Experimental Study on Hydrogen Recombination Characteristics of a Passive Autocatalytic Recombiner during Spray Operation
Abstract
:1. Introduction
2. SPARC Test Facility
2.1. SPARC Pressure Vessel
2.2. SPARC Measurement System
2.3. Spray System
2.4. Test PAR and Measurement
3. Postprocessing of Experimental Data
3.1. Gas Species Concentrations
3.2. PAR Recombination Rate
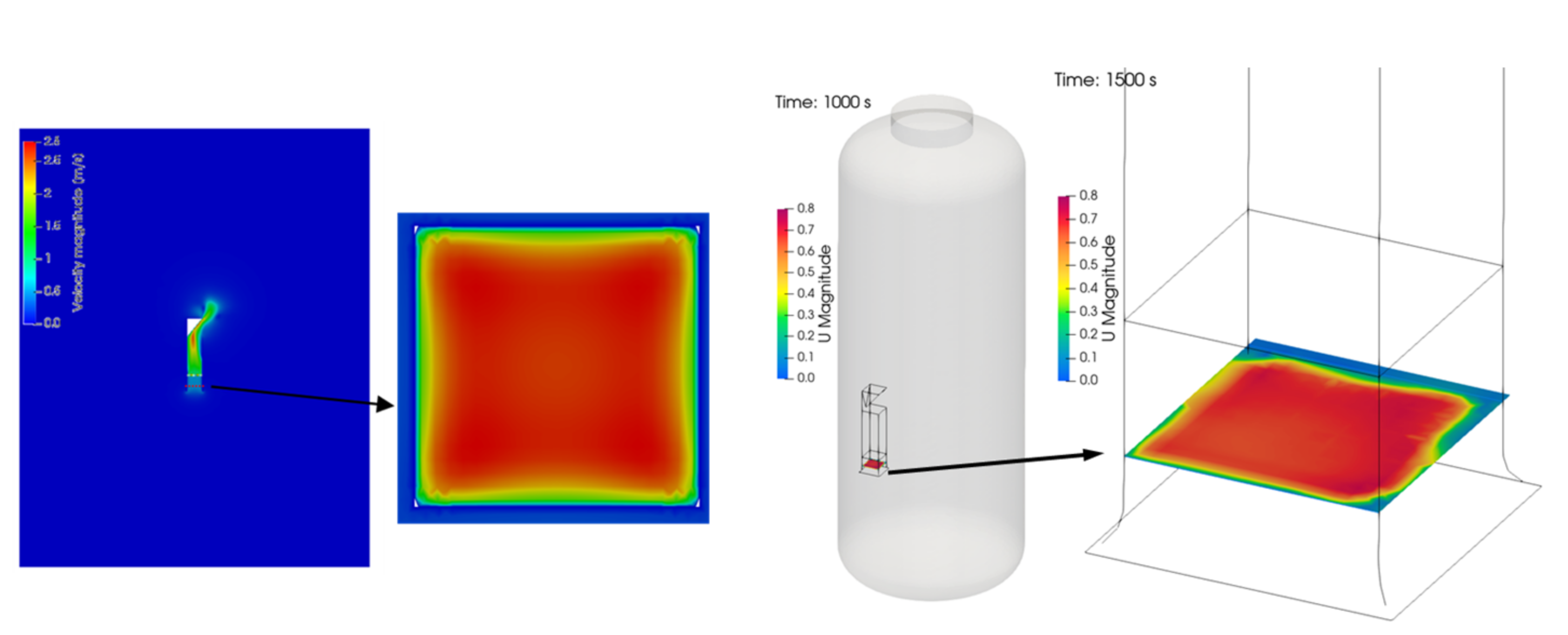
4. SPARC-SPRAY-PAR Experiment
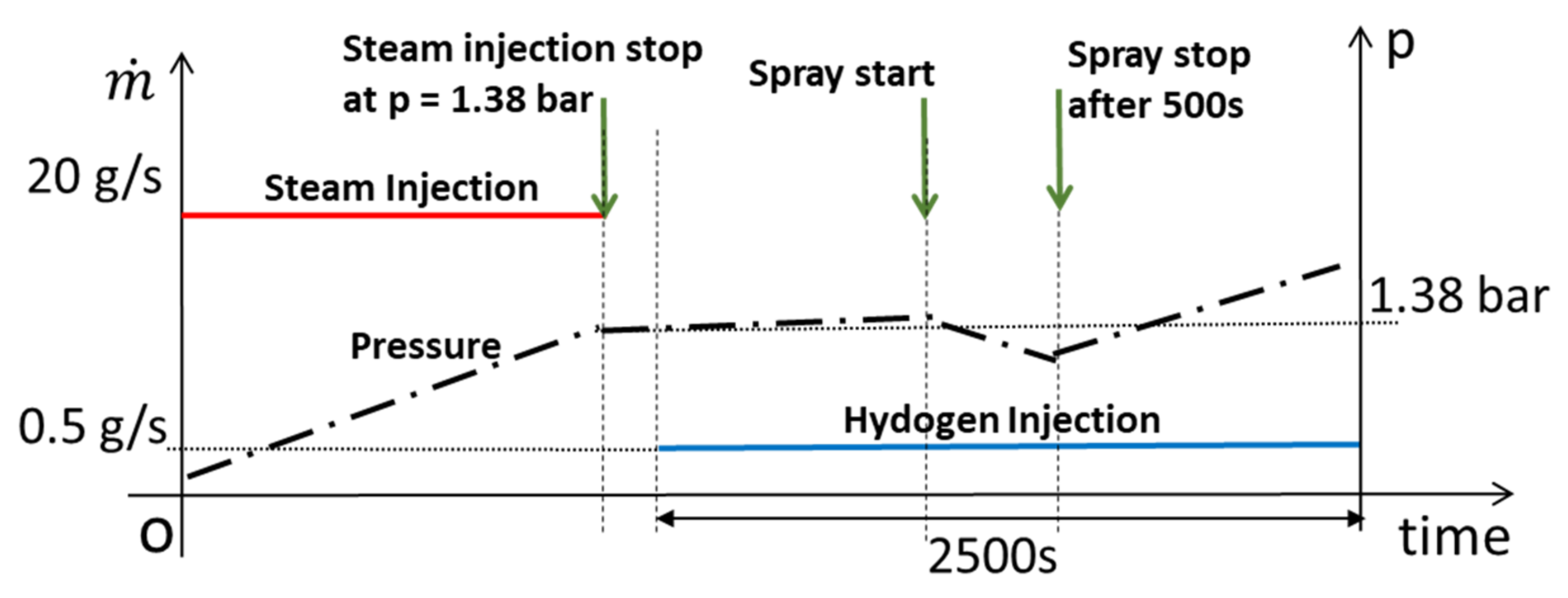
4.1. Test Conditions
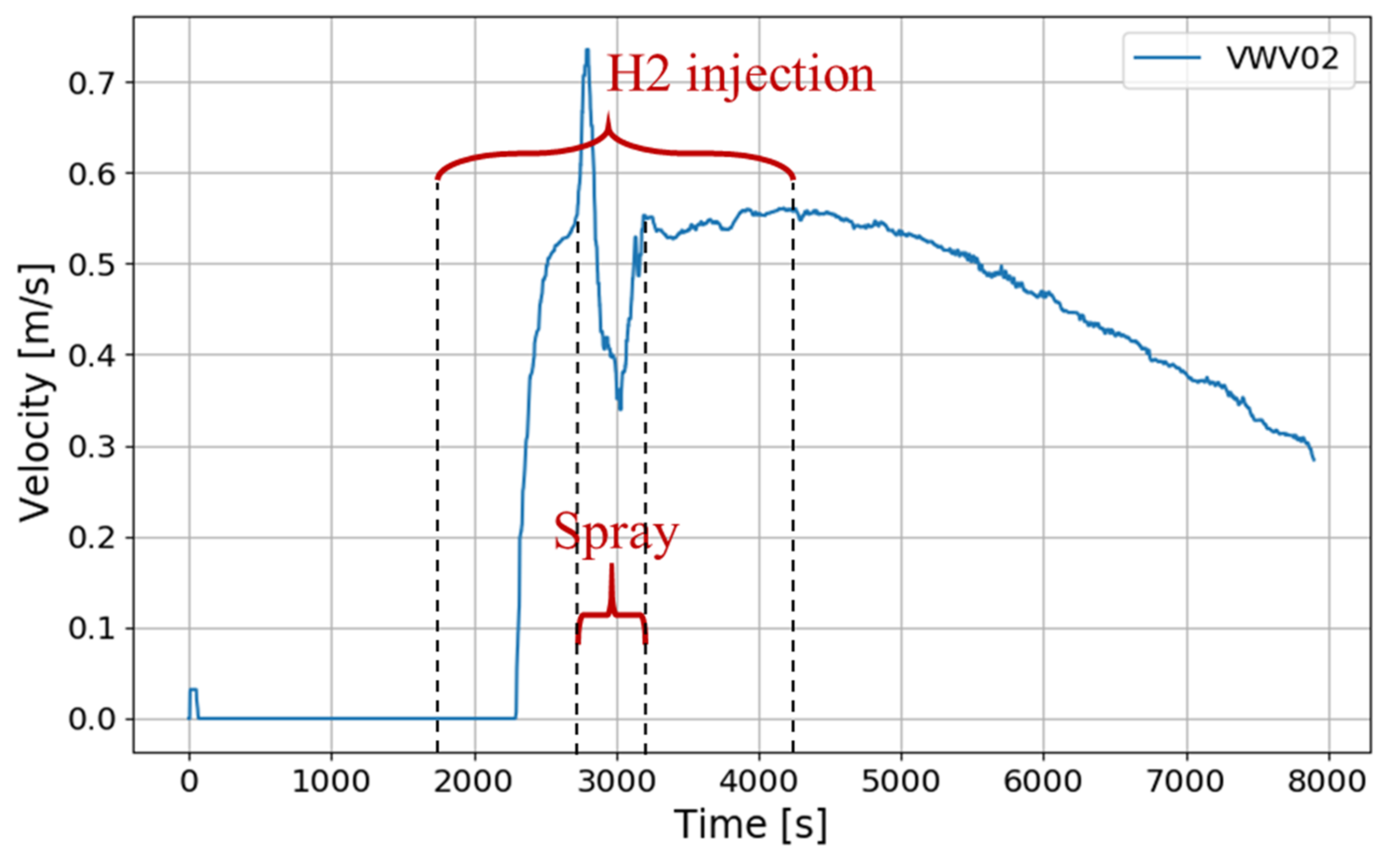
4.2. SSP3 Test
4.3. SSP4 Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | Area (m2) |
| m | Mass (kg) |
| Mass flow (kg/s) | |
| N | Number of mole (kmole) |
| p | Pressure (Pa) |
| R | Universal gas constant (J/kmol-K) |
| Hydrogen recombination rate (kg/s) | |
| RH | Relative humidity (−) |
| T | Temperature (K) |
| U | Velocity (m/s) |
| W | Molecular weight (kg/kmole) |
| x | Mole fraction of a gas species (−) |
| Y | Mass fraction of a gas species (−) |
| ρ | Density (kg/m3) |
| Subscripts | |
| h2o | Water vapor (Steam) |
| h2 | Hydrogen |
| o2 | Oxygen |
| in | Inlet |
| out | Outlet |
| m | Mixture |
References
- Korea Institute of Nuclear Safety. Light Water Reactor Nuclear Power Plant Regulatory Standards and Regulatory Guidelines; Korea Institute of Nuclear Safety: Daejeon, Korea, 2015. [Google Scholar]
- US-NRC. Combustible Gas Control for Nuclear Power Reactors, NRC Regulations 10CFR 50.44. 2020. Available online: https://www.nrc.gov/reading-rm/doc-collections/cfr/part050/part050-0044.html (accessed on 1 May 2020).
- AREVA. AREVA Passive Autocatalytic Recombiner. 2020. Available online: https://us.areva.com/home/liblocal/docs/Solutions/literature/G-008-V1PB-2011-ENG_PAR_reader.pdf (accessed on 3 April 2021).
- Kelm, S.; Jahn, W.; Reinecke, E.-A. Operational Behaviour of Catalytic Recombiners—Experimental Results and Modelling Approaches. In Proceedings of the OECD/NEA workshop on the Experiments and CFD Code Application to Nuclear reactor Safety, Grenoble, France, 10–12 September 2008. [Google Scholar]
- Bachellerie, E.; Arnould, F.; Auglaire, M.; De Boeck, B.; Braillard, O.; Eckardt, B.; Ferroni, F.; Moffett, R. Generic Approach for Designing and Implementing a Passive Autocatalytic Recombiner PAR-System in Nuclear Power Plant Containments. Nucl. Eng. Des. 2003, 221, 151–165. [Google Scholar] [CrossRef]
- Dewit, W.A.; Koroll, G.W.; Sitar, L. Hydrogen Recombiner Development at AECL. In Proceedings of the OECD/NEA/CSNI Workshop on the Implementation of Hydrogen Mitigation Techniques, Winnipeg, MB, Canada, 13–15 May 1996. [Google Scholar]
- Passive Autocatalytic Recombiner (PARs), SNC-LAVALIN AECL. 2021. Available online: www.snclavalin.com/nuclear (accessed on 3 April 2021).
- Park, J.W.; Koh, B.R.; Suh, K.Y. Demonstrative Testing of Honeycomb Passive Autocatalytic Recombiner for Nuclear Power Plant. Nucl. Eng. Des. 2011, 241, 4280–4288. [Google Scholar] [CrossRef]
- Kim, C.H.; Sung, J.J.; Ha, S.J.; Yeo, I.S. Analysis Method for the Design of a Hydrogen Mitigation System with Passive Autocatalytic Recombiners in OPR1000. In Proceedings of the 19th Pacific Basin Nuclear Conference and 38th Annual Student Conference of the Canadian Nuclear Society and Canadian Nuclear Association, Vancouver, BC, Canada, 24–28 August 2014. [Google Scholar]
- Reinecke, E.-A.; Bentaib, A.; Kelm, S.; Jahn, W.; Meynet, N.; Caroli, C. Open Issues in the Applicability of Recombiner Experiments and Modeling to Reactor Simulations. Prog. Nucl. Energy 2010, 52, 136–147. [Google Scholar] [CrossRef]
- Arnould, F.; Bachellerie, E.; Auglaire, M.; De Boeck, B.; Braillard, O.; Eckardt, B.; Ferroni, F.; Moffett, R.; Van Goethem, G. State of the Art on Hydrogen Passive Autocatalytic Recombiner. In Proceedings of the 9th International Conference on Nuclear Engineering, Nice, France, 8–12 April 2001. [Google Scholar]
- Simon, B.; Reinecke, E.-A.; Kubelt, C.; Allelein, H.-J. Start-up Behaviour of a Passive Auto-catalytic Recombiner under Counter flow conditions: Results of a first orienting experimental Study. Nucl. Eng. Des. 2013, 278, 317–322. [Google Scholar] [CrossRef]
- Reinecke, E.-A.; Kelm, S.; Heidelberg, D.; Klauck, M.; Steffen, P.-M.; Alleleina, H.-J. Modeling the start-up behavior of passive auto-catalytic recombiners in REKO-DIREKT. In Proceedings of the 8th European Review Meeting on Severe Accident Research—ERMSAR-2017, Warsaw, Poland, 16–18 May 2017. [Google Scholar]
- Gupta, S.; Kanzleiter, T.; Pos, G. Passive Autocatalytic Recombiners (PAR) Induced Ignition and the Resulting Hydrogen Deflagration Behaviour in LWR Containments. In Proceedings of the NURETH-16, Chicago, IL, USA, 30 August–4 September 2015. [Google Scholar]
- Chakraborty, A.; Reinecke, E.-A.; Meynet, N.; Bentaib, A.; Chaumeix, N.; Allelein, H.-J. Investigation of Ignition Characteristics of Passive Autocatalytic Recombiners. In Proceedings of the ICAPP 2017, Kyoto, Japan, 24–28 April 2017. [Google Scholar]
- Gardner, L.; Liang, Z.; Clouthier, T.; MacCoy, R. A Large-scale Study on the Effect of Ambient Conditions on Hydrogen Recombiner-Induced Ignition. Int. J. Hydrog. Energy 2021, 46, 12594–12604. [Google Scholar] [CrossRef]
- Kim, J.; Hong, S.; Park, K.H.; Kim, J.H.; Oh, J.Y. Experimental Study on a Hydrogen Stratification Induced by PARs Installed in a Containment. Energies 2020, 13, 5552. [Google Scholar] [CrossRef]
- Schmidt, E.; Freitag, M. Passive Autocatalytic Recombiner Operation under Counter Current Flow Conditions (Test Series HR-46–HR-50); Reactor Safety Research Project 1501516; OECD-NEA: Paris, France, 2017. [Google Scholar]
- Blanchat, T.K.; Malliakos, A.C. Testing a Passive Autocatalytic Recombiner in the Surtsey Facility. Nucl. Technol. 2000, 129, 356–373. [Google Scholar] [CrossRef] [Green Version]
- Klauck, M.; Reinecke, E.-A.; Allelein, H.-J. Effect of par deactivation by carbon monoxide in the late phase of a severe accident. Ann. Nucl. Energy 2021, 151, 107887. [Google Scholar] [CrossRef]
- Meynet, N.; Bentaib, A.; Giovangigli, V. Impact of Oxygen Starvation on Operation and Potential Gas-phase Ignition of Passive Auto-catalytic Recombiners. Combust. Flame 2014, 161, 2192–2202. [Google Scholar] [CrossRef]
- Kanzleiter, T.; Gupta, S.; Fischer, K.; Ahrens, G.; Langer, G.; Kühnel, A.; Poss, G.; Langrock, G.; Funke, F. Hydrogen and Fission Product Issues Relevant for Containment Safety Assessment under Severe Accident Conditions; Reactor Safety Research Project 1501326; OECD-NEA: Paris, France, 2010. [Google Scholar]
- Gupta, S.; Poss, G.; Freitag, M.; Schmidt, E.; Colombet, M.; Von Laufenberg, B.; Kuhnel, A.; Langer, G.; Funke, F.; Langrock, G.; et al. Aerosol and Iodine Issues and Hydrogen Mitigation under Accidental Conditions in Water Cooled Reactors; Reactor Safety Research Project 1501420; OECD-NEA: Paris, France, 2015. [Google Scholar]
- Kim, J.; Hong, S.; Park, K.H.; Kim, J.H.; Oh, J.Y. Experimental Study on the Grid-Type Passive Auto-Catalytic Recombiner for Hydrogen Mitigation in a Containment; KAERI Technical Report, TR-9069; KAERI: Daejeon, Korea, 2021. [Google Scholar]
- Korea Hydro and Nuclear Power Company (KHNP). Preliminary Safety Analysis Report for Shin-Ulchin 1 and 2 Nuclear Power Plants; Korea Hydro and Nuclear Power Company (KHNP): Gyeongju, Korea, 2007. [Google Scholar]
- Na, Y.S.; Hong, S.; Hong, S.W. Commissioning of the SPARC Test Facility; KAERI Technical Report, TR-6392; KAERI: Daejeon, Korea, 2016. [Google Scholar]

























Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Hong, S.; Park, K.-H.; Kim, J.-H.; Oh, J.-Y. Experimental Study on Hydrogen Recombination Characteristics of a Passive Autocatalytic Recombiner during Spray Operation. Hydrogen 2022, 3, 197-217. https://doi.org/10.3390/hydrogen3020013
Kim J, Hong S, Park K-H, Kim J-H, Oh J-Y. Experimental Study on Hydrogen Recombination Characteristics of a Passive Autocatalytic Recombiner during Spray Operation. Hydrogen. 2022; 3(2):197-217. https://doi.org/10.3390/hydrogen3020013
Chicago/Turabian StyleKim, Jongtae, Seongho Hong, Ki-Han Park, Jin-Hyeok Kim, and Jeong-Yun Oh. 2022. "Experimental Study on Hydrogen Recombination Characteristics of a Passive Autocatalytic Recombiner during Spray Operation" Hydrogen 3, no. 2: 197-217. https://doi.org/10.3390/hydrogen3020013
APA StyleKim, J., Hong, S., Park, K.-H., Kim, J.-H., & Oh, J.-Y. (2022). Experimental Study on Hydrogen Recombination Characteristics of a Passive Autocatalytic Recombiner during Spray Operation. Hydrogen, 3(2), 197-217. https://doi.org/10.3390/hydrogen3020013




