Nanoengineering of Catalysts for Enhanced Hydrogen Production
Abstract
1. Introduction
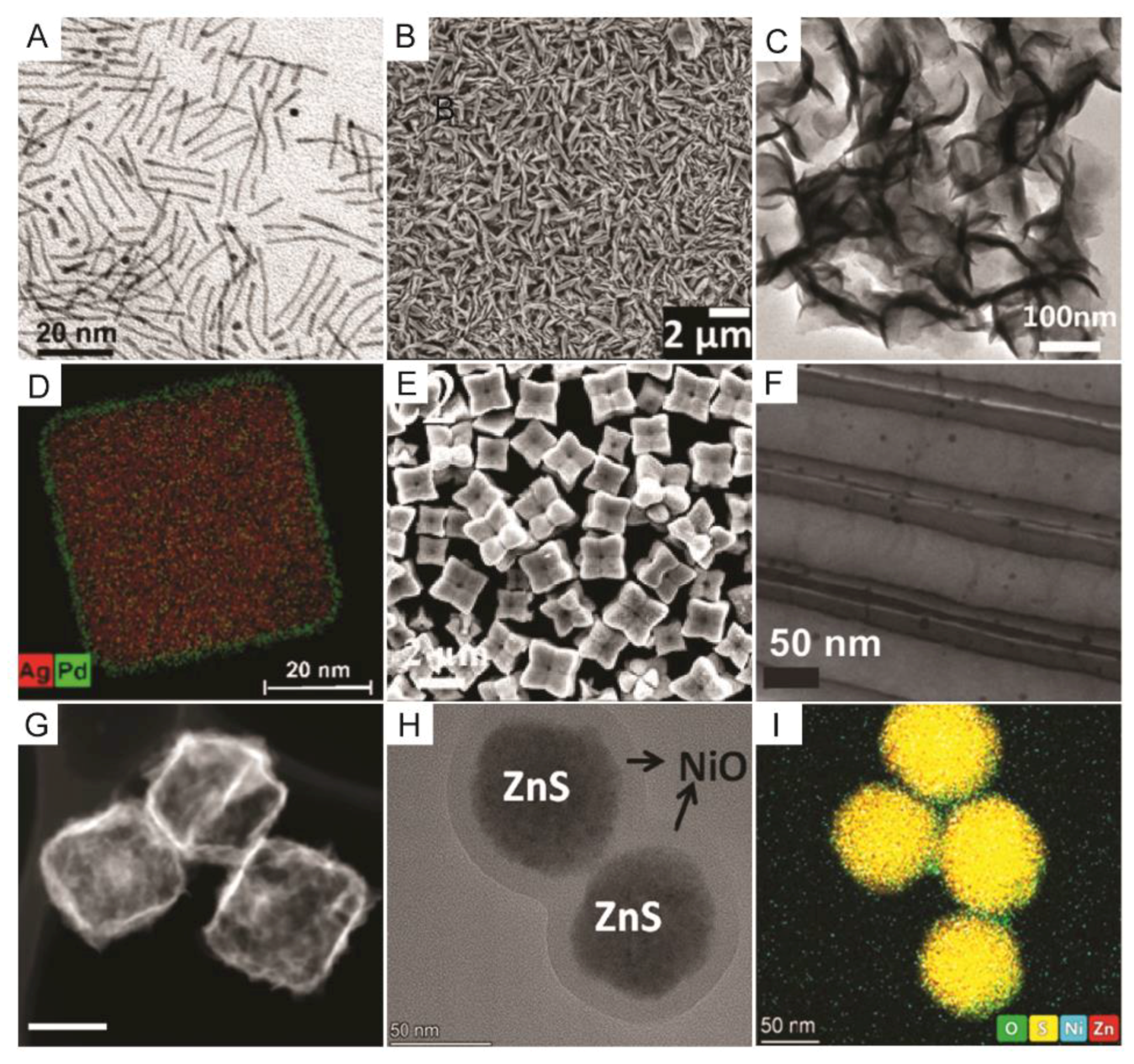
2. Nanoscale Design Concepts of Nanocatalysts Applied for H2 Production
3. Main Catalytic Processes for H2 Generation
4. Methane Reforming
5. Ethanol Steam Reforming (ESR)
6. Water–Gas Shift Reaction
7. Water Electrolysis
Selected Metal-Based HER Electrocatalysts
8. Photochemical Water-Splitting Reaction
9. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Griffiths, S.; Sovacool, B.K.; Kim, J.; Bazilian, M.; Uratani, J.M. Industrial decarbonization via hydrogen: A critical and systematic review of developments, socio-technical systems and policy options. Energy Res. Soc. Sci. 2021, 80, 102208. [Google Scholar] [CrossRef]
- Fan, L.; Tu, Z.; Chan, S.H. Recent development of hydrogen and fuel cell technologies: A review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar] [CrossRef]
- McCay, M.H.; Shafiee, S. Hydrogen: An energy carrier. Future Energy Improv. Sustain. Clean Options Our Planet 2020, 475–493. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen Production Technologies: Current State and Future Developments. Conf. Pap. Sci. 2013, 2013, 690627. [Google Scholar] [CrossRef]
- Kanz, O.; Bittkau, K.; Ding, K.; Rau, U.; Reinders, A. Review and Harmonization of the Life-Cycle Global Warming Impact of PV-Powered Hydrogen Production by Electrolysis. Front. Electron. 2021, 2, 11. [Google Scholar] [CrossRef]
- Bermudez, J.M.; Hannula, I. Hydrogen—More Efforts Are Needed; International Energy Agency (IEA): Paris, France, 2021. [Google Scholar]
- Dincer, I.; Ishaq, H. Chapter 1—Introduction. In Renewable Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–33. ISBN 9780323851763. [Google Scholar]
- Singh, V.; Das, D. Potential of Hydrogen Production from Biomass; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128142516. [Google Scholar]
- Kadier, A.; Kalil, M.S.; Abdeshahian, P.; Chandrasekhar, K.; Mohamed, A.; Azman, N.F.; Logroño, W.; Simayi, Y.; Hamid, A.A. Recent advances and emerging challenges in microbial electrolysis cells (MECs) for microbial production of hydrogen and value-added chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Koku, H.; Eroǧlu, I.; Gündüz, U.; Yücel, M.; Türker, L. Kinetics of biological hydrogen production by the photosynthetic bacterium Rhodobacter sphaeroides O.U. 001. Int. J. Hydrog. Energy 2003, 28, 381–388. [Google Scholar] [CrossRef]
- Eljack, F.; Kazi, M.-K. Prospects and Challenges of Green Hydrogen Economy via Multi-Sector Global Symbiosis in Qatar. Front. Sustain. 2021, 1, 612762. [Google Scholar] [CrossRef]
- Borgschulte, A. The hydrogen grand challenge. Front. Energy Res. 2016, 4, 11. [Google Scholar] [CrossRef]
- Martino, M.; Ruocco, C.; Meloni, E.; Pullumbi, P.; Palma, V. Main hydrogen production processes: An overview. Catalysts 2021, 11, 547. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Kumar, A.; Khraisheh, M. A review of recent advances in water-gas shift catalysis for hydrogen production. Emergent Mater. 2020, 3, 881–917. [Google Scholar] [CrossRef]
- Philippot, K.; Serp, P. Concepts in Nanocatalysis. In Nanomateriais in Catalysis; Serp, P., Philippot, K., Eds.; WILEY-VCH: Weinheim, Germany, 2013; pp. 1–54. [Google Scholar]
- Garcia, M.A.S.; Heyder, R.S.; Oliveira, K.C.B.; Costa, J.C.S.; Corio, P.; Gusevskaya, E.V.; dos Santos, E.N.; Bazito, R.C.; Rossi, L.M. Support Functionalization with a Phosphine-Containing Hyperbranched Polymer: A Strategy to Enhance Phosphine Grafting and Metal Loading in a Hydroformylation Catalyst. ChemCatChem 2016, 8, 1951–1960. [Google Scholar] [CrossRef]
- Garcia, M.A.S.; Ibrahim, M.; Costa, J.C.S.; Corio, P.; Gusevskaya, E.V.; dos Santos, E.N.; Philippot, K.; Rossi, L.M. Study of the influence of PPh3 used as capping ligand or as reaction modifier for hydroformylation reaction involving Rh NPs as precatalyst. Appl. Catal. A Gen. 2017, 548, 136–142. [Google Scholar] [CrossRef]
- Itaciara, I.E.M.; de Sousa, S.A.A.; Laíse, L.N.; Oliveira, J.M.; Castro, K.P.R.; Costa, J.C.S.; de Moura, E.M.; de Moura, C.V.R.; Garcia, M.A.S. Au−Pd Selectivity-switchable Alcohol-oxidation Catalyst: Controlling the Duality of the Mechanism using a Multivariate Approach. ChemCatChem 2019, 11, 3022–3034. [Google Scholar] [CrossRef]
- Franca, M.C.; Ferreira, R.M.; dos Santos Pereira, F.; e Silva, F.A.; Silva, A.C.A.; Cunha, L.C.S.; de Jesus Gomes Varela Júnior, J.; de Lima Neto, P.; Takana, A.A.; Rodrigues, T.S.; et al. Galvanic replacement managing direct methanol fuel cells: AgPt nanotubes as a strategy for methanol crossover effect tolerance. J. Mater. Sci. 2022, 57, 8225–8240. [Google Scholar] [CrossRef]
- Cui, X.; Li, W.; Ryabchuk, P.; Junge, K.; Beller, M. Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts. Nat. Catal. 2018, 1, 385–397. [Google Scholar] [CrossRef]
- Camargo, P.H.C.; Rodrigues, T.S.; da Silva, A.G.M.; Wang, J. Controlled Synthesis: Nucleation and Growth in Solution. In Metallic Nanostructures; Springer: Berlin/Heidelberg, Germany, 2015; pp. 49–74. [Google Scholar]
- Costa, J.C.S.; Franco, N.; Soares, T.A.S.; Saraiva, N.A.M.; Garcia, M.A.S.; Gonzalez, J.R.; Machado, G. TiO2 nanotubes decorated with au nanoparticles for photocatalytic hydrogen generation under UV-visible and visible light irradiations. An. Acad. Bras. Cienc. 2020, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Huang, B.; Shao, Q.; Huang, X. Universal Strategy for Ultrathin Pt–M (M = Fe, Co, Ni) Nanowires for Efficient Catalytic Hydrogen Generation. ACS Appl. Mater. Interfaces 2018, 10, 22257–22263. [Google Scholar] [CrossRef]
- Shinde, D.V.; Kokumai, T.M.; Buha, J.; Prato, M.; De Trizio, L.; Manna, L. A robust and highly active hydrogen evolution catalyst based on Ru nanocrystals supported on vertically oriented Cu nanoplates. J. Mater. Chem. A 2020, 8, 10787–10795. [Google Scholar] [CrossRef]
- Xu, P.; Lu, W.; Zhang, J.; Zhang, L. Efficient Hydrolysis of Ammonia Borane for Hydrogen Evolution Catalyzed by Plasmonic Ag@Pd Core–Shell Nanocubes. ACS Sustain. Chem. Eng. 2020, 8, 12366–12377. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, B.; Ge, X.; Liu, Z.; Wang, J.-Y.; Wang, X. Ultrathin MoS2 Nanoplates with Rich Active Sites as Highly Efficient Catalyst for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2013, 5, 12794–12798. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Hahn, R.; Altomare, M.; Selli, E.; Schmuki, P. Intrinsic Au Decoration of Growing TiO2 Nanotubes and Formation of a High-Efficiency Photocatalyst for H2 Production. Adv. Mater. 2013, 25, 6133–6137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Jiu, B.-B.; Gong, F.-L.; Chen, J.-L.; Zhang, H.-L. Morphology-controllable Cu2O supercrystals: Facile synthesis, facet etching mechanism and comparative photocatalytic H2 production. J. Alloys Compd. 2017, 729, 563–570. [Google Scholar] [CrossRef]
- Li, M.; Zhang, S.; Zhao, J.; Wang, H. Maximizing Metal–Support Interactions in Pt/Co3O4 Nanocages to Simultaneously Boost Hydrogen Production Activity and Durability. ACS Appl. Mater. Interfaces 2021, 13, 57362–57371. [Google Scholar] [CrossRef]
- Navakoteswara Rao, V.; Ravi, P.; Sathish, M.; Lakshmana Reddy, N.; Lee, K.; Sakar, M.; Prathap, P.; Mamatha Kumari, M.; Raghava Reddy, K.; Nadagouda, M.N.; et al. Monodispersed core/shell nanospheres of ZnS/NiO with enhanced H2 generation and quantum efficiency at versatile photocatalytic conditions. J. Hazard. Mater. 2021, 413, 125359. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Sun, W.; Cheng, P. Spectroscopic and theoretical studies of quantum and electronic confinement effects in nanostructured materials. Molecules 2003, 8, 207–222. [Google Scholar] [CrossRef]
- Xie, C.; Niu, Z.; Kim, D.; Li, M.; Yang, P. Surface and Interface Control in Nanoparticle Catalysis. Chem. Rev. 2020, 120, 1184–1249. [Google Scholar] [CrossRef]
- Cao, S.; Tao, F.F.; Tang, Y.; Li, Y.; Yu, J. Size- and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts. Chem. Soc. Rev. 2016, 45, 4747–4765. [Google Scholar] [CrossRef]
- Rossi, L.M.; Fiorio, J.L.; Garcia, M.A.S.; Ferraz, C.P. The role and fate of capping ligands in colloidally prepared metal nanoparticle catalysts. Dalt. Trans. 2018, 47, 5889–5915. [Google Scholar] [CrossRef]
- Ibrahim, M.; Garcia, M.A.S.; Vono, L.L.R.; Guerrero, M.; Lecante, P.; Rossi, L.M.; Philippot, K. Polymer: Versus phosphine stabilized Rh nanoparticles as components of supported catalysts: Implication in the hydrogenation of cyclohexene model molecule. Dalt. Trans. 2016, 45, 17782–17791. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C. Supported Gold Nanoparticles as Catalysts for the Oxidation of Alcohols and Alkanes. Front. Chem. 2019, 7, 702. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.N.S.; Ribeiro, C.E.S.; Tofanello, A.; Costa, J.C.S.; De Moura, C.V.R.; Garcia, M.A.S.; De Moura, E.M. Gold Supported on Strontium Surface-Enriched CoFe2O4 Nanoparticles: A Strategy for the Selective Oxidation of Benzyl Alcohol. J. Brazilian Chem. Soc. 2019, 30, 1317–1325. [Google Scholar] [CrossRef]
- Ilieva, L.; Petrova, P.; Pantaleo, G.; Zanella, R.; Sobczak, J.W.; Lisowski, W.; Kaszkur, Z.; Munteanu, G.; Yordanova, I.; Liotta, L.F.; et al. Alumina supported Au/Y-doped ceria catalysts for pure hydrogen production via PROX. Int. J. Hydrogen Energy 2019, 44, 233–245. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Zhang, L.; Herrmann, L.O.; Baumberg, J.J. Size Dependent Plasmonic Effect on BiVO4 Photoanodes for Solar Water Splitting. Sci. Rep. 2015, 5, 16660. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef]
- Gao, G.; Niu, X.; Xu, B.; Wang, X.L.; Yao, Y.F. Shape and size effects on photocatalytic hydrogen production: Via Pd/C3N4photocatalysts under visible light. Catal. Sci. Technol. 2020, 10, 5438–5442. [Google Scholar] [CrossRef]
- Vitos, L.; Ruban, A.V.; Skriver, H.L.; Kolla, J. The surface energy of metals. Surf. Sci. 1998, 411, 186–202. [Google Scholar] [CrossRef]
- Geonmonond, R.S.; Da Silva, A.G.M.; Camargo, P.H.C. Controlled synthesis of noble metal nanomaterials: Motivation, principles, and opportunities in nanocatalysis. An. Da Acad. Bras. De Ciências 2018, 90, 719–744. [Google Scholar] [CrossRef]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, W.; Luo, N.; Xue, Z.; Hu, Q.; Zeng, W.; Xu, J. Bimetallic nanocrystals: Structure, controllable synthesis and applications in catalysis, energy and sensing. Nanomaterials 2021, 11, 1926. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.C.; Qin, D.; Xia, Y. Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Muthurasu, A.; Chhetri, K.; Dahal, B. Ruthenium Nanoparticles-Integrated Bimetallic Metal-Organic Frameworks Electrocatalysts for Multifunctional Electrode Materials and Practical Water Electrolysis in Seawater. Nanoscale 2022, 14, 6557–6569. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.; Wang, B.; Yang, T.; Yang, S. Electrochemical formation of PtRu bimetallic nanoparticles for highly efficient and pH-universal hydrogen evolution reaction. J. Mater. Chem. A 2020, 8, 2090–2098. [Google Scholar] [CrossRef]
- Lu, X.F.; Zhang, S.L.; Sim, W.L.; Gao, S.; Lou, X.W. Phosphorized CoNi2S4 Yolk-Shell Spheres for Highly Efficient Hydrogen Production via Water and Urea Electrolysis. Angew. Chem. Int. Ed. 2021, 60, 22885–22891. [Google Scholar] [CrossRef]
- Cortés, E.; Besteiro, L.V.; Alabastri, A.; Baldi, A.; Tagliabue, G.; Demetriadou, A.; Narang, P. Challenges in Plasmonic Catalysis. ACS Nano 2020, 14, 16202–16219. [Google Scholar] [CrossRef]
- Araujo, T.P.; Quiroz, J.; Barbosa, E.C.M.; Camargo, P.H.C. Understanding plasmonic catalysis with controlled nanomaterials based on catalytic and plasmonic metals. Curr. Opin. Colloid Interface Sci. 2019, 39, 110–122. [Google Scholar] [CrossRef]
- Ezendam, S.; Herran, M.; Nan, L.; Gruber, C.; Kang, Y.; Gröbmeyer, F.; Lin, R.; Gargiulo, J.; Sousa-Castillo, A.; Cortés, E. Hybrid Plasmonic Nanomaterials for Hydrogen Generation and Carbon Dioxide Reduction. ACS Energy Lett. 2022, 7, 778–815. [Google Scholar] [CrossRef]
- da Silva, A.G.M.; Rodrigues, T.S.; Wang, J.; Camargo, P.H.C. Plasmonic catalysis with designer nanoparticles. Chem. Commun. 2022, 58, 2055–2074. [Google Scholar] [CrossRef]
- Christopher, P.; Xin, H.; Marimuthu, A.; Linic, S. Singular characteristics and unique chemical bond activation mechanisms of photocatalytic reactions on plasmonic nanostructures. Nat. Mater. 2012, 11, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Barbosa, E.C.M.; Wu, S.; Li, Y.; Sun, Y.; Xiang, W.; Li, T.; Pu, S.; Robertson, A.; Wu, T.-S.; et al. Atomic-Precision Tailoring of Au–Ag Core–Shell Composite Nanoparticles for Direct Electrochemical-Plasmonic Hydrogen Evolution in Water Splitting. Adv. Funct. Mater. 2021, 31, 2102517. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen Production, Storage, Utilisation and Environmental Impacts: A Review; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; ISBN 0123456789. [Google Scholar]
- El-Shafie, M.; Kambara, S.; Hayakawa, Y. Hydrogen Production Technologies Overview. J. Power Energy Eng. 2019, 7, 107–154. [Google Scholar] [CrossRef]
- Basile, A.; Liguori, S.; Iulianelli, A. Membrane Reactors for Methane Steam Reforming (MSR); Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9781782422273. [Google Scholar]
- Mortensen, P.M.; Dybkjær, I. Industrial scale experience on steam reforming of CO2-rich gas. Appl. Catal. A Gen. 2015, 495, 141–151. [Google Scholar] [CrossRef]
- Han, B.; Wang, F.; Zhang, L.; Wang, Y.; Fan, W.; Xu, L.; Yu, H.; Li, Z. Syngas production from methane steam reforming and dry reforming reactions over sintering-resistant Ni@SiO2 catalyst. Res. Chem. Intermed. 2020, 46, 1735–1748. [Google Scholar] [CrossRef]
- Sasson Bitters, J.; He, T.; Nestler, E.; Senanayake, S.D.; Chen, J.G.; Zhang, C. Utilizing bimetallic catalysts to mitigate coke formation in dry reforming of methane. J. Energy Chem. 2022, 68, 124–142. [Google Scholar] [CrossRef]
- Nambo, A.; Atla, V.; Vasireddy, S.; Kumar, V.; Jasinski, J.B.; Upadhyayula, S.; Sunkara, M. Nanowire-based materials as coke-resistant catalyst supports for dry methane reforming. Catalysts 2021, 11, 175. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Vinum, M.G.; Almind, M.R.; Engbæk, J.S.; Vendelbo, S.B.; Hansen, M.F.; Frandsen, C.; Bendix, J.; Mortensen, P.M. Dual-Function Cobalt–Nickel Nanoparticles Tailored for High-Temperature Induction-Heated Steam Methane Reforming. Angew. Chemie Int. Ed. 2018, 57, 10569–10573. [Google Scholar] [CrossRef]
- Jang, W.J.; Shim, J.O.; Kim, H.M.; Yoo, S.Y.; Roh, H.S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Usman, M.; Wan Daud, W.M.A.; Abbas, H.F. Dry reforming of methane: Influence of process parameters—A review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef]
- Guczi, L.; Stefler, G.; Geszti, O.; Sajó, I.; Pászti, Z.; Tompos, A.; Schay, Z. Methane dry reforming with CO2: A study on surface carbon species. Appl. Catal. A Gen. 2010, 375, 236–246. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, L.; Zhang, X.; Wang, Y.; Pan, H.; Su, H. Modification effect of natural mixed rare earths on Co/γ-Al2O3 catalysts for CH4/CO2 reforming to synthesis gas. Int. J. Hydrog. Energy 2012, 37, 9994–10001. [Google Scholar] [CrossRef]
- Zhou, L.; Martirez, J.M.P.; Finzel, J.; Zhang, C.; Swearer, D.F.; Tian, S.; Robatjazi, H.; Lou, M.; Dong, L.; Henderson, L.; et al. Light-driven methane dry reforming with single atomic site antenna-reactor plasmonic photocatalysts. Nat. Energy 2020, 5, 61–70. [Google Scholar] [CrossRef]
- Niu, J.; Wang, Y.; Qi, Y.; Dam, A.H.; Wang, H.; Zhu, Y.A.; Holmen, A.; Ran, J.; Chen, D. New mechanism insights into methane steam reforming on Pt/Ni from DFT and experimental kinetic study. Fuel 2020, 266, 117143. [Google Scholar] [CrossRef]
- Azancot, L.; Bobadilla, L.F.; Santos, J.L.; Córdoba, J.M.; Centeno, M.A.; Odriozola, J.A. Influence of the preparation method in the metal-support interaction and reducibility of Ni-Mg-Al based catalysts for methane steam reforming. Int. J. Hydrog. Energy 2019, 44, 19827–19840. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Safavinia, B.; Wang, Y.; Jiang, C.; Roman, C.; Darapaneni, P.; Larriviere, J.; Cullen, D.A.; Dooley, K.M.; Dorman, J.A. Enhancing CexZr1−xO2 Activity for Methane Dry Reforming Using Subsurface Ni Dopants. ACS Catal. 2020, 10, 4070–4079. [Google Scholar] [CrossRef]
- Damaskinos, C.M.; Vasiliades, M.A.; Stathopoulos, V.N.; Efstathiou, A.M. The Effect of CeO2 Preparation Method on the Carbon Pathways in the Dry Reforming of Methane on Ni/CeO2 Studied by Transient Techniques. Caatalysts 2019, 9, 621. [Google Scholar] [CrossRef]
- Ni, Z.; Djitcheu, X.; Gao, X.; Wang, J.; Liu, H.; Zhang, Q. Effect of preparation methods of CeO2 on the properties and performance of Ni/CeO2 in CO2 reforming of CH4. Sci. Rep. 2022, 12, 5344. [Google Scholar] [CrossRef]
- Wang, N.; Qian, W.; Chu, W.; Wei, F. Crystal-plane effect of nanoscale CeO2 on the catalytic performance of Ni/CeO2 catalysts for methane dry reforming. Catal. Sci. Technol. 2016, 6, 3594–3605. [Google Scholar] [CrossRef]
- Tu, P.H.; Le, D.N.; Dao, T.D.; Tran, Q.T.; Doan, T.C.D.; Shiratori, Y.; Dang, C.M. Paper-structured catalyst containing CeO2–Ni flowers for dry reforming of methane. Int. J. Hydrog. Energy 2020, 45, 18363–18375. [Google Scholar] [CrossRef]
- He, L.; Ren, Y.; Fu, Y.; Yue, B.; Edman Tsang, S.C.; He, H. Morphology-dependent catalytic activity of Ru/CeO2 in dry reforming of methane. Molecules 2019, 24, 526. [Google Scholar] [CrossRef]
- Anil, S.; Indraja, S.; Singh, R.; Appari, S.; Roy, B. A review on ethanol steam reforming for hydrogen production over Ni/Al2O3 and Ni/CeO2 based catalyst powders. Int. J. Hydrog. Energy 2022, 47, 8177–8213. [Google Scholar] [CrossRef]
- Shtyka, O.; Dimitrova, Z.; Ciesielski, R.; Kedziora, A.; Mitukiewicz, G.; Leyko, J.; Maniukewicz, W.; Czylkowska, A.; Maniecki, T. Steam reforming of ethanol for hydrogen production: Influence of catalyst composition (Ni/Al2O3, Ni/Al2O3–CeO2, Ni/Al2O3–ZnO) and process conditions. React. Kinet. Mech. Catal. 2021, 132, 907–919. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, K.; Wang, L.; Wang, B.; Li, Y. Oxygen vacancy clusters promoting reducibility and activity of ceria nanorods. J. Am. Chem. Soc. 2009, 131, 3140–3141. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Y.; Hou, F.; Wu, C.; Pan, L.; Zou, J.; Wang, L.; Zhang, X.; Liu, G.; Li, G. Engineering oxygen vacancies and nickel dispersion on CeO2 by Pr doping for highly stable ethanol steam reforming. Appl. Catal. B Environ. 2019, 258, 117940. [Google Scholar] [CrossRef]
- Xiao, Z.; Wu, C.; Wang, L.; Xu, J.; Zheng, Q.; Pan, L.; Zou, J.; Zhang, X.; Li, G. Boosting hydrogen production from steam reforming of ethanol on nickel by lanthanum doped ceria. Appl. Catal. B Environ. 2021, 286, 119884. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Chen, L. Nanostructured ceria-based materials: Synthesis, properties, and applications. Energy Environ. Sci. 2012, 5, 8475–8505. [Google Scholar] [CrossRef]
- Sohn, H.; Ozkan, U.S. Cobalt-Based Catalysts for Ethanol Steam Reforming: An Overview. Energy Fuels 2016, 30, 5309–5322. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, S.; He, S.; Lu, J.; Liu, J.; Lu, H.; Song, D.; Luo, Y. Nanoarchitectonics of Ni/CeO2 Catalysts: The Effect of Pretreatment on the Low-Temperature Steam Reforming of Glycerol. Nanomaterials 2022, 12, 816. [Google Scholar] [CrossRef]
- Zanchet, D.; Santos, J.B.O.; Damyanova, S.; Gallo, J.M.R.; Bueno, J.M.C. Toward understanding metal-catalyzed ethanol reforming. ACS Catal. 2015, 5, 3841–3863. [Google Scholar] [CrossRef]
- Borges, L.R.; da Silva, A.G.M.; Braga, A.H.; Rossi, L.M.; Suller Garcia, M.A.; Vidinha, P. Towards the Effect of Pt0/Ptδ+ and Ce3+ Species at the Surface of CeO2 Crystals: Understanding the Nature of the Interactions under CO Oxidation Conditions. ChemCatChem 2021, 13, 1340–1354. [Google Scholar] [CrossRef]
- Araiza, D.G.; Gómez-Cortés, A.; Díaz, G. Effect of ceria morphology on the carbon deposition during steam reforming of ethanol over Ni/CeO2 catalysts. Catal. Today 2020, 349, 235–243. [Google Scholar] [CrossRef]
- Moraes, T.S.; Neto, R.C.R.; Ribeiro, M.C.; Mattos, L.V.; Kourtelesis, M.; Verykios, X.; Noronha, F.B. Effects of ceria morphology on catalytic performance of Ni/CeO2 catalysts for low temperature steam reforming of ethanol. Top. Catal. 2015, 58, 281–294. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, F.; Liu, Y.; Zhang, L.; Chen, Y.; Wang, H.; Tian, Y.; Zhang, C.; Liu, D. Morphology effect of ceria on the performance of CuO/CeO2 catalysts for hydrogen production by methanol steam reforming. Int. J. Hydrog. Energy 2019, 44, 7252–7261. [Google Scholar] [CrossRef]
- Silva, A.G.M.; Rodrigues, T.S.; Dias, A.; Fajardo, H.V.; Goncalves, R.F.; Godinho, M.; Robles-Dutenhefner, P.A. Ce1−xSmxO1.9-[small delta] nanoparticles obtained by microwave-assisted hydrothermal processing: An efficient application for catalytic oxidation of [small alpha]-bisabolol. Catal. Sci. Technol. 2014, 4, 814–821. [Google Scholar] [CrossRef]
- Da Silva, A.G.M.; Batalha, D.C.; Rodrigues, T.S.; Candido, E.G.; Luz, S.C.; De Freitas, I.C.; Fonseca, F.C.; De Oliveira, D.C.; Taylor, J.G.; De Torresi, S.I.C.; et al. Sub-15 nm CeO2 nanowires as an efficient non-noble metal catalyst in the room-temperature oxidation of aniline. Catal. Sci. Technol. 2018, 8, 1828–1839. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; de Moura, A.B.L.; e Silva, F.A.; Candido, E.G.; da Silva, A.G.M.; de Oliveira, D.C.; Quiroz, J.; Camargo, P.H.C.; Bergamaschi, V.S.; Ferreira, J.C.; et al. Ni supported Ce0.9Sm0.1O2-Δ nanowires: An efficient catalyst for ethanol steam reforming for hydrogen production. Fuel 2019, 237, 1244–1253. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Zhu, J.; Han, B.; Zhao, L.; Yu, H.; Deng, Z.; Shi, W. Study on different CeO2 structure stability during ethanol steam reforming reaction over Ir/CeO2 nanocatalysts. Appl. Catal. A Gen. 2018, 564, 226–233. [Google Scholar] [CrossRef]
- Kourtelesis, M.; Moraes, T.S.; Mattos, L.V.; Niakolas, D.K.; Noronha, F.B.; Verykios, X. The effects of support morphology on the performance of Pt/CeO2 catalysts for the low temperature steam reforming of ethanol. Appl. Catal. B Environ. 2021, 284, 119757. [Google Scholar] [CrossRef]
- Ratnasamy, C.; Wagner, J.P. Water Gas Shift Catalysis. Catal. Rev. 2009, 51, 325–440. [Google Scholar] [CrossRef]
- Liang, J.; Lin, J.; Liu, J.; Wang, X.; Zhang, T.; Li, J. Dual Metal Active Sites in an Ir1/FeOx Single-Atom Catalyst: A Redox Mechanism for the Water-Gas Shift Reaction. Angew. Chem. Int. Ed. 2020, 59, 12868–12875. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Chen, C.-Y. Water gas shift reaction for hydrogen production and carbon dioxide capture: A review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Ramírez, P.J.; Asara, G.G.; Viñes, F.; Evans, J.; Liu, P.; Ricart, J.M.; Illas, F. Charge Polarization at a Au-TiC Interface and the Generation of Highly Active and Selective Catalysts for the Low-Temperature Water-Gas Shift Reaction. Angew. Chem. Int. Ed. 2014, 53, 11270–11274. [Google Scholar] [CrossRef]
- Chen, W.-H.; Jheng, J.-G.; Yu, A.B. Hydrogen generation from a catalytic water gas shift reaction under microwave irradiation. Int. J. Hydrog. Energy 2008, 33, 4789–4797. [Google Scholar] [CrossRef]
- Chen, W.-H.; Syu, Y.-J. Hydrogen production from water gas shift reaction in a high gravity (Higee) environment using a rotating packed bed. Int. J. Hydrog. Energy 2010, 35, 10179–10189. [Google Scholar] [CrossRef]
- Sastre, F.; Oteri, M.; Corma, A.; García, H. Photocatalytic water gas shift using visible or simulated solar light for the efficient, room-temperature hydrogen generation. Energy Environ. Sci. 2013, 6, 2211. [Google Scholar] [CrossRef]
- Zhao, L.; Qi, Y.; Song, L.; Ning, S.; Ouyang, S.; Xu, H.; Ye, J. Solar-Driven Water–Gas Shift Reaction over CuOx/Al2O3 with 1.1 % of Light-to-Energy Storage. Angew. Chem. 2019, 131, 7790–7794. [Google Scholar] [CrossRef]
- de Lucas-Consuegra, A.; Caravaca, A.; González-Cobos, J.; Valverde, J.L.; Dorado, F. Electrochemical activation of a non noble metal catalyst for the water–gas shift reaction. Catal. Commun. 2011, 15, 6–9. [Google Scholar] [CrossRef]
- Cui, X.; Su, H.-Y.; Chen, R.; Yu, L.; Dong, J.; Ma, C.; Wang, S.; Li, J.; Yang, F.; Xiao, J.; et al. Room-temperature electrochemical water–gas shift reaction for high purity hydrogen production. Nat. Commun. 2019, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Chiu, T.-W.; Hung, C.-I. Hydrogen production from methane under the interaction of catalytic partial oxidation, water gas shift reaction and heat recovery. Int. J. Hydrog. Energy 2010, 35, 12808–12820. [Google Scholar] [CrossRef]
- Chou, C.Y.; Loiland, J.A.; Lobo, R.F. Reverse Water-Gas Shift Iron Catalyst Derived from Magnetite. Catalysts 2019, 9, 773. [Google Scholar] [CrossRef]
- Zhu, M.; Wachs, I.E. Iron-Based Catalysts for the High-Temperature Water–Gas Shift (HT-WGS) Reaction: A Review. ACS Catal. 2016, 6, 722–732. [Google Scholar] [CrossRef]
- Ren, Z.; Peng, F.; Li, J.; Liang, X.; Chen, B. Morphology-dependent properties of Cu/CEO2 catalysts for the water-gas shift reaction. Catalysts 2017, 7, 48. [Google Scholar] [CrossRef]
- Yan, H.; Qin, X.-T.; Yin, Y.; Teng, Y.-F.; Jin, Z.; Jia, C.-J. Promoted Cu-Fe3O4 catalysts for low-temperature water gas shift reaction: Optimization of Cu content. Appl. Catal. B Environ. 2018, 226, 182–193. [Google Scholar] [CrossRef]
- Jha, A.; Jeong, D.-W.; Shim, J.-O.; Jang, W.-J.; Lee, Y.-L.; Rode, C.V.; Roh, H.-S. Hydrogen production by the water-gas shift reaction using CuNi/Fe2O3 catalyst. Catal. Sci. Technol. 2015, 5, 2752–2760. [Google Scholar] [CrossRef]
- Ang, M.L.; Oemar, U.; Kathiraser, Y.; Saw, E.T.; Lew, C.H.K.; Du, Y.; Borgna, A.; Kawi, S. High-temperature water–gas shift reaction over Ni/xK/CeO2 catalysts: Suppression of methanation via formation of bridging carbonyls. J. Catal. 2015, 329, 130–143. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, H.; Wan, K.; Guo, L.-J.; He, T.; Shi, X. Water–Gas Shift Reaction on Titania-Supported Single-Metal-Atom Catalysts: The Role of Cation (Ti) and Oxygen Vacancy. J. Phys. Chem. C 2021, 125, 8620–8629. [Google Scholar] [CrossRef]
- Thouchprasitchai, N.; Luengnaruemitchai, A.; Pongstabodee, S. Water-gas shift reaction over Cu–Zn, Cu–Fe, and Cu–Zn–Fe composite-oxide catalysts prepared by urea-nitrate combustion. J. Ind. Eng. Chem. 2013, 19, 1483–1492. [Google Scholar] [CrossRef]
- Hu, J.; Guo, W.; Liu, Z.-H.; Lu, X.; Zhu, H.; Shi, F.; Yan, J.; Jiang, R. Unraveling the Mechanism of the Zn-Improved Catalytic Activity of Pd-Based Catalysts for Water–Gas Shift Reaction. J. Phys. Chem. C 2016, 120, 20181–20191. [Google Scholar] [CrossRef]
- Na, H.-S.; Ahn, C.-I.; Jha, A.; Park, K.S.; Jang, W.-J.; Shim, J.-O.; Jeong, D.-W.; Roh, H.-S.; Bae, J.W. The investigation of non-noble metal doped mesoporous cobalt oxide catalysts for the water–gas shift reaction. RSC Adv. 2016, 6, 52754–52760. [Google Scholar] [CrossRef]
- Majima, T.; Kono, E.; Ogo, S.; Sekine, Y. Pre-reduction and K loading effects on noble metal free Co-system catalyst for water gas shift reaction. Appl. Catal. A Gen. 2016, 523, 92–96. [Google Scholar] [CrossRef]
- Luo, W.; Chen, Y.; Du, Z.; Chen, C. Theoretical Study on PdCu/CeO2 -Catalyzed Water–Gas Shift Reaction: Crucial Role of the Metal/Ceria Interface and O2 Enhancement Effects. J. Phys. Chem. C 2018, 122, 28868–28883. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.-S.; Song, R.; Cao, T.; Luo, L.; Chen, X.; Gao, Y.; Lu, J.; Li, W.-X.; Huang, W. The most active Cu facet for low-temperature water gas shift reaction. Nat. Commun. 2017, 8, 488. [Google Scholar] [CrossRef]
- Li, Z.; Li, N.; Wang, N.; Zhou, B.; Yin, P.; Song, B.; Yu, J.; Yang, Y. Mechanism Investigations on Water Gas Shift Reaction over Cu(111), Cu(100), and Cu(211) Surfaces. ACS Omega 2022, 7, 3514–3521. [Google Scholar] [CrossRef]
- Lykaki, M.; Stefa, S.; Carabineiro, S.A.C.; Soria, M.A.; Madeira, L.M.; Konsolakis, M. Shape effects of ceria nanoparticles on the water-gas shift performance of CuOx/CeO2 catalysts. Catalysts 2021, 11, 753. [Google Scholar] [CrossRef]
- Knudsen, J.; Nilekar, A.U.; Vang, R.T.; Schnadt, J.; Kunkes, E.L.; Dumesic, J.A.; Mavrikakis, M.; Besenbacher, F. A Cu/Pt Near-Surface Alloy for Water−Gas Shift Catalysis. J. Am. Chem. Soc. 2007, 129, 6485–6490. [Google Scholar] [CrossRef]
- Fu, X.-P.; Guo, L.-W.; Wang, W.-W.; Ma, C.; Jia, C.-J.; Wu, K.; Si, R.; Sun, L.-D.; Yan, C.-H. Direct Identification of Active Surface Species for the Water–Gas Shift Reaction on a Gold–Ceria Catalyst. J. Am. Chem. Soc. 2019, 141, 4613–4623. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Li, L.; Qiao, B.; Liu, J.; Su, Y.; Wang, X. Identifying Size Effects of Pt as Single Atoms and Nanoparticles Supported on FeO x for the Water-Gas Shift Reaction. ACS Catal. 2018, 8, 859–868. [Google Scholar] [CrossRef]
- Rivero-Crespo, M.A.; Mon, M.; Ferrando-Soria, J.; Lopes, C.W.; Boronat, M.; Leyva-Pérez, A.; Corma, A.; Hernández-Garrido, J.C.; López-Haro, M.; Calvino, J.J.; et al. Confined Pt 1 1+ Water Clusters in a MOF Catalyze the Low-Temperature Water–Gas Shift Reaction with both CO2 Oxygen Atoms Coming from Water. Angew. Chem. Int. Ed. 2018, 57, 17094–17099. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Gulec, A.; Johnson, A.M.; Schweitzer, N.M.; Stucky, G.D.; Marks, L.D.; Stair, P.C. Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts. Science 2015, 350, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Ammal, S.C.; Heyden, A. Understanding the Nature and Activity of Supported Platinum Catalysts for the Water–Gas Shift Reaction: From Metallic Nanoclusters to Alkali-Stabilized Single-Atom Cations. ACS Catal. 2019, 9, 7721–7740. [Google Scholar] [CrossRef]
- Chen, J.-J.; Li, X.-N.; Liu, Q.-Y.; Wei, G.-P.; Yang, Y.; Li, Z.-Y.; He, S.-G. Water Gas Shift Reaction Catalyzed by Rhodium–Manganese Oxide Cluster Anions. J. Phys. Chem. Lett. 2021, 12, 8513–8520. [Google Scholar] [CrossRef]
- Lei, Y.; Cant, N.; Trimm, D. The origin of rhodium promotion of Fe3O4–Cr2O3 catalysts for the high-temperature water–gas shift reaction. J. Catal. 2006, 239, 227–236. [Google Scholar] [CrossRef]
- Shekhar, M.; Wang, J.; Lee, W.-S.; Williams, W.D.; Kim, S.M.; Stach, E.A.; Miller, J.T.; Delgass, W.N.; Ribeiro, F.H. Size and Support Effects for the Water–Gas Shift Catalysis over Gold Nanoparticles Supported on Model Al2O3 and TiO2. J. Am. Chem. Soc. 2012, 134, 4700–4708. [Google Scholar] [CrossRef]
- Fu, Q.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science 2003, 301, 935–938. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, X.; Zhou, W.; Gao, R.; Xu, W.; Ye, Y.; Lin, L.; Wen, X.; Liu, P.; Chen, B.; et al. Atomic-layered Au clusters on α-MoC as catalysts for the low-temperature water-gas shift reaction. Science 2017, 357, 389–393. [Google Scholar] [CrossRef]
- Tao, F.; Ma, Z. Water–gas shift on gold catalysts: Catalyst systems and fundamental studies. Phys. Chem. Chem. Phys. 2013, 15, 15260. [Google Scholar] [CrossRef]
- Arab, A.; Sharafie, D.; Fazli, M. Theoretical study of water-gas shift reaction on the silver nanocluster. J. Phys. Chem. Solids 2017, 109, 100–108. [Google Scholar] [CrossRef]
- Zugic, B.; Zhang, S.; Bell, D.C.; Tao, F.; Flytzani-Stephanopoulos, M. Probing the Low-Temperature Water–Gas Shift Activity of Alkali-Promoted Platinum Catalysts Stabilized on Carbon Supports. J. Am. Chem. Soc. 2014, 136, 3238–3245. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Xu, H.; Li, W.; Goldbach, A. Water–gas shift reaction in a Pd membrane reactor over Pt/Ce0.6Zr0.4O2 catalyst. Int. J. Hydrog. Energy 2009, 34, 2965–2971. [Google Scholar] [CrossRef]
- Huang, S.-C.; Lin, C.-H.; Wang, J.-H. Trends of Water Gas Shift Reaction on Close-Packed Transition Metal Surfaces. J. Phys. Chem. C 2010, 114, 9826–9834. [Google Scholar] [CrossRef]
- Schweitzer, N.M.; Schaidle, J.A.; Ezekoye, O.K.; Pan, X.; Linic, S.; Thompson, L.T. High Activity Carbide Supported Catalysts for Water Gas Shift. J. Am. Chem. Soc. 2011, 133, 2378–2381. [Google Scholar] [CrossRef]
- Si, R.; Flytzani-Stephanopoulos, M. Shape and Crystal-Plane Effects of Nanoscale Ceria on the Activity of Au-CeO2 Catalysts for the Water–Gas Shift Reaction. Angew. Chem. Int. Ed. 2008, 47, 2884–2887. [Google Scholar] [CrossRef]
- Guan, Y.; Ligthart, D.A.J.M.; Pirgon-Galin, Ö.; Pieterse, J.A.Z.; van Santen, R.A.; Hensen, E.J.M. Gold Stabilized by Nanostructured Ceria Supports: Nature of the Active Sites and Catalytic Performance. Top. Catal. 2011, 54, 424–438. [Google Scholar] [CrossRef]
- Lee, Y.L.; Mnoyan, A.; Na, H.S.; Ahn, S.Y.; Kim, K.J.; Shim, J.O.; Lee, K.; Roh, H.S. Comparison of the effects of the catalyst preparation method and CeO2 morphology on the catalytic activity of Pt/CeO2 catalysts for the water-gas shift reaction. Catal. Sci. Technol. 2020, 10, 6299–6308. [Google Scholar] [CrossRef]
- Li, Y.; Kottwitz, M.; Vincent, J.L.; Enright, M.J.; Liu, Z.; Zhang, L.; Huang, J.; Senanayake, S.D.; Yang, W.C.D.; Crozier, P.A.; et al. Dynamic structure of active sites in ceria-supported Pt catalysts for the water gas shift reaction. Nat. Commun. 2021, 12, 914. [Google Scholar] [CrossRef]
- Baschuk, J.J.; Li, X. Carbon monoxide poisoning of proton exchange membrane fuel cells. Int. J. Energy Res. 2001, 25, 695–713. [Google Scholar] [CrossRef]
- Teixeira, M.; Madeira, L.M.; Sousa, J.M.; Mendes, A. Modeling of a catalytic membrane reactor for CO removal from hydrogen streams—A theoretical study. Int. J. Hydrog. Energy 2010, 35, 11505–11513. [Google Scholar] [CrossRef]
- Dagle, R.A.; Karim, A.; Li, G.; Su, Y.; King, D.L. Syngas Conditioning; Elsevier B.V.: Amsterdam, The Netherlands, 2011; ISBN 9780444535634. [Google Scholar] [CrossRef]
- Tabakova, T. Recent Advances in Design of Gold-Based Catalysts for H2 Clean-Up Reactions. Front. Chem. 2019, 7, 517. [Google Scholar] [CrossRef] [PubMed]
- Al Soubaihi, R.M.; Saoud, K.M.; Dutta, J. Critical review of low-temperature CO oxidation and hysteresis phenomenon on heterogeneous catalysts. Catalysts 2018, 8, 660. [Google Scholar] [CrossRef]
- Hernández, J.A.; Gómez, S.A.; Zepeda, T.A.; Fierro-González, J.C.; Fuentes, G.A. Insight into the Deactivation of Au/CeO2 Catalysts Studied by in Situ Spectroscopy during the CO-PROX Reaction. ACS Catal. 2015, 5, 4003–4012. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, Q.; Stephanopoulos, M.F. Preferential oxidation of CO in H2 over CuO-CeO2 catalysts. Catal. Today 2004, 93, 241–246. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C.; Figueiredo, J.L. Hydrogen production by alkaline water electrolysis. Quim. Nova 2013, 36, 1176–1193. [Google Scholar] [CrossRef]
- Pagliaro, M.; Konstandopoulos, A.G.; Ciriminnaa, R.; Palmisano, G. Solar hydrogen: Fuel of the near future. Energy Environ. Sci. 2010, 3, 279–287. [Google Scholar] [CrossRef]
- Mahmood, N.; Yao, Y.; Zhang, J.W.; Pan, L.; Zhang, X.; Zou, J.J. Electrocatalysts for Hydrogen Evolution in Alkaline Electrolytes: Mechanisms, Challenges, and Prospective Solutions. Adv. Sci. 2018, 5, 1700464. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Vasileff, A.; Qiao, S.Z. The Hydrogen Evolution Reaction in Alkaline Solution: From Theory, Single Crystal Models, to Practical Electrocatalysts. Angew. Chem. Int. Ed. 2018, 57, 7568–7579. [Google Scholar] [CrossRef]
- Ďurovič, M.; Hnát, J.; Bouzek, K. Electrocatalysts for the hydrogen evolution reaction in alkaline and neutral media. A comparative review. J. Power Sources 2021, 493, 229708. [Google Scholar] [CrossRef]
- Santos, A.L.; Cebola, M.J.; Santos, D.M.F. Towards the hydrogen economy—A review of the parameters that influence the efficiency of alkaline water electrolyzers. Energies 2021, 14, 3193. [Google Scholar] [CrossRef]
- Dezem, V. Why Hydrogen Is the Hottest Thing in Green Energy 2021. Available online: https://illuminem.com/post/96ed8c14-6f8b-4c90-8602-d36f92d5af1b (accessed on 28 April 2022).
- Wang, S.; Lu, A.; Zhong, C.J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.M.; Nakajima, H.; Inada, A.; Sasaki, K.; Ito, K. Overpotentials and reaction mechanism in electrochemical hydrogen pumps. Electrochim. Acta 2019, 301, 274–283. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef] [PubMed]
- Roger, B.Y. The rate of electrolytic hydrogen and the heat of adsorption of hydrogen. Trans. Faraday Soc. 1957, 54, 1053–1063. [Google Scholar] [CrossRef]
- Sabatier, P. Hydroénatione et déhydrogénations par catalyse. Ber. Der Dtsch. Chem. Ges. 1911, 44, 1984–2001. [Google Scholar] [CrossRef]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 3. [Google Scholar] [CrossRef]
- Skúlason, E.; Tripkovic, V.; Björketun, M.E.; Gudmundsdóttir, S.; Karlberg, G.; Rossmeisl, J.; Bligaard, T.; Jónsson, H.; Nørskov, J.K. Modeling the electrochemical hydrogen oxidation and evolution reactions on the basis of density functional theory calculations. J. Phys. Chem. C 2010, 114, 18182–18197. [Google Scholar] [CrossRef]
- Li, C.; Baek, J.B. Recent Advances in Noble Metal (Pt, Ru, and Ir)-Based Electrocatalysts for Efficient Hydrogen Evolution Reaction. ACS Omega 2020, 5, 31–40. [Google Scholar] [CrossRef]
- Cai, J.; Javed, R.; Ye, D.; Zhao, H.; Zhang, J. Recent progress in noble metal nanocluster and single atom electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2020, 8, 22467–22487. [Google Scholar] [CrossRef]
- Dubouis, N.; Grimaud, A. The hydrogen evolution reaction: From material to interfacial descriptors. Chem. Sci. 2019, 10, 9165–9181. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Sheng, W.; Zhuang, Z.; Xu, B.; Yan, Y. Universal dependence of hydrogen oxidation and evolution reaction activity of platinum-group metals on pH and hydrogen binding energy. Sci. Adv. 2016, 2, e1501602. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ge, R.; Su, J.; Chen, L. Recent Progress in Low Pt Content Electrocatalysts for Hydrogen Evolution Reaction. Adv. Mater. Interfaces 2020, 7, 2000396. [Google Scholar] [CrossRef]
- Duan, S.; Du, Z.; Fan, H.; Wang, R. Nanostructure optimization of platinum-based nanomaterials for catalytic applications. Nanomaterials 2018, 8, 949. [Google Scholar] [CrossRef] [PubMed]
- Marković, N.M.; Sarraf, S.T.; Gasteiger, H.A.; Ross, P.N. Hydrogen electrochemistry on platinum low-index single-crystal surfaces in alkaline solution. J. Chem. Soc. Faraday Trans. 1996, 92, 3719–3725. [Google Scholar] [CrossRef]
- Gilroy, K.D.; Farzinpour, P.; Sundar, A.; Hughes, R.A.; Neretina, S. Sacrificial templates for galvanic replacement reactions: Design criteria for the synthesis of pure pt nanoshells with a smooth surface morphology. Chem. Mater. 2014, 26, 3340–3347. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, H.; Gao, W.; Xue, W.; Liu, Z.; Huang, J.; Pan, X.; Huang, Y. Surface-Engineered PtNi-O Nanostructure with Record-High Performance for Electrocatalytic Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2018, 140, 9046–9050. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, G.; Cui, X.; Chen, B.; Zhu, Y.; Gong, Y.; Saleem, F.; Xi, S.; Du, Y.; Borgna, A.; et al. Crystal Phase and Architecture Engineering of Lotus-Thalamus-Shaped Pt-Ni Anisotropic Superstructures for Highly Efficient Electrochemical Hydrogen Evolution. Adv. Mater. 2018, 30, 1801741. [Google Scholar] [CrossRef]
- Koo, B.; Chu, J.; Seo, J.; Jung, G.; Baek, S.H.; Nam, S.W.; Duah, C.; Lee, Y.K.; Jung, W.C.; Shin, B. Drop-casted Platinum Nanocube Catalysts for Hydrogen Evolution Reaction with Ultrahigh Mass Activity. ChemSusChem 2021, 14, 2585–2590. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Wang, H.; Lu, J. A Review on Particle Size Effect in Metal-Catalyzed Heterogeneous Reactions. Chin. J. Chem. 2020, 38, 1422–1444. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Guo, X.; Chen, C.; Dong, C.L.; Liu, R.S.; Han, C.P.; Li, Y.; Gogotsi, Y.; Wang, G. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 2018, 1, 985–992. [Google Scholar] [CrossRef]
- Yu, P.-W.; Elmas, S.; Roman, T.; Pan, X.; Yin, Y.; Gibson, C.T.; Andersson, G.G.; Andersson, M.R. Highly Active Platinum Single-Atom Catalyst Grafted onto 3D Carbon Cloth Support for the Electrocatalytic Hydrogen Evolution Reaction. Appl. Surf. Sci. 2022, 595, 153480. [Google Scholar] [CrossRef]
- Elmas, S.; Beelders, W.; Bradley, S.J.; Kroon, R.; Laufersky, G.; Andersson, M.; Nann, T. Platinum Terpyridine Metallopolymer Electrode as Cost-Effective Replacement for Bulk Platinum Catalysts in Oxygen Reduction Reaction and Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2017, 5, 10206–10214. [Google Scholar] [CrossRef]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.K.; Liu, L.M.; et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, G.; Gauquelin, N.; Chen, N.; Zhou, J.; Yang, S.; Chen, W.; Meng, X.; Geng, D.; Banis, M.N.; et al. Single-atom catalysis using Pt/graphene achieved through atomic layer deposition. Sci. Rep. 2013, 3, 1775. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, Z.R.; Xiao, Y.Y.; Yin, Y.C.; Huang, W.M.; Huang, Z.C.; Zheng, Y.Z.; Mu, F.Y.; Huang, R.; Shi, G.Y.; et al. Electronic metal–support interaction modulates single-atom platinum catalysis for hydrogen evolution reaction. Nat. Commun. 2021, 12, 3021. [Google Scholar] [CrossRef]
- Tavakkoli, M.; Holmberg, N.; Kronberg, R.; Jiang, H.; Sainio, J.; Kauppinen, E.I.; Kallio, T.; Laasonen, K.; Tavakkoli, M.; Holmberg, N.; et al. Electrochemical activation of single-walled carbon nanotubes with pseudo atomic-scale platinum for hydrogen evolution reaction. ACS Catal. 2017, 7, 3121–3130. [Google Scholar] [CrossRef]
- Pu, Z.; Amiinu, I.S.; Cheng, R.; Wang, P.; Zhang, C.; Mu, S.; Zhao, W.; Su, F.; Zhang, G.; Liao, S.; et al. Single-Atom Catalysts for Electrochemical Hydrogen Evolution Reaction: Recent Advances and Future Perspectives. Nano-Micro Lett. 2020, 12, 21. [Google Scholar] [CrossRef]
- Fang, S.; Zhu, X.; Liu, X.; Gu, J.; Liu, W.; Wang, D.; Zhang, W.; Lin, Y.; Lu, J.; Wei, S.; et al. Uncovering near-free platinum single-atom dynamics during electrochemical hydrogen evolution reaction. Nat. Commun. 2020, 11, 1029. [Google Scholar] [CrossRef]
- Xiong, B.Y.; Xia, Y. Shape-Controlled Synthesis of Metal Nanostructures: The Case of Palladium. Adv. Mater. 2007, 19, 3385–3391. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, S.; Liu, J.; Park, J.; Huang, C.Z.; Xia, Y. Shape-Controlled Synthesis of Palladium Nanocrystals: A Mechanistic Understanding of the Evolution from Octahedrons to Tetrahedrons. Nano Lett. 2013, 13, 2276–2281. [Google Scholar] [CrossRef]
- Janssen, A.; Shi, Y.; Xia, Y. Separating Growth from Nucleation for Facile Control over the Size and Shape of Palladium Nanocrystals. Chem. A Eur. J. 2020, 26, 13890–13895. [Google Scholar] [CrossRef] [PubMed]
- Zalineeva, A.; Baranton, S.; Coutanceau, C.; Jerkiewicz, G. Octahedral palladium nanoparticles as excellent hosts for electrochemically adsorbed and absorbed hydrogen. Sci. Adv. 2017, 3, e1600542. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, F.; Guo, S.X.; Zhang, J.; Ma, J. PdCu@Pd Nanocube with Pt-like Activity for Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 8151–8160. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, J.; Sui, Y.; Wang, M.; Qiao, L.; Du, F.; Zou, B. Palladium structure engineering induced by electrochemical H intercalation boosts hydrogen evolution catalysis. J. Mater. Chem. A 2019, 7, 14876–14881. [Google Scholar] [CrossRef]
- Liang, Z.; Ahn, H.S.; Bard, A.J. A Study of the Mechanism of the Hydrogen Evolution Reaction on Nickel by Surface Interrogation Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2017, 139, 4854–4858. [Google Scholar] [CrossRef]
- Vij, V.; Sultan, S.; Harzandi, A.M.; Meena, A.; Tiwari, J.N.; Lee, W.G.; Yoon, T.; Kim, K.S. Nickel-based electrocatalysts for energy-related applications: Oxygen reduction, oxygen evolution, and hydrogen evolution reactions. ACS Catal. 2017, 7, 7196–7225. [Google Scholar] [CrossRef]
- Li, Z.; Yu, C.; Wen, Y.; Gao, Y.; Xing, X.; Wei, Z.; Sun, H.; Zhang, Y.W.; Song, W. Mesoporous Hollow Cu-Ni Alloy Nanocage from Core-Shell Cu@Ni Nanocube for Efficient Hydrogen Evolution Reaction. ACS Catal. 2019, 9, 5084–5095. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, Q.; Sun, Y.; Ning, G.; Fan, X.; Wang, H.; Lu, H.; Zhang, Y.; Wang, H. Ni–Fe nanocubes embedded with Pt nanoparticles for hydrogen and oxygen evolution reactions. Int. J. Hydrog. Energy 2020, 45, 20832–20842. [Google Scholar] [CrossRef]
- Hong, Y.; Choi, C.H.; Choi, S.-I. Catalytic Surface Specificity of Ni(OH)2-Decorated Pt Nanocubes for the Hydrogen Evolution Reaction in an Alkaline Electrolyte. ChemSusChem 2019, 12, 4021–4028. [Google Scholar] [CrossRef] [PubMed]
- Kavian, R.; Choi, S.-I.; Park, J.; Liu, T.; Peng, H.C.; Lu, N.; Wang, J.; Kim, M.J.; Xia, Y.; Lee, S.W. Pt-Ni octahedral nanocrystals as a class of highly active electrocatalysts toward the hydrogen evolution reaction in an alkaline electrolyte. J. Mater. Chem. A 2016, 4, 12392–12397. [Google Scholar] [CrossRef]
- Seo, B.; Baek, D.S.; Sa, Y.J.; Joo, S.H. Shape effects of nickel phosphide nanocrystals on hydrogen evolution reaction. CrystEngComm 2016, 18, 6083–6089. [Google Scholar] [CrossRef]
- Xiang, D.; Zhang, B.; Zhang, H.; Shen, L. One-Step Synthesis of Bifunctional Nickel Phosphide Nanowires as Electrocatalysts for Hydrogen and Oxygen Evolution Reactions. Front. Chem. 2021, 9, 773018. [Google Scholar] [CrossRef]
- Li, R.; Li, C. Photocatalytic Water Splitting on Semiconductor-Based Photocatalysts, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 60. [Google Scholar]
- Li, X.; Wang, Z.; Wang, L. Metal–Organic Framework-Based Materials for Solar Water Splitting. Small Sci. 2021, 1, 2000074. [Google Scholar] [CrossRef]
- Qiao, M.; Liu, J.; Wang, Y.; Li, Y.; Chen, Z. PdSeO3 Monolayer: Promising Inorganic 2D Photocatalyst for Direct Overall Water Splitting Without Using Sacrificial Reagents and Cocatalysts. J. Am. Chem. Soc. 2018, 140, 12256–12262. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic water splitting—The untamed dream: A review of recent advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Teixeira, I.F.; Barbosa, E.C.M.; Tsang, S.C.E.; Camargo, P.H.C. Carbon nitrides and metal nanoparticles: From controlled synthesis to design principles for improved photocatalysis. Chem. Soc. Rev. 2018, 47, 7783–7817. [Google Scholar] [CrossRef]
- Prasad, S.; Schumacher, H.; Gopinath, A. Review of Semiconductor Materials and Physics; Cambridge University Press: Cambridge, UK, 2010; ISBN 9780511626517. [Google Scholar] [CrossRef]
- Chou, H.L.; Hwang, B.J.; Sun, C.L. Catalysis in Fuel Cells and Hydrogen Production; Elsevier B.V.: Amsterdam, The Netherlands, 2013; ISBN 9780444538802. [Google Scholar]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mater. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Jiang, M.Y.; Liu, M.; Wu, L.K.; Hou, G.Y.; Tang, Y.P. An Fe-V@NiO heterostructure electrocatalyst towards the oxygen evolution reaction. Nanoscale 2020, 12, 3803–3811. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Qiu, C.; Jiang, J.; Ai, L. Hierarchical CoP-FeP Branched Heterostructures for Highly Efficient Electrocatalytic Water Splitting. ACS Sustain. Chem. Eng. 2019, 7, 2335–2342. [Google Scholar] [CrossRef]
- Shit, S.; Chhetri, S.; Jang, W.; Murmu, N.C.; Koo, H.; Samanta, P.; Kuila, T. Cobalt Sulfide/Nickel Sulfide Heterostructure Directly Grown on Nickel Foam: An Efficient and Durable Electrocatalyst for Overall Water Splitting Application. ACS Appl. Mater. Interfaces 2018, 10, 27712–27722. [Google Scholar] [CrossRef] [PubMed]
- Shit, S.; Chhetri, S.; Bolar, S.; Murmu, N.C.; Jang, W.; Koo, H.; Kuila, T. Hierarchical Cobalt Sulfide/Molybdenum Sulfide Heterostructure as Bifunctional Electrocatalyst towards Overall Water Splitting. ChemElectroChem 2019, 6, 430–438. [Google Scholar] [CrossRef]
- Zhang, H.; Maijenburg, A.W.; Li, X.; Schweizer, S.L.; Wehrspohn, R.B. Bifunctional Heterostructured Transition Metal Phosphides for Efficient Electrochemical Water Splitting. Adv. Funct. Mater. 2020, 30, 2003261. [Google Scholar] [CrossRef]
- Sitara, E.; Nasir, H.; Mumtaz, A.; Ehsan, M.F.; Sohail, M.; Iram, S.; Bukhari, S.A.B. Efficient photoelectrochemical water splitting by tailoring MoS2/CoTe heterojunction in a photoelectrochemical cell. Nanomaterials 2020, 10, 2341. [Google Scholar] [CrossRef]
- Lucas, T.T.A.; Melo, M.A.; Freitas, A.L.M.; Souza, F.L.; Gonçalves, R.V. Enhancing the solar water splitting activity of TiO2 nanotube-array photoanode by surface coating with La-doped SrTiO3. Sol. Energy Mater. Sol. Cells 2020, 208, 110428. [Google Scholar] [CrossRef]
- Ge, J.; Liu, Y.; Jiang, D.; Zhang, L.; Du, P. Integrating non-precious-metal cocatalyst Ni3N with g-C3N4 for enhanced photocatalytic H2 production in water under visible-light irradiation. Cuihua Xuebao/Chin. J. Catal. 2019, 40, 160–167. [Google Scholar] [CrossRef]
- Ma, S.; Deng, Y.; Xie, J.; He, K.; Liu, W.; Chen, X.; Li, X. Noble-metal-free Ni3C cocatalysts decorated CdS nanosheets for high-efficiency visible-light-driven photocatalytic H2 evolution. Appl. Catal. B Environ. 2018, 227, 218–228. [Google Scholar] [CrossRef]
- Shen, R.; Xie, J.; Xiang, Q.; Chen, X.; Jiang, J.; Li, X. Ni-based photocatalytic H2-production cocatalysts. Cuihua Xuebao/Chin. J. Catal. 2019, 40, 240–288. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Y.; Hu, X.; Liu, E.; Fan, J. Enhanced photocatalytic H2 production over dual-cocatalyst-modified g-C3N4 heterojunctions. Cuihua Xuebao/Chin. J. Catal. 2019, 40, 434–445. [Google Scholar] [CrossRef]
- Fujishima, A.; Kohayakawa, K.; Honda, K. Hydrogen Production under Sunlight with an Electrochemical Photocell. J. Electrochem. Soc. 1975, 122, 1487–1489. [Google Scholar] [CrossRef]
- Chandra, M.; Pradhan, D. Engineering the Morphology and Crystal Phase of 3D Hierarchical TiO2 with Excellent Photochemical and Photoelectrochemical Solar Water Splitting. ChemSusChem 2020, 13, 3005–3016. [Google Scholar] [CrossRef]
- Peng, Y.W.; Shan, C.; Wang, H.J.; Hong, L.Y.; Yao, S.; Wu, R.J.; Zhang, Z.M.; Lu, T.B. Polyoxometalate-Derived Ultrasmall Pt2W/WO3 Heterostructure Outperforms Platinum for Large-Current-Density H2 Evolution. Adv. Energy Mater. 2019, 9, 1900597. [Google Scholar] [CrossRef]
- Zhao, G.; Li, P.; Cheng, N.; Dou, S.X.; Sun, W. An Ir/Ni(OH)2 Heterostructured Electrocatalyst for the Oxygen Evolution Reaction: Breaking the Scaling Relation, Stabilizing Iridium(V), and Beyond. Adv. Mater. 2020, 32, 2000872. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C.; Liu, H.; Sun, J.; Xie, R.; Qiu, Y.; Lü, F.; Liu, Y.; Zhuo, L.; Liu, X.; et al. Amorphous MoOX-Stabilized single platinum atoms with ultrahigh mass activity for acidic hydrogen evolution. Nano Energy 2020, 70, 104529. [Google Scholar] [CrossRef]
- Yi, S.S.; Zhang, X.B.; Wulan, B.R.; Yan, J.M.; Jiang, Q. Non-noble metals applied to solar water splitting. Energy Environ. Sci. 2018, 11, 3128–3156. [Google Scholar] [CrossRef]
- Li, T.; Ding, D.; Dong, Z.; Ning, C. Photoelectrochemicalwater splitting properties of Ti-Ni-Si-O nanostructures on Ti-Ni-Si alloy. Nanomaterials 2017, 7, 359. [Google Scholar] [CrossRef]
- Singh, S.B. Nanomaterials for Water Splitting: A Greener Approach to Generate Hydrogen. In Nanomaterials for Water Splitting: A Greener Approach to Generate Hydrogen; Springer: Cham, Denmark, 2021; pp. 1201–1220. [Google Scholar]
- Grewe, T.; Meggouh, M.; Tüysüz, H. Nanocatalysts for Solar Water Splitting and a Perspective on Hydrogen Economy. Chem. Asian J. 2016, 11, 22–42. [Google Scholar] [CrossRef]
- da Silva, A.G.M.; Rodrigues, T.S.; Candido, E.G.; de Freitas, I.C.; da Silva, A.H.M.; Fajardo, H.V.; Balzer, R.; Gomes, J.F.; Assaf, J.M.; de Oliveira, D.C.; et al. Combining active phase and support optimization in MnO2-Au nanoflowers: Enabling high activities towards green oxidations. J. Colloid Interface Sci. 2018, 530, 282–291. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.G.M.; Rodrigues, T.S.; Macedo, A.; da Silva, R.T.P.; Camargo, P.H.C. An Undergraduate Level Experiment on the Synthesis of Au Nanoparticles and Their Size-Dependent Optical and Catalytic Properties. Quim. Nov. 2014, 37, 1716–1720. [Google Scholar] [CrossRef]
- Lu, M.; Cui, X.; Song, B.; Ouyang, H.; Wang, K.; Wang, Y. Studying the Effect of CuCo2S4 Morphology on the Oxygen Evolution Reaction using a Flexible Carbon Cloth Substrate. ChemElectroChem 2020, 7, 1080–1083. [Google Scholar] [CrossRef]
- Fang, L.; Jiang, Z.; Xu, H.; Liu, L.; Guan, Y.; Gu, X.; Wang, Y. Crystal-plane engineering of NiCo2O4 electrocatalysts towards efficient overall water splitting. J. Catal. 2018, 357, 238–246. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Qin, Y.; Zhang, W.; Wang, L.; Luo, M.; Yang, H.; Guo, S. Recent Advances on Water-Splitting Electrocatalysis Mediated by Noble-Metal-Based Nanostructured Materials. Adv. Energy Mater. 2020, 10, 1903120. [Google Scholar] [CrossRef]
- da Silva, A.G.M.; Fernandes, C.G.; Hood, Z.D.; Peng, R.; Wu, Z.; Dourado, A.H.B.; Parreira, L.S.; de Oliveira, D.C.; Camargo, P.H.C.; de Torresi, S.I.C. PdPt-TiO2 nanowires: Correlating composition, electronic effects and O-vacancies with activities towards water splitting and oxygen reduction. Appl. Catal. B Environ. 2020, 277, 119177. [Google Scholar] [CrossRef]
- Zheng, D.; Cao, X.; Wang, X. Precise Formation of a Hollow Carbon Nitride Structure with a Janus Surface To Promote Water Splitting by Photoredox Catalysis. Angew. Chem. 2016, 128, 11684–11688. [Google Scholar] [CrossRef]
- Wei, Z.; Mogan, T.R.; Wang, K.; Janczarek, M.; Kowalska, E. Morphology-governed performance of multi-dimensional photocatalysts for hydrogen generation. Energies 2021, 14, 7223. [Google Scholar] [CrossRef]
- Samuel, E.; Joshi, B.; Kim, M.W.; Swihart, M.T.; Yoon, S.S. Morphology engineering of photoelectrodes for efficient photoelectrochemical water splitting. Nano Energy 2020, 72, 104648. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Fateev, V.N.; Bessarabov, D.G.; Millet, P. Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrog. Energy 2020, 45, 26036–26058. [Google Scholar] [CrossRef]
- Wang, Q.; Hisatomi, T.; Jia, Q.; Tokudome, H.; Zhong, M.; Wang, C.; Pan, Z.; Takata, T.; Nakabayashi, M.; Shibata, N.; et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 2016, 15, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.; Tzimas, E.; Willis, P.; Arendorf, J.; Tercero Espinoza, L. Critical Metals in the Path towards the Decarbonisation of the EU Energy Sector: Assessing Rare Metals as Supply-Chain Bottlenecks in Low-Carbon Energy Technologies; Publications Office of the European Union: Luxembourg, 2013. [Google Scholar]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorio, J.L.; Gothe, M.L.; Kohlrausch, E.C.; Zardo, M.L.; Tanaka, A.A.; de Lima, R.B.; da Silva, A.G.M.; Garcia, M.A.S.; Vidinha, P.; Machado, G. Nanoengineering of Catalysts for Enhanced Hydrogen Production. Hydrogen 2022, 3, 218-254. https://doi.org/10.3390/hydrogen3020014
Fiorio JL, Gothe ML, Kohlrausch EC, Zardo ML, Tanaka AA, de Lima RB, da Silva AGM, Garcia MAS, Vidinha P, Machado G. Nanoengineering of Catalysts for Enhanced Hydrogen Production. Hydrogen. 2022; 3(2):218-254. https://doi.org/10.3390/hydrogen3020014
Chicago/Turabian StyleFiorio, Jhonatan Luiz, Maitê Lippel Gothe, Emerson Cristofer Kohlrausch, Maria Luísa Zardo, Auro Atsushi Tanaka, Roberto Batista de Lima, Anderson Gabriel Marques da Silva, Marco Aurélio Suller Garcia, Pedro Vidinha, and Giovanna Machado. 2022. "Nanoengineering of Catalysts for Enhanced Hydrogen Production" Hydrogen 3, no. 2: 218-254. https://doi.org/10.3390/hydrogen3020014
APA StyleFiorio, J. L., Gothe, M. L., Kohlrausch, E. C., Zardo, M. L., Tanaka, A. A., de Lima, R. B., da Silva, A. G. M., Garcia, M. A. S., Vidinha, P., & Machado, G. (2022). Nanoengineering of Catalysts for Enhanced Hydrogen Production. Hydrogen, 3(2), 218-254. https://doi.org/10.3390/hydrogen3020014






