Abstract
Mount Falakro in Northern Greece historically hosted populations of the Balkan-endemic butterfly Polyommatus orphicus and its larval host plant Onobrychis alba. In this study, we surveyed six historically confirmed localities during the peak flight period of P. orphicus in 2024, but neither the butterfly nor the host plant were detected. While the historical data on both species are scarce and often imprecise, our field observations indicate severe habitat degradation, dominated by overgrazing and suspected climate-driven shifts. Habitat conditions were assessed qualitatively, with special attention to limestone substrates previously known to support O. alba. Although definitive absence cannot be statistically confirmed, the lack of detection in previously occupied sites raises urgent concerns about possible local extinction. Our findings suggest that both species may already be extirpated from parts of their former range. This case study underscores the conservation relevance of absence data and highlights the importance of site-based monitoring in mountainous ecosystems undergoing rapid environmental change. Long-term surveys, regulated grazing, and post-disturbance habitat restoration are urgently needed to clarify the conservation status of these species and guide future management strategies.
1. Introduction
Biodiversity loss in mountainous ecosystems is accelerating under the combined pressures of climate change, land-use alteration, and intensified grazing regimes. Endemic insects, especially habitat specialists with narrow ecological tolerances, are among the most vulnerable to these disturbances [1]. Butterflies, in particular, are widely used as indicators of environmental change due to their sensitivity to microhabitat quality, host plant availability, and phenological shifts driven by climate variability [2,3].
Polyommatus orphicus Kolev, 2005 (Lepidoptera, Lycaenidae), known as Kolev’s anomalous blue, is a little-known Balkan endemic butterfly belonging to the subgenus Agrodiaetus. It is restricted to open, rocky grasslands, often on limestone substrates, and depends exclusively on its larval host plant Onobrychis alba Waldst. & Kit. (Fabaceae), a species typically found in xeric calcareous habitats [4,5]. The butterfly lays its eggs on the senescent stems of O. alba, and the hatching larvae likely enter diapause, relying on the annual sprouting of the plant to complete their development [6,7,8]. This tightly coupled life cycle suggests high vulnerability to changes in vegetation phenology or disturbance to host plant populations. Therefore, it is s assessed as Endangered (EN) at both the European and national levels, underscoring its high conservation priority in Greece [9,10].
Although originally believed to be endemic to the Greek-Bulgarian border region of the Rhodope Mountains, P. orphicus has recently been recorded in Albania and North Macedonia [5,11], and its occurrence in other countries of the northern Balkans is theoretically possible [12,13], suggesting a wider but still fragmented distribution. The Greek population is considered localized and potentially declining, with previous records from Falakro Mountain and nearby sites [9]. However, these records are often dated and lack precise geolocation, complicating current status assessments.
The survival of P. orphicus relies on the availability of its host plant. However, O. alba is highly sensitive to drought, as it depends on timely germination and flowering to complete its life cycle. Increased grazing pressure in combination with climatic extremes can further exacerbate habitat degradation, leaving host plants and dependent species vulnerable to local extinction [14,15]. For example, overgrazing has been shown to reduce seed production and plant recruitment in Mediterranean grasslands, directly impacting butterfly species relying on these plants for larval development [16].
Mountains serve as climate refugia for many taxa, but the upward shift in suitable habitats caused by warming is a growing concern. Overgrazing, often transitioning from traditional livestock grazing to more intensive practices, can degrade montane ecosystems, reducing plant diversity and cover [17,18]. Wildfires further threaten these ecosystems by altering soil structure and destroying both flora and fauna, including the seed banks critical for perennials like O. alba. For instance, research indicates that in cold desert regions, fire can significantly alter seed bank species composition, with effects persisting for over 30 years post-fire [19]. Similarly, in Mediterranean ecosystems, frequent fires have been shown to deplete seed availability for species relying on long-lived soil seed banks, thereby influencing plant community dynamics [20]. These alterations in the seed bank and soil structure have cascading effects on higher trophic levels, including larval host specialists such as P. orphicus. The destruction of specific host plants, like O. alba, due to fire-induced seed bank depletion, directly threatens the survival of these monophagous larvae. Moreover, studies have demonstrated that wildfires can disrupt essential pollination processes, increasing the risk of local extinctions for both plants and their associated larvae [21]. Collectively, these findings underscore the vulnerability of specialized plant-insect interactions to wildfire disturbances, emphasizing the need for targeted conservation strategies to protect these intricate ecological relationships.
In 2024, we conducted a field survey targeting known and ecologically plausible localities of P. orphicus and O. alba on Falakro Mountain The aim of this study was to investigate the current presence of both P. orphicus and its larval host plant in selected montane habitats, and to document the environmental conditions of these sites in order to assess potential threats to their persistence.
2. Materials and Methods
2.1. Study Area
This study was conducted on Mount Falakro, located in Eastern Macedonia, northern Greece. Historically referred to as Boz Dağ (Turkish for “cold mountain”), Falakro forms the westernmost part of the Rhodope mountain range (Figure 1). The mountain is characterized by steep limestone slopes, rocky outcrops, and a mosaic of alpine and subalpine grasslands and beech (Fagus sylvatica) forests. The peak, Prophet Elias, reaches 2232 m.
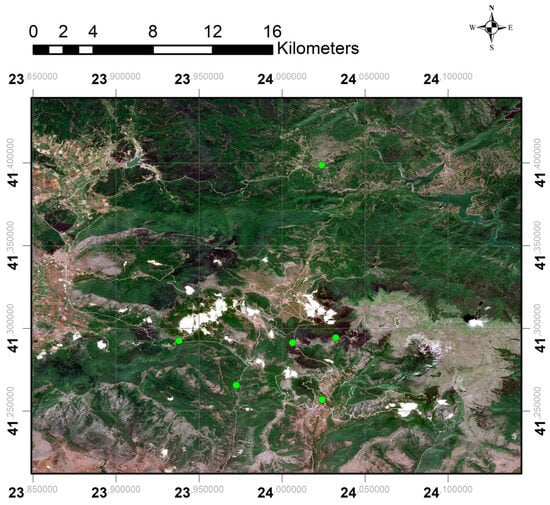
Figure 1.
Map of the study area in Falakro Mountain based on Sentinel-2 L2A imagery acquired on 11 June 2024, sourced from the Copernicus Data Space Ecosystem, referenced in WGS 84 (EPSG:4326). Precise coordinates of sampling sites are not disclosed for conservation reasons.
The study area lies within the Falakro Mountain Natura 2000 network, designated both as a Site of Community Interest (SCI; GR1140004) under the Habitats Directive and as a Special Protection Area (SPA; GR1140009) under the Birds Directive. Vegetation types range from shrublands and alpine meadows at higher elevations to broadleaf and coniferous forests at lower altitudes. The climate is Mediterranean with continental influence. Winter temperatures range from −15 °C to −5 °C (extremes down to −26 °C), while summer temperatures range from 5 °C to 20 °C. Snowfall is frequent between October and April and may occasionally extend into June (Hellenic National Meteorological Service).
2.2. Survey Design and Methods
The survey was conducted between 20 June and 25 July 2024, targeting the expected adult flight period of P. orphicus and the flowering season of O. alba. A total of six sites were selected based on historical occurrence, habitat suitability, presence of limestone substrates, and biodiversity records covering a range of montane grassland and shrubland habitats. Site 1 comprised open calcareous grassland interspersed with Onobrychis patches; Site 2 was dominated by low shrubs with scattered herbaceous vegetation; Site 3 was a mosaic of grassland and rocky outcrops; Site 4 featured grazed subalpine meadows; Site 5 consisted of lightly grazed montane pastures bordered by Quercus scrub; and Site 6 included abandoned pasture undergoing natural succession with tall grasses. The minimum distance between nearest neighboring sites was approximately 2 km (SD ± 0.5) so that each site effectively represents an independent sampling unit, and permanent transect routes were established. Selection criteria included:
- Historical butterfly and plant records from literature [7,22];
- Occurrence data from GBIF and unpublished botanical surveys from the Laboratory of Systematic Botany and Phytogeography (AUTH);
- Habitat classifications from national monitoring projects (1999–2001 and 2014–2015)
- Geological maps emphasizing limestone substrates.
Each site was visited thrice. Surveys were carried out periodically but were interrupted between 17 and 23 July due to an uncontrolled wildfire that burned approximately 1100 hectares before being extinguished by rainfall. At each site, 60 min timed visual searches were carried out for adult butterflies and O. alba; a transect was walked at a steady pace under favorable conditions (sunny weather, wind < 3 Bft, temperature > 18 °C) that were suitable for butterfly activity [23], recording all butterflies observed above and in front of the observer along a standardized length and width of 300 × 5 m [24]. Surveys also included additional suitable habitats beyond the predefined locations. Habitat conditions (e.g., drought stress, grazing signs) and basic environmental descriptors, including air temperature, cloud cover, wind category, relative ground cover by grasses/forbs/shrubs, substrate, slope, aspect, elevation, visible grazing intensity and presence of Fabaceae inflorescences were noted.
Butterfly identification followed field guides [4,7]. Photographs were taken with a Canon EOS R50 macro setup. In difficult cases, non-lethal in situ genitalia examination was used per Lafranchis, whereby light abdominal pressure revealed genitalia, which were inspected using a magnifying lens [4]. All butterflies were released unharmed, and no specimens were killed.
In parallel, we searched for O. alba individuals within and around the butterfly survey zones. Searches covered a minimum 200 m radius from historical GPS points, recording all individuals or clusters, their phenological stage (vegetative, flowering, fruiting), and associated plant communities. Special care was taken to avoid trampling or disturbing vegetation, especially due to the potential presence of immature insect stages. No plant material was collected. All fieldwork was conducted in compliance with Greek legislation on biodiversity protection (Law 3937/2011) and relevant ethical standards. Research activities were carried out under permit No. ΥΠΕΝ/ΔΔΔ/12345/22-03-2024, issued by the Ministry of Environment and Energy of Greece.
Habitat quality was assessed qualitatively based on visible indicators of degradation (soil erosion, trampling, grazing intensity, presence of invasive species, shrub encroachment). Grazing pressure was estimated by counting livestock presence and signs (dung, hoofprints) along 100 m transects within each site. Although no quantitative vegetation cover measurements were conducted, observations were made on floral abundance and grassland structure to provide context for potential decline drivers. Temperature and precipitation data for June–July 2024 were obtained from the Hellenic National Meteorological Service (station: Drama) and compared with 10-year climatic averages.
3. Results
During the field surveys, no individuals of Polyommatus orphicus nor flowering or vegetative individuals of its host plant Onobrychis alba were recorded in any of the six surveyed sites on Mount Falakro. Despite high observer effort (~18 h total), the absence of both species persisted across varied altitudes (1300–2100 m.a.s.l.) and microhabitats.
Low-elevation sites were characterized by visibly stressed herbaceous vegetation, with widespread drought symptoms including premature withering and desiccation of Fabaceae species. The survey followed an unusually dry/cold season transition (2024 cumulative precipitation at the Drama station ≈ 505.8 mm vs. a 2009–2017 mean of 660.2 mm; National Observatory of Athens; Institute of Environmental Research and Sustainable Development) that likely compressed spring phenology. According to the National Observatory of Athens, precipitation between October 2023 and April 2024 was only 50% of the 2012–2022 average. Concurrently, the European Centre for Medium-Range Weather Forecasts identified summer 2024 as the hottest since 1993. These anomalies manifested in the field as early senescence or complete lack of flowering in Fabaceae species, particularly those presumed to be O. alba. In several cases, legumes displayed no stem elongation or floral development, suggesting failed reproduction (Figure 2). These observations align with findings in legume species, where drought stress significantly reduces plant productivity through diminished growth, lower photosynthetic activity, impaired carbon assimilation, and alterations in flowering and reproductive success [25,26,27,28].

Figure 2.
Close-up photo of a legume without floral development, showing drought impact. The pictured legume shows truncated apical stems consistent with recent grazing, along with the widespread desiccation of senescent shoots.
Higher-elevation sites appeared greener but were heavily impacted by recent livestock activity, particularly cattle and equines, resulting in trampled vegetation and grazed patches (Figure 3). Since plants of the genus Onobrychis are highly palatable to sheep, intensive grazing restricts the growth and spread of O. alba, sometimes leading to the plant’s disappearance [6]. While traditional sheep grazing is beneficial and can help maintain open landscapes, uncontrolled and overgrazing can have devastating effects on butterflies and other insects such as bees, an issue increasingly reported across various parts of Europe [29,30,31,32]. Although overgrazing by sheep is not common in Greece, the transition from traditional extensive livestock farming (sheep and goats) to large cattle farming has resulted in plants being heavily grazed and trampled by cattle [9]. Over recent years, cattle farming has increasingly replaced traditional sheep and goat pastoralism in the Falakro region, and equines (primarily mules) are now commonly used for forest product transport in managed woodland areas. Current grazing can cause trampling and selective foraging damage to herbaceous plants. Historically, however, the region also supported wild grazers (e.g., equids, bovids, cervids and caprines), and the arrival of domestic sheep and goats in the Neolithic brought a different grazing regime. While legumes likely adapted to moderate grazing, poorly managed cattle and horse grazing may exert greater disturbance, particularly if seasonal timing overlaps with host plant flowering. Management that mimics intermediate disturbance (rotational, low-intensity stocking; seasonal rests) is more likely to maintain O. alba stands than prolonged, high-pressure grazing.

Figure 3.
Field photos showing heavily grazed vegetation.
In the locus typicus area of P. eleniae (a junior synonym of P. orphicus) in Granitis [33] (Figure 4), site conditions were further compromised by quarry infrastructure and ongoing logging [10]. No evidence of O. alba or P. orphicus was found despite extensive habitat searching in surrounding microhabitats and adjacent limestone slopes. The failure to detect even a single individual, despite intensive effort, suggests a marked local decline or possible extirpation of both taxa from this locality.

Figure 4.
Locus typicus of P. eleniae in the summer of 2024, showing severe drought. P. eleniae is currently treated as a junior synonym of P. orphicus.
4. Discussion
The complete lack of Polyommatus orphicus and Onobrychis alba detections across all six surveyed sites is cause for concern, although it cannot be interpreted as definitive evidence of local extinction. The limited temporal window of the survey (June–July 2024) represents a snapshot in time, and detection failure could result from temporal mismatch, population fluctuations, or interannual variability. One optimistic explanation is temporal mismatch between survey dates and species’ peak activity. The exceptionally hot and dry conditions in 2024 may have advanced O. alba flowering and P. orphicus flight periods into early June, before surveys began. Such shifts have been documented in other Onobrychis-feeding lycaenids [34,35]. However, the consistent pattern of non-detection across sites with historically suitable habitat suggests more than a sampling artifact. Future work should include repeated visits from late May onward to account for phenological variability.
Environmental degradation observed in situ, particularly signs of prolonged drought and intensive grazing pressure, supports the hypothesis of habitat deterioration. At low elevations, herbaceous vegetation displayed extreme desiccation, and Fabaceae species exhibited stunted growth or failed flowering. These patterns are consistent with known drought impacts on legumes, which affect photosynthesis, biomass allocation, and reproductive output [36,37].
Drought and rising temperatures are among the most significant consequences of climate change, directly affecting butterflies such as P. orphicus. High temperatures can negatively impact larval development and survival by reducing the availability of suitable host plants due to dehydration. Across survey dates, Fabaceae inflorescences were scarce to absent at most sites, consistent with advanced senescence and drought-compressed phenology. We therefore consider phenological asynchrony between O. alba flowering and P. orphicus flight as the most parsimonious explanation for non-detection in 2024, while not excluding localized plant decline. Additionally, drought decreases soil moisture and plant regeneration, further affecting the habitats that support the species. Butterflies in areas experiencing frequent droughts show significant population declines, as extreme events disrupt their natural life cycles [38,39].
Climate change forces many butterfly species to migrate to higher elevations or latitudes in search of cooler and more humid conditions, leading to shifts in their distribution. However, the limited availability of suitable habitats at higher elevations increases competition between species and the risk of local extinctions. Rising temperatures could potentially cause asynchrony between the flight period of P. orphicus and the flowering of its host plants, which in turn may influence reproductive success. While such phenological mismatches have been documented in other plant–insect systems, no direct measurements were conducted in this study to confirm this pattern [40,41,42]. Climate change can alter plant life cycles, including flowering and fruiting periods, causing mismatches with pollinators and impacting reproductive success. This can have severe consequences for plants dependent on specific pollinators, particularly in high-altitude ecosystems where biodiversity is already limited [43,44]. Because P. orphicus is larval-host dependent on O. alba, any reduction in flowering/seed set that lowers stand persistence will indirectly depress butterfly oviposition resources, independent of adult nectar availability. Data indicate that climate change is already contributing to biodiversity loss among butterflies with restricted geographic ranges, making P. orphicus particularly vulnerable [2,45].
Moreover, at higher elevations—where thermal conditions should be more favorable—the presence of cattle and equines likely contributes to trampling and overgrazing, affecting both the plant community and larval host availability. This shift from traditional sheep grazing to intensive cattle use has been noted in the region and likely exacerbates pressures on specialist flora and fauna [9]. Of course, climate change impacts plants at all elevations. However, at higher elevations, certain conditions may mitigate some of these effects. Still, plants in such environments are adapted to specific climatic conditions, and climate change could disrupt these balances [46,47]. Studies have shown that climate change may cause shifts in plant distribution by elevation, with some species moving higher in search of more favorable conditions [48]. However, the availability of suitable habitats at higher elevations is limited, which can lead to increased competition between species and biodiversity loss [49]. Additionally, plants at higher elevations remain vulnerable to other impacts, such as changes in ecological interactions and habitat loss [50]. Understanding these effects is crucial for developing conservation and adaptation strategies.
Another climate change-related phenomenon expected to have devastating consequences for the butterfly population is wildfires. At the time of sampling, a wildfire was raging on Falakro Mountain, burning approximately 1100 hectares. Although a natural part of Mediterranean ecosystems, wildfires may become more frequent and severe under climate change. Fires dramatically affect butterfly communities by rapidly destroying habitats and host plants. O. alba is particularly vulnerable to wildfires, as soil and seed destruction can drastically reduce its regeneration capacity. While many perennial plants have evolved resistance mechanisms such as producing a high number of seeds or growing rapidly under favorable conditions [51], frequent and intense fires can surpass these strategies, leading to seed mortality or reduced viability [52]. This destruction of the host plant limits food availability for butterfly larvae, leading to population declines. Additionally, post-fire ecosystem recovery is often slow, leaving P. orphicus with limited available habitats [53,54]. In some cases, controlled post-fire grazing could maintain early-successional calcareous grasslands beneficial to Onobrychis species, but its role requires careful site-specific evaluation.
The survey relied on presence-absence detection through visual methods but did not incorporate formal occupancy modeling or environmental covariates (e.g., soil analysis, climate layers), which limits the strength of the absence inference. While such techniques would add statistical rigor, the observed habitat degradation, consistent detection failure across suitable habitat, and loss of flowering phenology in O. alba warrant conservation attention.
Future work should incorporate repeat surveys across multiple years, ecological niche modeling based on historic and current bioclimatic variables, and detection probability estimation to refine conservation conclusions. Integrating climatic data (e.g., from WorldClim or ECMWF), land-use layers, and grazing intensity maps would allow for a stronger linkage between species patterns and environmental pressures.
Despite methodological limitations, this study raises a red flag for the conservation status of P. orphicus and its host plant in Greece. It supports the need for long-term monitoring programs, population modeling, and habitat restoration efforts to safeguard these threatened montane species.
5. Conclusions
In this study, we documented the complete non-detection of the endemic butterfly Polyommatus orphicus and its larval host plant Onobrychis alba in historically confirmed localities on Mount Falakro, despite multiple site visits and targeted surveys. While we cannot conclusively demonstrate species extinction based on these absences alone, our findings raise significant concern about a potential local extirpation event.
The concurrent absence of both butterfly and host plant highlights the possibility of compounded ecological pressures, including habitat degradation, overgrazing, and climatic extremes. These threats are well-documented drivers of biodiversity decline in montane ecosystems, which are particularly vulnerable due to their spatial isolation and narrow ecological niches.
We recommend the implementation of long-term monitoring programs focused on both species and their habitat quality, including the establishment of baseline phenological data and repeat surveys across seasons and years. In parallel, adaptive grazing management, fire prevention strategies, and legal protection of remnant limestone habitats should be prioritized. Our case study underscores the urgent need to reassess and protect neglected biodiversity hotspots in the Balkans before losses become irreversible.
Author Contributions
Conceptualization, A.T. and C.C.; methodology, A.T. and C.C.; investigation, A.T. and C.C.; writing—original draft preparation, A.T.; writing—review and editing, A.T. and C.C.; project administration, A.T.; funding acquisition, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was implemented as part of a scholarship awarded by WWF Greece, within the framework of the “Greek Wildlife Alliance” initiative, grant number 1169/22-12-2023.
Institutional Review Board Statement
Not applicable. The study involved non-destructive field observations and brief in-hand examination of a subset of adult males, which were immediately released. No specimens were collected or euthanized.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (A.T.) upon reasonable request.
Acknowledgments
We are thankful to the Hellenic Botanical Society Ioannis Tsiripidis for the dataset of the O. alba localities. The authors gratefully acknowledge the Ministry of Environment and Energy of Greece for granting the research permit (Permit No. ΥΠΕΝ/ΔΔΔ/12345/22-03-2024, issued 22 March 2024), which ensured that all field activities complied with national legislation and ethical standards.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Settele, J.; Kudrna, O.; Harpke, A.; Kühn, I.; Van Swaay, C.; Verovnik, R.; Warren, M.; Wiemers, M.; Hanspach, J.; Hickler, T.; et al. Climatic Risk Atlas of European Butterflies. BioRisk 2008, 1, 1–712. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and Evolutionary Responses to Recent Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Forrest, J.R. Complex Responses of Insect Phenology to Climate Change. Curr. Opin. Insect Sci. 2016, 17, 49–54. [Google Scholar] [CrossRef]
- Lafranchis, T. Butterflies of Europe: New Field Guide and Key; Diatheo: Paris, France, 2004. [Google Scholar]
- Cuvelier, S.; Parmentier, L.; Qirinxhi, X.; Paparisto, A. Butterflies of Albania New Data and Going Online (Lepidoptera: Papilionoidae). Bul. I Shk. Te Nat. Tirana Univ. 2023, 32, 5–31. [Google Scholar]
- Lafranchis, T.; Gil, T.F.; Lafranchis, A. New Data on the Ecology of 8 Taxa of Agrodiaetus Hübner, 1822 from Greece and Spain: Hostplants, Associated Ants and Parasitoids. Atalanta 2007, 38, 189–197. [Google Scholar]
- Pamperis, L.N. The Butterflies of Greece, 2nd ed.; Editions Pamperis: Athens, Greece, 2009. [Google Scholar]
- Lafranchis, T. Notes on the Biology of Some Butterflies in Greece (Lepidoptera: Papilionoidea). Entomol. Gaz. 2019, 70, 113–134. [Google Scholar] [CrossRef]
- Tzortzakaki, O.; Zografou, K.; Pamperis, L. Polyommatus orphicus. In The Greek Red List of Threatened Species; 2024. Available online: https://redlist.necca.gov.gr/ (accessed on 4 September 2025).
- IUCN. Polyommatus orphicus. In The IUCN Red List of Threatened Species 2025: E.T213648831A201853807; Van Swaay, C., Ellis, S., Warren, M., Eds.; IUCN: Gland, Switzerland, 2023. [Google Scholar] [CrossRef]
- Kudrna, O. Distribution Atlas of Butterflies in Europe; Ges. für Schmetterlingsschutz: Halle (Saale), Germany, 2011. [Google Scholar]
- Vishnevskaya, M.S.; Saifitdinova, A.F.; Lukhtanov, V.A. Karyosystematics and Molecular Taxonomy of the Anomalous Blue Butterflies (Lepidoptera, Lycaenidae) from the Balkan Peninsula. Comp. Cytogenet. 2016, 10, 1–85. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, L.; Vila, R.; Lukhtanov, V. Integrative Analysis Reveals Cryptic Speciation Linked to Habitat Differentiation within Albanian Populations of the Anomalous Blues (Lepidoptera, Lycaenidae, Polyommatus Latreille, 1804). Comp. Cytogenet. 2022, 16, 211–242. [Google Scholar] [CrossRef]
- Harrison, P.A.; Berry, P.M.; Butt, N.; New, M. Modelling Climate Change Impacts on Species’ Distributions at the European Scale: Implications for Conservation Policy. Environ. Sci. Policy 2006, 9, 116–128. [Google Scholar] [CrossRef]
- Dirnböck, T.; Essl, F.; Rabitsch, W. Disproportional Risk for Habitat Loss of High-altitude Endemic Species under Climate Change. Glob. Change Biol. 2011, 17, 990–996. [Google Scholar] [CrossRef]
- Rundlöf, M.; Nilsson, H.; Smith, H.G. Interacting Effects of Farming Practice and Landscape Context on Bumble Bees. Biol. Conserv. 2008, 141, 417–426. [Google Scholar] [CrossRef]
- Papanastasis, V.P.; Mantzanas, K.; Dini-Papanastasi, O.; Ispikoudis, I. Traditional Agroforestry Systems and Their Evolution in Greece. In Agroforestry in Europe; Rigueiro-Rodróguez, A., McAdam, J., Mosquera-Losada, M.R., Nair, P.K.R., Eds.; Advances in Agroforestry; Springer: Dordrecht, The Netherlands, 2009; Volume 6, pp. 89–109. [Google Scholar] [CrossRef]
- Chen, M.; Niu, X.; Li, W.; Liu, P.; Zhang, C.; Huang, Z. Effects of Grazing Disturbances on Soil Seed Bank Diversity in Two Vegetation Types. Glob. Ecol. Conserv. 2024, 53, e03047. [Google Scholar] [CrossRef]
- Hosna, R.K.; Reed, S.C.; Faist, A.M. Long-term Relationships between Seed Bank Communities and Wildfire across Four North American Desert Sites. Ecosphere 2023, 14, e4398. [Google Scholar] [CrossRef]
- Plumanns Pouton, E.; Kasel, S.; Penman, T.D.; Swan, M.; Kelly, L.T. Soil Seedbanks Are Shaped by the Timing of Fires in a Mediterranean-type Ecosystem. J. Appl. Ecol. 2024, 61, 2335–2349. [Google Scholar] [CrossRef]
- Brown, J.; York, A.; Christie, F.; McCarthy, M. Effects of Fire on Pollinators and Pollination. J. Appl. Ecol. 2017, 54, 313–322. [Google Scholar] [CrossRef]
- Quézel, P. Contribution à l’étude Phytosociologique Des Pelouses Ecorchées Culminales Du Massif Du Falakron. Bios 1989, 1, 187–193. [Google Scholar]
- Pollard, E.; Yates, T.J. Monitoring Butterflies for Ecology and Conservation: The British Butterfly Monitoring Scheme; Conservation Biology Series; Chapman & Hall: London, UK, 1993. [Google Scholar]
- Pollard, E. A Method for Assessing Changes in the Abundance of Butterflies. Biol. Conserv. 1977, 12, 115–134. [Google Scholar] [CrossRef]
- Croser, J.S.; Ahmad, F.; Clarke, H.J.; Siddique, K.H.M. Utilisation of Wild Cicer in Chickpea Improvement—Progress, Constraints, and Prospects. Aust. J. Agric. Res. 2003, 54, 429. [Google Scholar] [CrossRef]
- Samarah, N.H.; Haddad, N.; Alqudah, A.M. Yield Potential Evaluation in Chickpea Genotypes under Late Terminal Drought in Relation to the Length of Reproductive Stage. Ital. J. Agron. 2009, 4, 111–117. [Google Scholar] [CrossRef]
- Mondal, M.M.A.; Fakir, S.A.; Juraimi, A.S.; Hakim, M.A.; Islam, M.M.; Shamsuddoha, A.T.M. Effects of Flowering Behavior and Pod Maturity Synchrony on Yield of Mungbean [Vigna radiata (L.) Wilczek]. Aust. J. Crop Sci. 2011, 5, 945–953. [Google Scholar]
- Duan, D.; Jiang, F.; Lin, W.; Tian, Z.; Wu, N.; Feng, X.; Chen, T.; Nan, Z. Effects of Drought on the Growth of Lespedeza davurica through the Alteration of Soil Microbial Communities and Nutrient Availability. J. Fungi 2022, 8, 384. [Google Scholar] [CrossRef]
- Kruess, A.; Tscharntke, T. Grazing Intensity and the Diversity of Grasshoppers, Butterflies, and Trap-Nesting Bees and Wasps. Conserv. Biol. 2002, 16, 1570–1580. [Google Scholar] [CrossRef]
- Potts, S.G.; Woodcock, B.A.; Roberts, S.P.M.; Tscheulin, T.; Pilgrim, E.S.; Brown, V.K.; Tallowin, J.R. Enhancing Pollinator Biodiversity in Intensive Grasslands. J. Appl. Ecol. 2009, 46, 369–379. [Google Scholar] [CrossRef]
- European Commission; Directorate-General for the Environment; International Union for Conservation of Nature; Butterfly Conservation Europe. European Red List of Butterflies; European Commission: Brussels, Belgium, 2010. [Google Scholar]
- Verbrugge, L.N.H.; Bjarnason, G.; Fagerholm, N.; Magnussen, E.; Mortensen, L.; Olsen, E.; Plieninger, T.; Raymond, C.M.; Olafsson, A.S. Navigating Overgrazing and Cultural Values through Narratives and Participatory Mapping: A Socio-Cultural Analysis of Sheep Grazing in the Faroe Islands. Ecosyst. People 2022, 18, 289–302. [Google Scholar] [CrossRef]
- Coutsis, J.G.; Prins, J.D. A New Brown Polyommatus (Agrodiaetus) from Northern Greece (Lepidoptera: Lycaenidae). Phegea 2005, 33, 129–137. [Google Scholar]
- Šlancarová, J.; Bednářová, B.; Beneš, J.; Konvička, M. How Life History Affects Threat Status: Requirements of Two Onobrychis-Feeding Lycaenid Butterflies, Polyommatus damon and Polyommatus thersites, in the Czech Republic. Biologia 2012, 67, 1175–1185. [Google Scholar] [CrossRef]
- Sucháčková Bartoňová, A.; Konvička, M.; Marešová, J.; Bláhová, D.; Číp, D.; Skala, P.; Andres, M.; Hula, V.; Dolek, M.; Geyer, A.; et al. Extremely Endangered Butterflies of Scattered Central European Dry Grasslands Under Current Habitat Alteration. Insect Syst. Divers. 2021, 5, 6. [Google Scholar] [CrossRef]
- Chowdhury, J.; Karim, M.; Khaliq, Q.; Ahmed, A.; Khan, M. Effect of Drought Stress on Gas Exchange Characteristics of Four Soybean Genotypes. Bangladesh J. Agric. Res. 2016, 41, 195–205. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and Heat-Stress Effects on Seed Filling in Food Crops: Impacts on Functional Biochemistry, Seed Yields, and Nutritional Quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef]
- Wilson, R.J.; Maclean, I.M.D. Recent Evidence for the Climate Change Threat to Lepidoptera and Other Insects. J. Insect Conserv. 2011, 15, 259–268. [Google Scholar] [CrossRef]
- Oliver, T.H.; Isaac, N.J.B.; August, T.A.; Woodcock, B.A.; Roy, D.B.; Bullock, J.M. Declining Resilience of Ecosystem Functions under Biodiversity Loss. Nat. Commun. 2015, 6, 10122. [Google Scholar] [CrossRef]
- Donoso, I.; Stefanescu, C.; Martínez-Abraín, A.; Traveset, A. Phenological Asynchrony in Plant–Butterfly Interactions Associated with Climate: A Community-wide Perspective. Oikos 2016, 125, 1434–1444. [Google Scholar] [CrossRef]
- Posledovich, D.; Toftegaard, T.; Wiklund, C.; Ehrlén, J.; Gotthard, K. Phenological Synchrony between a Butterfly and Its Host Plants: Experimental Test of Effects of Spring Temperature. J. Anim. Ecol. 2018, 87, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Colom, P.; Ninyerola, M.; Pons, X.; Traveset, A.; Stefanescu, C. Phenological Sensitivity and Seasonal Variability Explain Climate-Driven Trends in Mediterranean Butterflies. Proc. R. Soc. B 2022, 289, 20220251. [Google Scholar] [CrossRef] [PubMed]
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M.V. Global Warming and the Disruption of Plant–Pollinator Interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef]
- Rafferty, N.E.; Ives, A.R. Effects of Experimental Shifts in Flowering Phenology on Plant-Pollinator Interactions: Experimental Shifts in Flowering Phenology. Ecol. Lett. 2011, 14, 69–74. [Google Scholar] [CrossRef]
- Forister, M.L.; McCall, A.C.; Sanders, N.J.; Fordyce, J.A.; Thorne, J.H.; O’Brien, J.; Waetjen, D.P.; Shapiro, A.M. Compounded Effects of Climate Change and Habitat Alteration Shift Patterns of Butterfly Diversity. Proc. Natl. Acad. Sci. USA 2010, 107, 2088–2092. [Google Scholar] [CrossRef]
- Dave, K.; Kumar, A.; Dave, N.; Jain, M.; Dhanda, P.S.; Yadav, A.; Kaushik, P. Climate Change Impacts on Legume Physiology and Ecosystem Dynamics: A Multifaceted Perspective. Sustainability 2024, 16, 6026. [Google Scholar] [CrossRef]
- Lepcha, P. Climate Change and Its Impact on Mountainous Plant Species: A Review. In Environmental Sciences; Kulshreshtha, S.N., Ed.; IntechOpen: London, UK, 2024; Volume 19. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.C.; Marquet, P.A.; De Ruffray, P.; Brisse, H. A Significant Upward Shift in Plant Species Optimum Elevation During the 20th Century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef]
- Engler, R.; Randin, C.F.; Thuiller, W.; Dullinger, S.; Zimmermann, N.E.; Araújo, M.B.; Pearman, P.B.; Le Lay, G.; Piedallu, C.; Albert, C.H.; et al. 21st Century Climate Change Threatens Mountain Flora Unequally across Europe: Climate Change Impacts on Mountain Florae. Glob. Change Biol. 2011, 17, 2330–2341. [Google Scholar] [CrossRef]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barančok, P.; Benito Alonso, J.L.; Coldea, G.; Dick, J.; Erschbamer, B.; Fernández Calzado, M.R.; et al. Continent-Wide Response of Mountain Vegetation to Climate Change. Nat. Clim. Change 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Sakhraoui, A.; Ltaeif, H.B.; Sakhraoui, A.; Villalba, J.J.; Castillo, J.M.; Rouz, S. Sainfoin (Onobrychis viciifolia) a Legume with Great Ecological and Agronomical Potential under Climate Change. J. Agric. Sci. 2024, 162, 307–331. [Google Scholar] [CrossRef]
- Enright, N.J.; Fontaine, J.B.; Bowman, D.M.; Bradstock, R.A.; Williams, R.J. Interval Squeeze: Altered Fire Regimes and Demographic Responses Interact to Threaten Woody Species Persistence as Climate Changes. Fron. Ecol. Environ. 2015, 13, 265–272. [Google Scholar] [CrossRef]
- Moreira, F.; Rego, F.C.; Ferreira, P.G. Temporal (1958–1995) Pattern of Change in a Cultural Landscape of Northwestern Portugal: Implications for Fire Occurrence. Landsc. Ecol. 2001, 16, 557–567. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. A Burning Story: The Role of Fire in the History of Life. BioScience 2009, 59, 593–601. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).