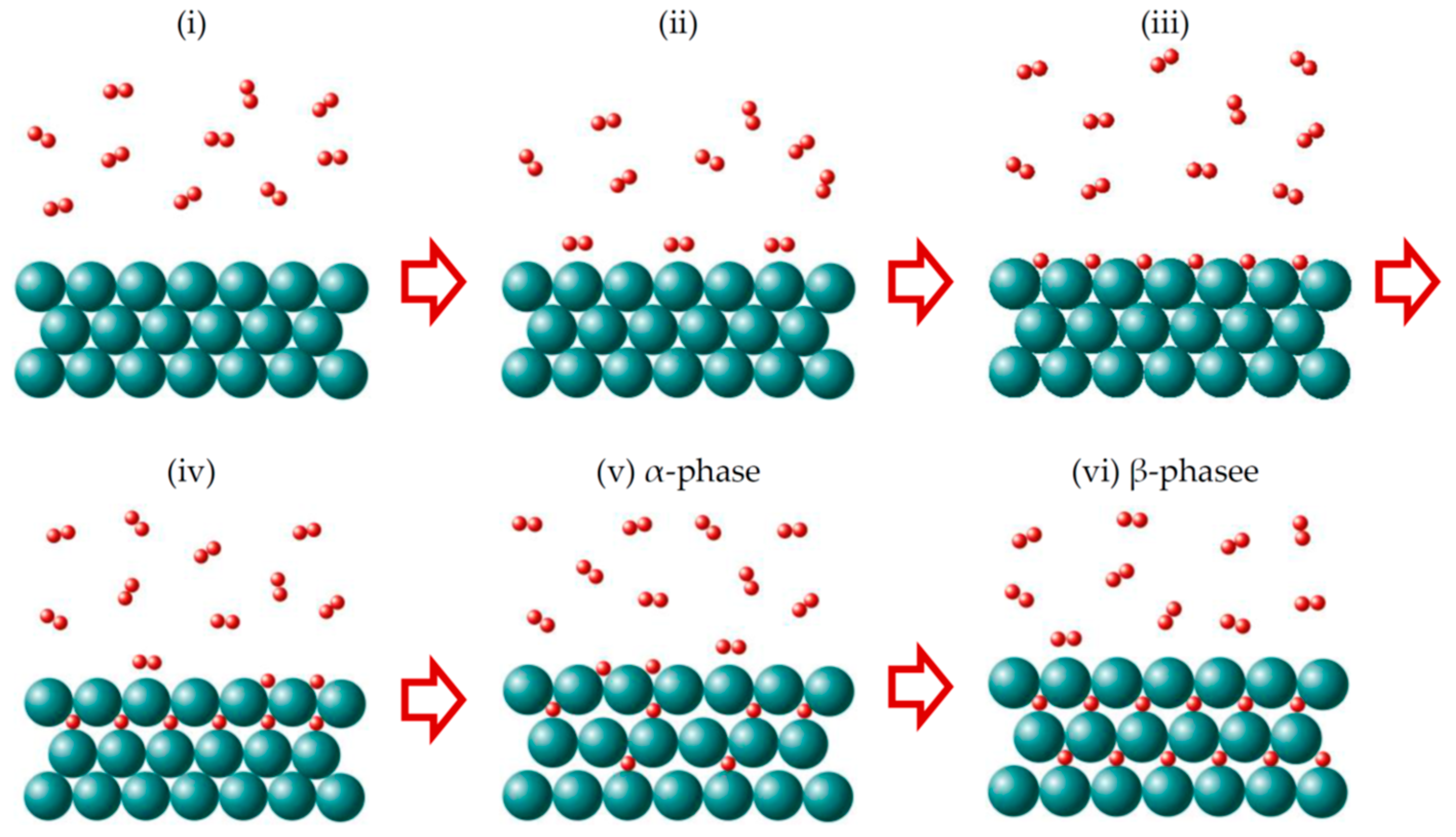
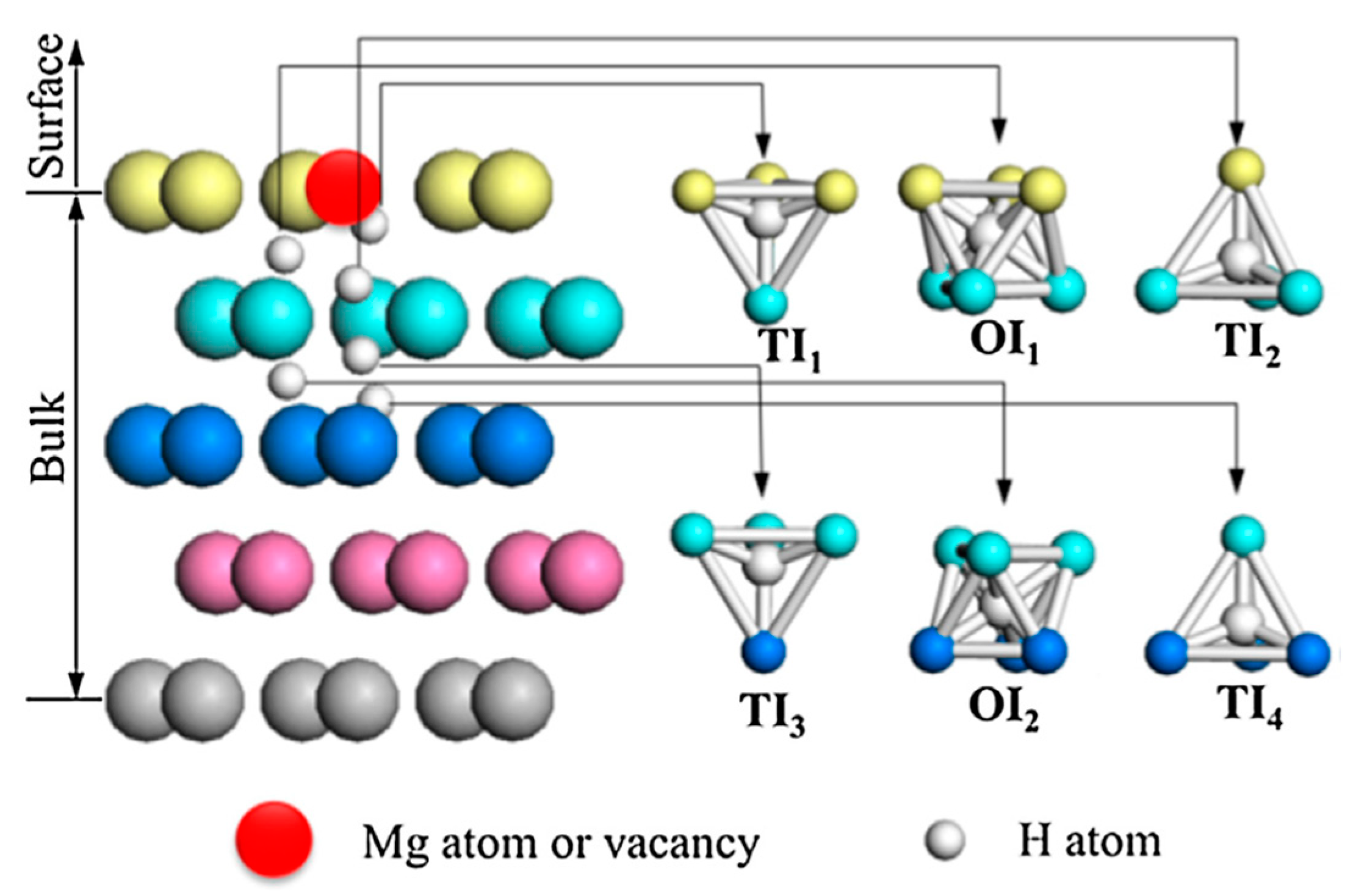
4.3. Hydrogen Molecule Dissociation on Mg(0001) Surfaces and Migration into the Subsurface
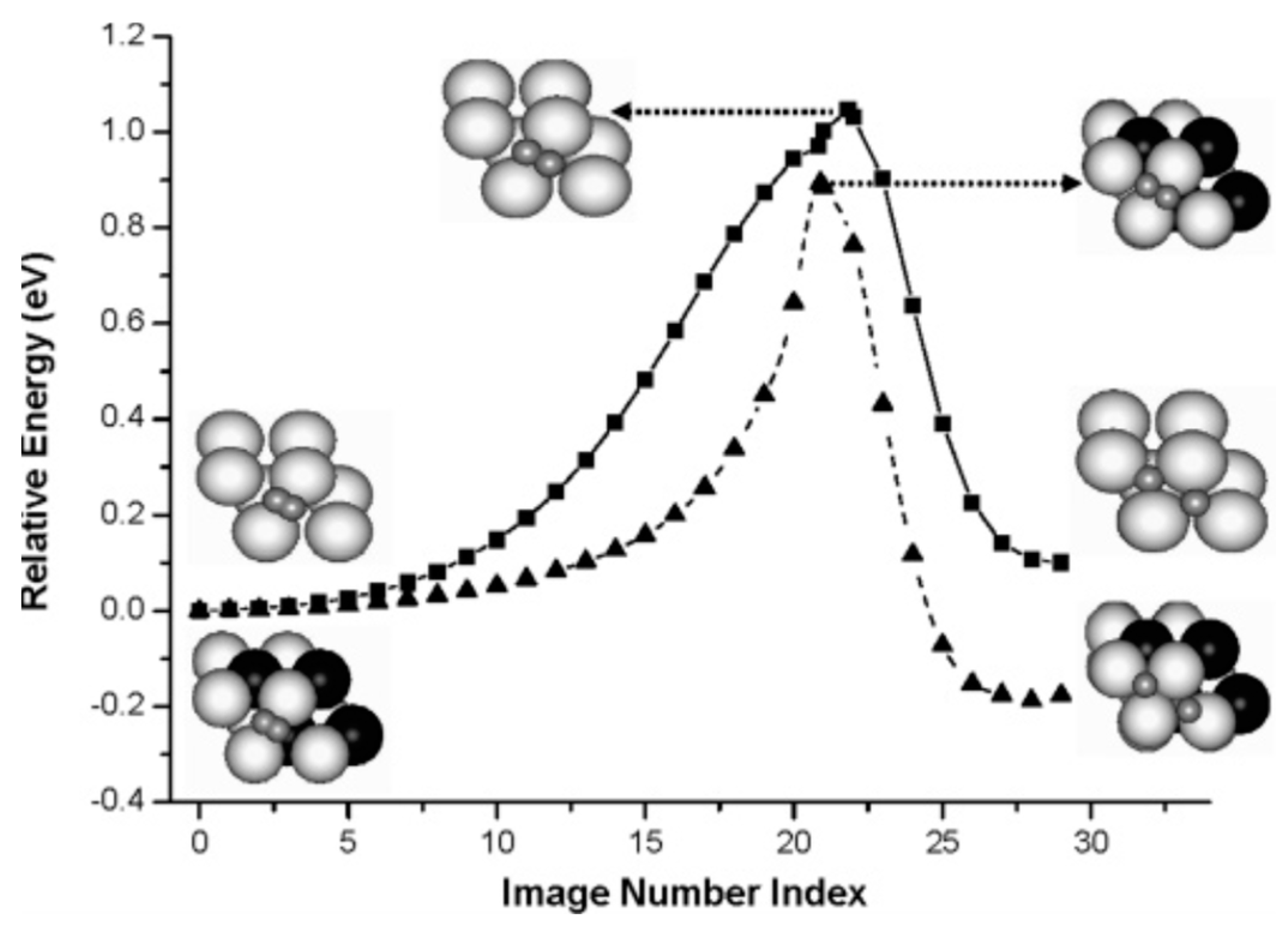
4.4. Hydrogen Induced Phase Transformations in MgHx
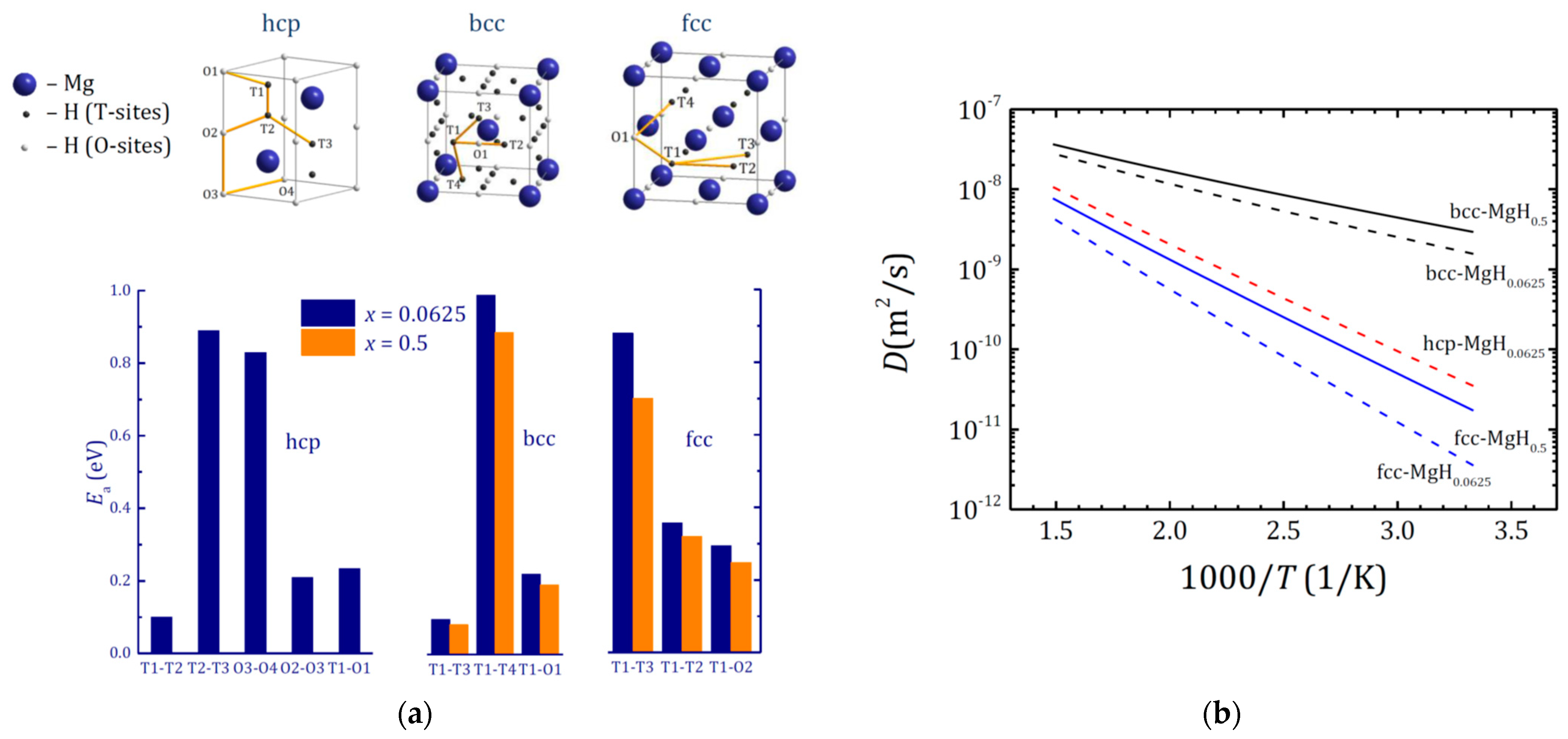
4.5. Modelling of Hydrogen Diffusion in Bulk MgHx
4.6. Hydrogen Desorption from (001) MgH2 Surfaces
5. Molecular Dynamics Simulation of Mg-H Systems
6. Conclusions
Funding
Conflicts of Interest
References
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.K. Hydrogen storage: The major technological barrier to the development of hydrogen fuel cell cars. Vacuum 2006, 80, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H. Hydrogen—A sustainable energy carrier. Prog. Nat. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Shelyapina, M.G. Metal hydrides for energy storage. In Handbook of Ecomaterials; Martínez, L., Kharissova, O., Kharisov, B., Eds.; Springer: Cham, Switzerland, 2019; pp. 775–810. ISBN 978-331968255-6. [Google Scholar] [CrossRef]
- de Rango, P.; Chaise, A.; Charbonnier, J.; Fruchart, D.; Jehan, M.; Marty, P.; Miraglia, S.; Rivoirard, S.; Skryabina, N. Nanostructured magnesium hydride for pilot tank development. J. Alloys Compd. 2007, 446-467, 52–57. [Google Scholar] [CrossRef]
- Jehan, M.; Fruchart, D. McPhy-Energy’s proposal for solid state hydrogen storage materials and systems. J. Alloys Compd. 2013, 580, S343–S348. [Google Scholar] [CrossRef]
- Pedersen, A.S.; Kjoller, J.; Larsen, B.; Vigeholm, B. Magnesium for hydrogen storage. Int. J. Hydrogen Energy 1983, 8, 205–211. [Google Scholar] [CrossRef]
- Armbrüster, M.; Behrens, M.; Cinquini, F.; Föttinger, K.; Grin, Y.; Haghofer, A.; Klötzer, B.; Knop-Gericke, A.; Lorenz, H.; Ota, A.; et al. How to control the selectivity of palladium-based catalysts in hydrogenation reactions: The role of subsurface chemistry. ChemCatChem 2012, 4, 1048–1063. [Google Scholar] [CrossRef]
- Klose, W.; Stuke, V. Investigation of the thermodynamic equilibrium in the hydrogen-magnesium-magnesium hydride system. Int. J. Hydrogen Energy 1995, 20, 309–316. [Google Scholar] [CrossRef]
- Grbović Novaković, J.; Novaković, N.; Kurko, S.; Milošević Govedarović, S.; Pantić, T.; Paskaš Mamula, B.; Batalović, K.; Radaković, J.; Rmuš, J.; Shelyapina, M.; et al. Influence of defects on the stability and hydrogen-sorption behavior of Mg-based hydrides. ChemPhysChem 2019, 20, 1216–1247. [Google Scholar] [CrossRef]
- Shang, Y.; Pistidda, C.; Gizer, G.; Klassen, T.; Dornheim, M. Mg-based materials for hydrogen storage. J. Magnes. Alloys 2021, 9, 1837–1860. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2–Tm (Tm = Ti, V, Mn, Fe and Ni) systems. J. Alloys Compd. 1999, 292, 247–252. [Google Scholar] [CrossRef]
- Charbonnier, J.; De Rango, P.; Fruchart, D.; Miraglia, S.; Pontonnier, L.; Rivoirard, S.; Skryabina, N.; Vulliet, P. Hydrogenation of transition element additives (Ti, V) during ball milling of magnesium hydride. J. Alloys Compd. 2004, 383, 205–208. [Google Scholar] [CrossRef]
- Rivoirard, S.; de Rango, P.; Fruchart, D.; Charbonnier, J.; Vempaire, D. Catalytic effect of additives on the hydrogen absorption properties of nano-crystalline MgH2(X) composites. J. Alloys Compd. 2003, 356-357, 622–625. [Google Scholar] [CrossRef]
- Oelerich, W.; Klassen, T.; Bormann, R. Metal oxides as catalysts for improved hydrogen sorption in nanocrystalline Mg-based materials. J. Alloys Compd. 2001, 315, 237–242. [Google Scholar] [CrossRef]
- Song, M.Y.; Bobet, J.L.; Darriet, B. Improvement in hydrogen sorption properties of Mg by reactive mechanical grinding with Cr2O3, Al2O3 and CeO2. J. Alloys Compd. 2002, 340, 256–262. [Google Scholar] [CrossRef]
- Floriano, R.; Leiva, D.R.; Deledda, S.; Hauback, B.C.; Botta, W.J. Cold rolling of MgH2 powders containing different additives. Int. J. Hydrogen Energy 2013, 38, 16193–16198. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Ström-Olsen, J.O. Nanocrystalline magnesium for hydrogen storage. J. Alloys Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- Botta, W.J.; Zepon, G.; Ishikawa, T.T.; Leiva, D.R. Metallurgical processing of Mg alloys and MgH2 for hydrogen storage. J. Alloys Compd. 2022, 897, 162798. [Google Scholar] [CrossRef]
- Huot, J.; Skryabina, N.Y.; Fruchart, D. Application of severe plastic deformation techniques to magnesium for enhanced hydrogen sorption properties. Metals 2012, 2, 329–343. [Google Scholar] [CrossRef] [Green Version]
- Uchida, H. Surface processes of H2 on rare earth based hydrogen storage alloys with various surface modifications. Int. J. Hydrogen Energy 1999, 24, 861–869. [Google Scholar] [CrossRef]
- Grbović Novaković, J.; Matović, L.; Drvendžija, M.; Novaković, N.; Rajnović, D.; Šiljegović, M.; Kačarević Popović, Z.; Milovanović, S.; Ivanović, N. Changes of hydrogen storage properties of MgH2 induced by heavy ion irradiation. Int. J. Hydrogen Energy 2008, 33, 1876–1879. [Google Scholar] [CrossRef]
- Fukai, Y. The Metal-Hydrogen System. Basic Bulk Properties; Springer: Berlin/Heidelberg, Germany, 2005; ISBN 3-540-00494-7. [Google Scholar]
- Völkl, J.; Alefeld, G. Diffusion of hydrogen in metals. In Hydrogen in Metals I: Basic Properties; Alefeld, G., Völkl, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1978; pp. 321–348. ISBN 978-3-540-35892-3. [Google Scholar]
- Wipf, H. Diffusion of hydrogen in metals. In Hydrogen in Metals III: Properties and Applications; Wipf, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 51–91. ISBN 978-3-540-69988-0. [Google Scholar]
- Hao, S.; Sholl, D.S. Self-diffusion and macroscopic diffusion of hydrogen in amorphous metals from first-principles calculations. J. Chem. Phys. 2009, 130, 244705. [Google Scholar] [CrossRef] [PubMed]
- Skripov, A.V.; Shelyapina, M.G. Nuclear magnetic resonance. In Neutron Scattering and Other Nuclear Techniques for Hydrogen in Materials; Fritzsche, H., Huot, J., Fruchart, D., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 337–376. ISBN 978-3-319-22792-4. [Google Scholar]
- Kasperovich, V.S.; Shelyapina, M.G.; Khar’Kov, B.; Rykov, I.; Osipov, V.; Kurenkova, E.; Ievlev, A.V.; Skryabina, N.E.; Fruchart, D.; Miraglia, S.; et al. NMR study of metal-hydrogen systems for hydrogen storage. J. Alloys Compd. 2011, 509, S804–S808. [Google Scholar] [CrossRef]
- Vyvodtceva, A.V.; Shelyapina, M.G.; Privalov, A.F.; Chernyshev, Y.S.; Fruchart, D. 1H NMR study of hydrogen self-diffusion in ternary Ti-V-Cr alloys. J. Alloys Compd. 2014, 614, 364–367. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Vyvodtceva, A.V.; Klyukin, K.A.; Bavrina, O.O.; Chernyshev, Y.S.S.; Privalov, A.F.; Fruchart, D. Hydrogen diffusion in metal-hydrogen systems via NMR and DFT. Int. J. Hydrogen Energy 2015, 40, 17038–17050. [Google Scholar] [CrossRef]
- Vajeeston, P.; Ravindran, P.; Kjekshus, A.; Fjellvåg, H. Pressure-induced structural transitions in MgH2. Phys. Rev. Lett. 2002, 89, 175506. [Google Scholar] [CrossRef] [Green Version]
- Vajeeston, P.; Ravindran, P.; Hauback, B.C.; Fjellvåg, H.; Kjekshus, A.; Furuseth, S.; Hanfland, M. Structural stability and pressure-induced phase transitions in MgH2. Phys. Rev. B 2006, 73, 224102. [Google Scholar] [CrossRef]
- Landolt-Börnstein-Group IV Physical chemistry. In Thermodynamic Properties of Inorganic Materials. Pure Substances. Part 4_Compounds from HgH_g to ZnTe_g; Springer: Berlin/Heidelberg, Germany, 2001; Volume 19A4, ISBN 978-3-540-41025-6.
- Tsuneda, T. Density Functional Theory in Quantum Chemistry; Springer: Tokyo, Japan, 2014; ISBN 978-4-431-54824-9. [Google Scholar]
- Jonsson, H.; Mills, G.; Jacobsen, K.W. Classical and quantum dynamics in condensed phase simulations. In Nudged Elastic Band Method for Finding Minimum Energy Paths of Transitions; Berne, B.J., Ciccotti, G., Coker, D.F., Eds.; World Scientific Publishing: Singapore, 1998; pp. 385–404. [Google Scholar]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9903. [Google Scholar] [CrossRef] [Green Version]
- Henkelman, G.; Jónsson, H.; Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; et al. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef] [Green Version]
- Peterson, A.A. Acceleration of saddle-point searches with machine learning. J. Chem. Phys. 2016, 145, 074106. [Google Scholar] [CrossRef] [PubMed]
- Garrido Torres, J.A.; Jennings, P.C.; Hansen, M.H.; Boes, J.R.; Bligaard, T. Low-scaling algorithm for nudged elastic band calculations using a surrogate machine learning model. Phys. Rev. Lett. 2019, 122, 156001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deringer, V.L.; Bartók, A.P.; Bernstein, N.; Wilkins, D.M.; Ceriotti, M.; Csányi, G. Gaussian process regression for materials and molecules. Chem. Rev. 2021, 121, 10073–10141. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, D.; Terrell, R.; Henkelman, G. Optimization methods for finding minimum energy paths. J. Chem. Phys. 2008, 128, 134106. [Google Scholar] [CrossRef] [Green Version]
- Wert, C.; Zener, C. Interstitia atomic diffusion coefficients. Phys. Rev. 1949, 76, 1169–1175. [Google Scholar] [CrossRef]
- Wimmer, E.; Wolf, W.; Sticht, J.; Saxe, P.; Geller, C.B.; Najafabadi, R.; Young, G.A. Temperature-dependent diffusion coefficients from ab initio computations: Hydrogen, deuterium, and tritium in nickel. Phys. Rev. B Condens. Matter Mater. Phys. 2008, 77, 134305. [Google Scholar] [CrossRef]
- Lasave, J.; Dominguez, F.; Koval, S.; Stachiotti, M.G.; Migoni, R.L. Shell-model description of lattice dynamical properties of MgH2. J. Phys. Condens. Matter 2005, 17, 7133–7141. [Google Scholar] [CrossRef]
- Kehr, K.W. Theory of the diffusion of hydrogen in metals. In Hydrogen in Metals I.; Alefeld, G., Völkl, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1978; pp. 197–226. [Google Scholar]
- Zener, C. Theory of D0 for atomic diffusion in metals. J. Appl. Phys. 1951, 22, 372–375. [Google Scholar] [CrossRef]
- Klyukin, K.; Shelyapina, M.G.; Fruchart, D. DFT calculations of hydrogen diffusion and phase transformations in magnesium. J. Alloys Compd. 2015, 644, 371–377. [Google Scholar] [CrossRef]
- Kulabukhova, N.A.; Poletaev, G.M.; Starostenkov, M.D.; Kulagina, V.V.; Potekaev, A.I. A molecular dynamics study of hydrogen-atom diffusion in fcc-metals. Russ. Phys. J. 2011, 12, 1394–1400. [Google Scholar] [CrossRef]
- Kirchheim, R. Solubility, diffusivity and trapping of hydrogen in dilute alloys. Deformed and amorphous metals-II. Acta Metall. 1982, 30, 1069–1078. [Google Scholar] [CrossRef]
- Brouwer, R.C.; Griessen, R. Heat of solution and site energies of hydrogen in disordered transition-metal alloys. Phys. Rev. B 1989, 40, 1481–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bavrina, O.O.; Shelyapina, M.G.; Klyukin, K.A.; Fruchart, D. First-principle modelling of hydrogen site solubility and diffusion in disordered Ti-V-Cr alloys. Int. J. Hydrogen Energy 2018, 43, 17338–17345. [Google Scholar] [CrossRef]
- Bavrina, O.O.; Shelyapina, M.G.; Fruchart, D.; Novaković, N. DFT calculations to study hydrogen localization and diffusion in disordered bcc Ti-V-Cr alloys. Solid State Phenom. 2019, 289, 205–211. [Google Scholar] [CrossRef]
- Kunc, K. Recent results in semiconductor dynamics by ab initio ‘direct’ approach. In Electronic Structure, Dynamics, and Quantum Structural Properties of Condensed Matter; Devreese, J.T., van Camp, P., Eds.; Plenum: New York, NY, USA, 1985; pp. 227–312. [Google Scholar]
- Wei, S.; Chou, M.Y. Ab initio calculation of force constants and full phonon dispersions. Phys. Rev. Lett. 1992, 69, 2799–2802. [Google Scholar] [CrossRef] [PubMed]
- Frank, W.; Elsässer, C.; Fähnle, M. Ab initio force-constant method for phonon dispersions in alkali metals. Phys. Rev. Lett. 1995, 74, 1791–1794. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J.; Hafner, J. Ab initio force constant approach to phonon dispersion relations of diamond and graphite. Europhys. Lett. 1995, 32, 729–734. [Google Scholar] [CrossRef]
- Parlinski, K.; Li, Z.; Kawazoe, Y. First-principles determination of the soft mode in cubic ZrO2. Phys. Rev. Lett. 1997, 78, 4063–4066. [Google Scholar] [CrossRef]
- Baroni, S.; Giannozzi, P.; Testa, A. Green’s-function approach to linear response in solids. Phys. Rev. Lett. 1987, 58, 1861–1864. [Google Scholar] [CrossRef]
- Giannozzi, P.; De Gironcoli, S.; Pavone, P.; Baroni, S. Ab initio calculation of phonon dispersions in semiconductors. Phys. Rev. B 1991, 43, 7231–7242. [Google Scholar] [CrossRef]
- Savrasov, S.Y. Linear-response calculations of lattice dynamics using muffin-tin basis sets. Phys. Rev. Lett. 1992, 69, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Savrasov, D.Y.; Savrasov, S.Y. Electron-phonon interactions and related physical properties of metals from linear-response theory. Phys. Rev. B 1996, 54, 16487–16501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail; Plummer, E.W.; Lazzeri, M.; de Gironcoli, S. Surface oscillatory thermal expansion: Mg(1010). Phys. Rev. B 2001, 63, 233401. [Google Scholar] [CrossRef]
- Sun, T.; Umemoto, K.; Wu, Z.; Zheng, J.C.; Wentzcovitch, R.M. Lattice dynamics and thermal equation of state of platinum. Phys. Rev. B 2008, 78, 024304. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, B.; Ismer, L.; Hickel, T.; Neugebauer, J. Ab initio up to the melting point: Anharmonicity and vacancies in aluminum. Phys. Rev. B 2009, 79, 134106. [Google Scholar] [CrossRef] [Green Version]
- Ohba, N.; Miwa, K.; Noritake, T.; Fukumoto, A. First-principles study on thermal vibration effects of MgH2. Phys. Rev. B Condens. Matter Mater. Phys. 2004, 70, 035102. [Google Scholar] [CrossRef]
- Subedi, A.; Singh, D.J. Bonding in Zintl phase hydrides: Density functional calculations for SrAlSiH, SrAl2H2, SrGa2H2, and BaGa2H2. Phys. Rev. B 2008, 78, 045106. [Google Scholar] [CrossRef] [Green Version]
- Vajeeston, P.; Ravindran, P.; Fjellvåg, H. Phonon, IR, and raman spectra, NMR parameters, and elastic constant calculations for AlH3 polymorphs. J. Phys. Chem. A 2011, 115, 10708–10719. [Google Scholar] [CrossRef]
- Yu, R.; Lam, P. Electronic and structural properties of MgH2. Phys. Rev. B 1988, 37, 8730–8737. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Fruchart, D.; Wolfers, P. Electronic structure and stability of new FCC magnesium hydrides Mg7MH16 and Mg6MH16 (M = Ti, V, Nb): An ab initio study. Int. J. Hydrogen Energy 2010, 35, 2025–2032. [Google Scholar] [CrossRef]
- Siretskiy, M.Y.; Shelyapina, M.G.; Fruchart, D.; Miraglia, S.; Skryabina, N.E. Influence of a transition metal atom on the geometry and electronic structure of Mg and Mg-H clusters. J. Alloys Compd. 2009, 480, 114–116. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Fruchart, D. Role of transition elements in stability of magnesium hydride: A review of theoretical studies. Solid State Phenom. 2011, 170, 227–231. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Siretskiy, M.Y. Influence of 3d metal atoms on the geometry, electronic structure, and stability of a Mg13H26 cluster. Phys. Solid State 2010, 52, 1992–1998. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Fruchart, D.; Miraglia, S.; Girard, G. Electronic structure and stability of Mg6TiM (M = Mg, Al, Zn) and their hydrides. Phys. Solid State 2011, 53, 6–12. [Google Scholar] [CrossRef]
- Larsson, P.; Araújo, C.M.; Larsson, J.A.; Jena, P.; Ahuja, R. Role of catalysts in dehydrogenation of MgH2 nanoclusters. Proc. Natl. Acad. Sci. USA 2008, 105, 8227–8231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyoi, D.; Sato, T.; Rönnebro, E.; Tsuji, Y.; Kitamura, N.; Ueda, A.; Ito, M.; Katsuyama, S.; Hara, S.; Noréus, D.; et al. A novel magnesium-vanadium hydride synthesized by a gigapascal-high- pressure technique. J. Alloys Compd. 2004, 375, 253–258. [Google Scholar] [CrossRef]
- Kyoi, D.; Sato, T.; Rönnebro, E.; Kitamura, N.; Ueda, A.; Ito, M.; Katsuyama, S.; Hara, S.; Noréus, D.; Sakai, T. A new ternary magnesium-titanium hydride Mg7TiHx with hydrogen desorption properties better than both binary magnesium and titanium hydrides. J. Alloys Compd. 2004, 372, 213–217. [Google Scholar] [CrossRef]
- Kyoi, D.; Kitamura, N.; Tanaka, H.; Ueda, A.; Tanase, S.; Sakai, T. Hydrogen desorption properties of FCC super-lattice hydride Mg7NbHx prepared by ultra-high pressure techniques. J. Alloys Compd. 2007, 428, 268–273. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Pinyugzhanin, V.M.; Skryabina, N.E.; Hauback, B.C. Electronic structure and stability of complex hydrides Mg2MHx (M = Fe, Co). Phys. Solid State 2013, 55, 12–20. [Google Scholar] [CrossRef]
- Vermeulen, P.; Graat, P.C.J.; Wondergem, H.J.; Notten, P.H.L. Crystal structures of MgyTi100-y thin film alloys in the as-deposited and hydrogenated state. Int. J. Hydrogen Energy 2008, 33, 5646–5650. [Google Scholar] [CrossRef]
- Mengucci, P.; Barucca, G.; Majni, G.; Bazzanella, N.; Checchetto, R.; Miotello, A. Structure modification of Mg-Nb films under hydrogen sorption cycles. J. Alloys Compd. 2011, 509, S572–S575. [Google Scholar] [CrossRef]
- Klyukin, K.; Shelyapina, M.G.; Fruchart, D. Modelling of Mg/Ti and Mg/Nb thin films for hydrogen storage. Solid State Phenom. 2011, 170, 298–301. [Google Scholar] [CrossRef]
- Junkaew, A.; Ham, B.; Zhang, X.; Talapatra, A.; Arróyave, R. Stabilization of bcc Mg in thin films at ambient pressure: Experimental evidence and ab initio calculations. Mater. Res. Lett. 2013, 1, 161–167. [Google Scholar] [CrossRef]
- Kumar, A.; Beyerlein, I.J.; Wang, J. First-principles study of the structure of Mg/Nb multilayers. Appl. Phys. Lett. 2014, 105, 071602. [Google Scholar] [CrossRef]
- Klyukin, K.; Shelyapina, M.G.; Fruchart, D. Hydrogen induced phase transition in magnesium: An ab initio study. J. Alloys Compd. 2013, 580, S10–S12. [Google Scholar] [CrossRef]
- Burgers, W.G. On the process of transition of the cubic-body-centered modification into the hexagonal-close-packed modification of zirconium. Physica 1934, 1, 561–586. [Google Scholar] [CrossRef]
- Vegge, T. Locating the rate-limiting step for the interaction of hydrogen with Mg (0001) using Density-Functional Theory calculations and rate theory. Phys. Rev. B Condens. Matter Mater. Phys. 2004, 70, 035412. [Google Scholar] [CrossRef] [Green Version]
- Du, A.J.; Smith, S.C.; Yao, X.D.; He, X.D.; Lu, G.Q. Atomic hydrogen diffusion in novel magnesium nanostructures: The impact of incorporated subsurface carbon atoms. J. Phys. Conf. Ser. 2006, 29, 167–172. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, J.; Wu, Y.; Li, Q.; Chou, K.; Bao, X. First-principle calculations of the adsorption, dissociation and diffusion of hydrogen on the Mg (0001) surface. Acta Phys. Chim. Sin. 2008, 24, 55–60. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, X.; Wu, M.; Sun, Q.; Jia, Y. First-principles study of hydrogen dissociation and diffusion on transition metal-doped Mg (0001) surfaces. Appl. Surf. Sci. 2014, 305, 40–45. [Google Scholar] [CrossRef]
- Han, Z.; Chen, H.; Zhou, S. Dissociation and diffusion of hydrogen on defect-free and vacancy defective Mg (0001) surfaces: A density functional theory study. Appl. Surf. Sci. 2017, 394, 371–377. [Google Scholar] [CrossRef]
- Jiang, T.; Sun, L.X.; Li, W.X. First-principles study of hydrogen absorption on Mg(0001) and formation of magnesium hydride. Phys. Rev. B Condens. Matter Mater. Phys. 2010, 81, 035416. [Google Scholar] [CrossRef] [Green Version]
- Du, A.J.; Smith, S.C.; Yao, X.D.; Lu, G.Q. Hydrogen spillover mechanism on a Pd-doped Mg surface as revealed by ab initio density functional calculation. J. Am. Chem. Soc. 2007, 129, 10201–10204. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, C.G.S.; Majumder, C. Dissociation and diffusion of hydrogen on the Mg(0001) surface: Catalytic effect of V and Ni double substitution. J. Phys. Chem. C 2009, 113, 10574–10579. [Google Scholar] [CrossRef]
- Han, Z.; Wu, Y.; Yu, H.; Zhou, S. Location-dependent effect of nickel on hydrogen dissociation and diffusion on Mg (0001) surface: Insights into hydrogen storage material design. J. Magnes. Alloys, 2021; in press. [Google Scholar] [CrossRef]
- Pozzo, M.; Alfè, D. Hydrogen dissociation and diffusion on transition metal (=Ti, Zr, V, Fe, Ru, Co, Rh, Ni, Pd, Cu, Ag)-doped Mg (0001) surfaces. Int. J. Hydrogen Energy 2009, 34, 1922–1930. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.J.; Paul Kung, W.-C. Confinement of Mg-MgH2 systems into carbon nanotubes changes hydrogen sorption energetics. J. Phys. Chem. B 2005, 109, 17837–17841. [Google Scholar] [CrossRef] [PubMed]
- Du, A.J.; Smith, S.C.; Yao, X.D.; Lu, G.Q. Ab initio studies of hydrogen desorption from low index magnesium hydride surface. Surf. Sci. 2006, 600, 1854–1859. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, B.W.J.; Peng, G.; Mavrikakis, M. Density functional theory study of thermodynamic and kinetic isotope effects of H2/D2 dissociative adsorption on transition metals. Catal. Sci. Technol. 2018, 8, 3321–3335. [Google Scholar] [CrossRef]
- Imamura, H.; Tabata, S.; Shigetomi, N.; Takesue, Y.; Sakata, Y. Composites for hydrogen storage by mechanical grinding of graphite carbon and magnesium. J. Alloys Compd. 2002, 330-332, 579–583. [Google Scholar] [CrossRef]
- Shang, C.X.; Guo, Z.X. Effect of carbon on hydrogen desorption and absorption of mechanically milled MgH2. J. Power Source 2004, 129, 73–80. [Google Scholar] [CrossRef]
- Du, A.J.; Smith, S.C.; Yao, X.D.; Lu, G.Q. Catalytic effects of subsurface carbon in the chemisorption of hydrogen on a Mg(0001) surface: an ab-initio study. J. Phys. Chem. B 2006, 110, 1814–1819. [Google Scholar] [CrossRef] [PubMed]
- San-Martin, A.; Manchester, F.D. The H-Mg (Hydrogen-Magnesium) system. J. Phase Equilibria 1987, 8, 431–437. [Google Scholar] [CrossRef]
- Schimmel, H.G.; Kearley, G.J.; Huot, J.; Mulder, F.M. Hydrogen diffusion in magnesium metal (α phase) studied by ab initio computer simulations. J. Alloys Compd. 2005, 404-406, 235–237. [Google Scholar] [CrossRef]
- Olijnyk, H.; Holzapfel, W.B. High-pressure structural phase transition in Mg. Phys. Rev. B 1985, 31, 4682–4683. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Notten, P.; van Santen, R.; Jansen, A. Density functional theory studies of the hydrogenation properties of Mg and Ti. Phys. Rev. B 2009, 79, 144121. [Google Scholar] [CrossRef] [Green Version]
- El-Eskandarany, M.S.; Banyan, M.; Al-Ajmi, F. Discovering a new MgH2 metastable phase. RSC Adv. 2018, 8, 32003–32008. [Google Scholar] [CrossRef] [Green Version]
- Fruchart, D.; Rango, P.; de Charbonnier, J.; Skryabina, N.; Jehan, M. Nanocrystalline Composite for Storage of Hydrogen. U.S. Patent Patent No 2009/0278086 A1, 12 November 2009. [Google Scholar]
- Ham, B.; Junkaew, A.; Arroyave, R.; Chen, J.; Wang, H.; Wang, P.; Majewski, J.; Park, J.; Zhou, H.C.; Arvapally, R.K.; et al. Hydrogen sorption in orthorhombic Mg hydride at ultra-low temperature. Int. J. Hydrogen Energy 2013, 38, 8328–8341. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Hydrogen storage properties of the mechanically milled MgH2-V nanocomposite. J. Alloys Compd. 1999, 291, 295–299. [Google Scholar] [CrossRef]
- Shang, C.X.; Bououdina, M.; Guo, Z.X. Structural stability of mechanically alloyed (Mg + 10Nb) and (MgH2 + 10Nb) powder mixtures. J. Alloys Compd. 2003, 349, 217–223. [Google Scholar] [CrossRef]
- Halilov, S.V.; Singh, D.J.; Gupta, M.; Gupta, R. Stability and electronic structure of the complex K2PtCl6-structure hydrides DMH6 (D=Mg, Ca, Sr; M=Fe, Ru, Os). Phys. Rev. B. 2004, 70, 195117. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, C.; Komaki, M.; Amano, M. Hydrogen permeation through magnesium. J. Alloys Compd. 1999, 293, 329–333. [Google Scholar] [CrossRef]
- Kappes, M.; Iannuzzi, M.; Carranza, R.M. Hydrogen embrittlement of magnesium and magnesium alloys: A review. J. Electrochem. Soc. 2013, 160, C168–C178. [Google Scholar] [CrossRef]
- Higuchi, K.; Yamamoto, K.; Kajioka, H.; Toiyama, K.; Honda, M.; Orimo, S.; Fujii, H. Remarkable hydrogen storage properties in three-layered Pd/Mg/Pd thin films. J. Alloys Compd. 2002, 330-332, 526–530. [Google Scholar] [CrossRef]
- Kelekar, R.; Giffard, H.; Kelly, S.T.; Clemens, B.M. Formation and dissociation of MgH2 in epitaxial Mg thin films. J. Appl. Phys. 2007, 101, 114311. [Google Scholar] [CrossRef]
- Chen, W.Y.; Tang, J.J.; Lu, Z.W.; Huang, M.X.; Liu, L.; He, C.C.; Zhao, Y.J. Theoretical investigation of the surface orientation impact on the hydrogen vacancy formation of MgH2. Surf. Sci. 2021, 710, 121850. [Google Scholar] [CrossRef]
- Fernández, J.F.; Sánchez, C.R. Rate determining step in the absorption and desorption of hydrogen by magnesium. J. Alloys Compd. 2002, 340, 189–198. [Google Scholar] [CrossRef]
- Huot, J.; Liang, G.; Boily, S.; Van Neste, A.; Schulz, R. Structural study and hydrogen sorption kinetics of ball-milled magnesium hydride. J. Alloys Compd. 1999, 293, 495–500. [Google Scholar] [CrossRef]
- Du, A.J.; Smith, S.C.; Lu, G.Q. First-principle studies of the formation and diffusion of hydrogen vacancies in magnesium hydride. J. Phys. Chem. C 2007, 111, 8360–8365. [Google Scholar] [CrossRef]
- Kurko, S.; Milanović, I.; Grbović Novaković, J.; Ivanović, N.; Novaković, N. Investigation of surface and near-surface effects on hydrogen desorption kinetics of MgH2. Int. J. Hydrogen Energy 2014, 39, 862–867. [Google Scholar] [CrossRef]
- Reich, J.M.; Wang, L.L.; Johnson, D.D. H2 desorption from MgH2 surfaces with steps and catalyst-dopants. J. Phys. Chem. C 2014, 118, 6641–6649. [Google Scholar] [CrossRef] [Green Version]
- Giusepponi, S.; Celino, M. DFT model of hydrogen desorption from MgH2: The role of iron catalyst. Int. J. Hydrogen Energy 2013, 38, 15254–15263. [Google Scholar] [CrossRef]
- Ri, S.-I.; Um, K.J.; Wi, J.H.; Kim, J.C.; Kim, N.H. Effects of single-and co-substitution of Ti and Fe on vacancy formation and dehydrogenation from MgH2 (110) surface: Ab initio study. Int. J. Hydrogen Energy 2019, 44, 22761–22769. [Google Scholar] [CrossRef]
- El Khatabi, M.; Bhihi, M.; Lakhal, M.; Abdellaoui, M.; Benyoussef, A.; El Kenz, A.; Loulidi, M. Enhanced hydrogen sorption kinetics of co-doped MgH2 hydrides. Comput. Mater. Sci. 2018, 152, 192–195. [Google Scholar] [CrossRef]
- Kurko, S.; Paskaš Mamula, B.; Rmuš, J.; Grbović Novaković, J.; Novaković, N. DFT study of boron doped MgH2: Bonding mechanism, hydrogen diffusion and desorption. Int. J. Hydrogen Energy 2020, 45, 7947–7957. [Google Scholar] [CrossRef]
- Cheung, S.; Deng, W.Q.; Van Duin, A.C.T.; Goddard, W.A. ReaxFFMgH reactive force field for magnesium hydride systems. J. Phys. Chem. A 2005, 109, 851–859. [Google Scholar] [CrossRef] [Green Version]
- Tanguy, D.; Magnin, T. Atomic-scale simulation of intergranular segregation of H in Al-Mg: Implications for H-induced damage. Philos. Mag. 2003, 83, 3995–4009. [Google Scholar] [CrossRef]
- Smirnova, D.E.; Starikov, S.V.; Vlasova, A.M. New interatomic potential for simulation of pure magnesium and magnesium hydrides. Comput. Mater. Sci. 2018, 154, 295–302. [Google Scholar] [CrossRef]
- Zhou, X.; Kang, S.; Heo, T.W.; Wood, B.C.; Stavila, V.; Allendorf, M.D. An analytical bond order potential for Mg−H systems. ChemPhysChem 2019, 20, 1404–1411. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Huang, S. Molecular dynamics study on magnesium hydride nanoclusters with machine-learning interatomic potential. Phys. Rev. B 2020, 102, 094111. [Google Scholar] [CrossRef]
- Rokhmanenkov, A. Modeling of nonlinear hydrogen diffusion in titanium hydrides TiHx. Int. J.Hydrogen Energy 2017, 42, 22610–22614. [Google Scholar] [CrossRef]
- Mazitov, A.B.; Oganov, A.R.; Yanilkin, A.V. Titanium-hydrogen interaction at high pressure. J. Appl. Phys. 2018, 123, 235901. [Google Scholar] [CrossRef] [Green Version]
- Iftimie, R.; Minary, P.; Tuckerman, M.E. Ab initio molecular dynamics: Concepts, recent developments, and future trends. Proc. Natl. Acad. Sci. USA 2005, 102, 6654–6659. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shelyapina, M.G. Hydrogen Diffusion on, into and in Magnesium Probed by DFT: A Review. Hydrogen 2022, 3, 285-302. https://doi.org/10.3390/hydrogen3030017
Shelyapina MG. Hydrogen Diffusion on, into and in Magnesium Probed by DFT: A Review. Hydrogen. 2022; 3(3):285-302. https://doi.org/10.3390/hydrogen3030017
Chicago/Turabian StyleShelyapina, Marina G. 2022. "Hydrogen Diffusion on, into and in Magnesium Probed by DFT: A Review" Hydrogen 3, no. 3: 285-302. https://doi.org/10.3390/hydrogen3030017
APA StyleShelyapina, M. G. (2022). Hydrogen Diffusion on, into and in Magnesium Probed by DFT: A Review. Hydrogen, 3(3), 285-302. https://doi.org/10.3390/hydrogen3030017





