Abstract
Phytophthora species are known as water molds and are widespread in rivers and riparian habitats, but the distribution of these oomycetes in coastal and sea ecosystems is not well explored. The present study aims to investigate salt tolerance and potential to survive in marine environment of thirteen Phytophthora species, including P. citricola, P. plurivora, P. pseudosyringae, P. inundata, P. chlamydospora, P. gonapodyides, P. bilorbang, P. lacustris, P. pseudocryptogea, P. syringae, P. polonica, P. honggalleglyana, and P. gallica. The effect of varying concentrations of sodium chloride and the impact of sea water from the Black Sea and the Aegean Sea on mycelial growth, colony type, and formation of different morphological structures by Phytophthora species were studied. The tested isolates belong to different clades of the genus and members of clade 6 stand out with more extensive colony growth on media with elevated salt content compared to the growth on the control medium. A number of Phytophthora isolates produced morphological structures for sexual and/or asexual reproduction under salt stress conditions. The ability of the studied Phytophthora species to exist in marine environment is discussed.
1. Introduction
Only a few Phytophthora species have been isolated from marine ecosystems worldwide and knowledge about the distribution of the genus in saline habitats is limited. Phytophthora rhizophorae and P. estuarina have been recovered from mangrove swamps in Brazil and were determined as estuarine oomycetes [1,2]. The species P. insolita has been isolated also from a mangrove habitat from fallen senescent leaves in the Philippines [3,4]. A widespread infection caused by P. gemini on eelgrass in six countries across the North Atlantic Ocean and the Mediterranean Sea has been reported too [5]. More recently, P. gemini, P. inundata, and a new Phytophthora species P. chesapeakensis have been associated with disease on seagrasses and other salt marsh plants in several locations, including the Wadden Sea (Denmark), the Dutch Delta area (The Netherlands), Thau lagoon (France), Lindholmen (Sweden), and Chesapeake Bay (Virginia, USA) [6]. In addition, the first report for the occurrence of Phytophthora species in Alaska waters was documented lately [7]. A subsequent investigation by metabarcoding of the DNA extracted from the water, sediment, and tissue samples from eelgrass meadows in Alaska proved a wide distribution of P. gemini in the Izembek, Chignik, and Safety Lagoons [8]. No other Phytophthora species from coastal habitats have been described up to now and the most likely reason for this may be insufficient research. Therefore, a hypothesis that the distribution of Phytophthora in marine ecosystems is expected to be wider than currently known is supported by some authors [5,8], but further studies needed to be conducted to confirm or reject this assumption.
The salinity tolerance of some pathogen species from the genus Phytophthora has been explored previously. P. cryptogea and P. parasitica demonstrated the ability to produce sporangia and zoospores at salinity levels that are demanding to most crop plants [9]. The salinity stress of tomato plants before or after inoculation with P. parasitica resulted in an increase in root and crown rot severity [10]. Experimental data showed that saline soils lead to a reduction in zoospore release by the pathogen as well as their motility, but no inhibition of the disease has been observed. Wilkens and Field demonstrated that P. polymorphica tolerates a wide range of sea water salinities, but biomass and hyphal growth have been affected by certain salinity levels [11]. Based on the experimental data, the authors suggest that the species could be adapted to the estuarine habitat. Analyses of the survival and factors affecting the aggressiveness of one of the most devastating members of the genus Phytophthora, P. ramorum, showed that the pathogen can infect plant tissue at high salt concentrations [12]. Nevertheless the growth of P. ramorum has been negatively correlated with salt concentration (from 0 to 45 g/L), as the species tolerates even the highest tested salinity. Most of the studies using salt stress up to now are focused on the potential to manage diseases caused by Phytophthora and have been limited to a few species. Although some authors have suggested the adaptation of single Phytophthora isolates to environments with increased salt content, the salinity tolerance of most members of the genus is unknown.
The distribution of species on a global scale is closely linked to climate change. This particularly affects species that inhabit transitional zones. It is well known that the level of the world’s oceans has been rising every year for the last century and the rate of these changes is increasing over time [13]. Coastal areas are most affected by sea level rise. Frequent flooding also causes saltwater intrusion, which changes estuaries and freshwater areas. This affects biodiversity of species, including oomycetes, some of which thrive in aquatic habitats, while others are soil-born [14,15,16]. All of them are subject to salt stress, but regular flooding with saltwater is a prerequisite for the natural adaptation of species that are tolerant to salinity. However, some species may not be able to live in an environment with increased salinity, and this will lead to changes in the ecosystem.
The present study explores the ability of a number of Phytophthora species to survive at different salinity levels. The effect of varying concentrations of NaCl in a culture medium on the mycelial growth rate of 13 species was tested. The impact of sea water from the Black Sea and the Aegean Sea on the same oomycetes by using in vitro tests was also analyzed. The influence of salinity on the formation of different morphological structures was observed and documented. The Phytophthora species that have the potential to survival in marine habitats were determined.
2. Materials and Methods
2.1. Phytophthora Isolates
Sixteen Phytophthora isolates of thirteen diverse species were selected for the study. They are part of the oomycetes collected in last 10 years by a phytopathology team of the AgroBioInstitute, some of which have been reported for the first time in Bulgaria [17,18]. For the purpose of this experiment, Phytophthora species that are members of different clades of the genus were selected as follows: P. citricola (RPlov2016/40c), P. plurivora (RKal2016/80f), P. pseudosyringae (RVit2016/6d), P. inundata (RVel2021/119b), P. chlamydospora (RKal2016/80a), P. gonapodyides (RChOs2016/83a), P. bilorbang (RVel2021/119a), P. lacustris (RPlov2016/44a/1/; RVel2021/113d/2/), P. pseudocryptogea (RLKam2016/63b), P. syringae (RDobrod2016/82b), P. polonica (SVel2022/5b), P. honggalleglyana (RHar2016/55b/1/; RVel2021/120c/2/; SVel2022/5f/3/), and P. gallica (RKal2016/80b). Fourteen isolates were recovered from natural water sources from various locations throughout Bulgaria. Two isolates (P. polonica and P. honggalleglyana-3) originate from soil samples collected in a close distance to the Black Sea coast [19]. Four isolates (P. bilorbang, P. inundata, P. lacustris-1, and P. honggalleglyana-2) were recovered from a river estuary next to the Black Sea [20]. For the species P. lacustris and P. honggalleglyana, more than one isolate was included in the study, as they differ in their origin (isolated in a region near the sea coast or from inland).
2.2. In Vitro Tests for Salt Tolerance of Phytophthora Species
All Phytophthora isolates are maintained on CA medium (carrot agar; 16 g agar, 3 g CaCO3, 100 mL carrot juice/1L). Two variants of the salt tolerance test were applied. In the first one, the CA medium was supplemented with NaCl in four different concentrations (4.5‰, 9‰, 18‰, and 36‰). For the second experiment, the carrot agar medium was prepared with sea water instead of distilled water. Two types of sea water were tested, from the Black Sea and the Aegean Sea, as their salinity is about 18‰ and 36‰, respectively [21]. All Phytophthora isolates were cultivated on CA medium as the control and the six variants of salinity at 20 °C in the dark. Radial mycelial growth (mm) of the tested oomycetes was measured after 6 days of incubation. Each variant of the treatments was prepared in 3 replicates and experiments were performed 2 times. The statistical significance of the differences between the control and each variant in the conducted tests was assessed by two-tailed t-tests at probability level of p ≤ 0.01.
2.3. Morphology of Phytophthora Species at Salinity Stress
The effect of high salinity on colony type, growth, and formation of different morphological structures by Phytophthora species was studied. A spontaneous production of oogonia and chlamydospores during prolonged incubation (7 weeks) on control CA medium, CA supplemented with 18‰ and 36‰ NaCl, and CA medium prepared with water from the Black Sea and the Aegean Sea were investigated. For the observation of sporangia, all Phytophthora isolates were incubated on V8A (vegetable agar: 16 g agar, 3 g CaCO3, 100 mL Campbell’s V8 juice/1L) at 20 °C in the dark. Sporangia were produced from agar plugs (10 × 10 mm) of 5-day-old cultures on V8A that were flooded with non-sterile spring water and were incubated into Petri dishes at natural daylight and ambient temperature for 24–48 h [22]. Microscopic observation of morphological structures was performed under microscope at ×400 magnification (ZEISS Axio Imager A2; Carl Zeiss Micriscopy, Jena, Germany) with a digital camera (AxioCamERs 5S; Carl Zeiss Micriscopy, Jena, Germany) and biometric software (AxioVision LE, Version 4; Carl Zeiss Micriscopy, Jena, Germany).
3. Results
3.1. Effect of Salinity on Radial Mycelial Growth of Phytophthora Species
The radial mycelial growth of all studied Phytophthora isolates on CA and CA supplemented with different concentrations of NaCl or prepared with sea water is presented in Figure 1.
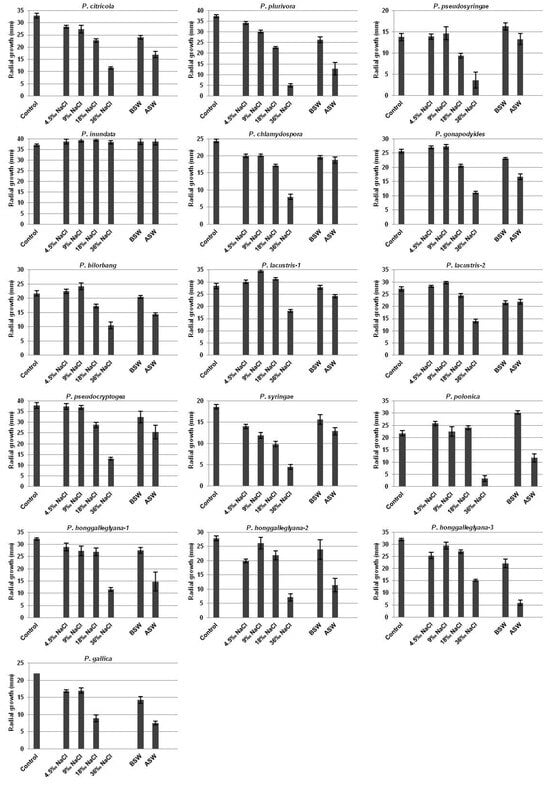
Figure 1.
Radial mycelial growth of Phytophthora species on the control CA medium, CA supplemented with different concentrations of NaCl or prepared with sea water and incubated at 20 °C for 6 days. Error bars indicate ±SD at p ≤ 0.01.
P. citricola and P. plurivora, both from clade 2, demonstrated similar tolerance to tested salinity conditions and the size of colonies inversely correlated to the concentration of NaCl in the medium.
The mycelial growth of P. pseudosyringae from clade 3 on CA and CA supplemented with 4.5‰ and 9‰ NaCl or prepared with the Aegean Sea water did not differ significantly, whereas the colony diameter was notably reduced at higher concentrations of NaCl. In contrast, growth was stimulated on CA prepared with the Black Sea water compared to the control.
The largest group of tested species, belonging to clade 6, showed a high variability in salinity tolerance among them. The radial mycelial growth of P. inundata is similar at all of the tested media and even a slightly stimulating effect of the salinity was monitored. The species P. chlamydospora demonstrated a dose-dependent reduction in mycelial growth on CA supplemented with increasing concentrations of NaCl compared to the control medium. The size of the colony on both CA media prepared with the Black Sea water or the Aegean Sea water was equal. A similar effect of the tested media with varying salt content on the radial growth of P. gonapodyides and P. bilorbang was monitored. A reduction in the colony diameter on medium with 18‰ and 36‰ NaCl was observed, while the growth on media prepared with sea water was less affected. For both P. lacustris isolates, low concentrations of NaCl in CA had a stimulating effect, but the mycelial growth was significantly restricted on the medium supplemented with 36‰ NaCl. The isolate P. lacustris-2 was more sensitive to the medium prepared with sea water compared to the isolate P. lacustris-1.
For the species P. pseudocryptogea, mycelial growth on CA supplemented with 4.5‰ and 9‰ NaCl did not differ from the control. The restriction of the colony diameter was slight on media supplemented with 18‰ NaCl or prepared with sea water, but a strong inhibition effect on CA with the highest tested amount of NaCl was observed. A clear dose-dependent reduction in the mycelial growth on CA supplemented with increasing concentrations of NaCl compared to the control medium for P. syringae was monitored. P. pseudocryptogea and P. syringae, both from clade 8, demonstrated similar growth rates on the two media prepared with sea water.
The impact of salinity on the growth of the species P. polonica belonging to clade 9 was characterized by slight stimulation of the growth on CA supplemented with NaCl up to 18‰, whereas a strong reduction in the colony diameter on CA with 36‰ NaCl was measured. Similarly, a totally different effect on the growth of the species was demonstrated by both media with sea water, with strong stimulation by the Black Sea water, but an inhibition effect by the Aegean Sea water was observed. The mycelial growth of all P. honggalleglyana isolates also from clade 9 was slightly restricted on the tested media, except for CA supplemented with 36‰ NaCl or prepared with the Aegean Sea water. It was noted that the colony size of P. honggalleglyana-2 and P. honggalleglyana-3 on the medium with 9‰ NaCl was significantly bigger than on CA with 4.5‰ NaCl.
Among the isolates included in the study, P. gallica from clade 10 is the only species that was totally inhibited on CA supplemented with 36‰ NaCl. A different level of the colony size reduction in all other tested variants was observed, but the growth of P. gallica was notable even on the medium prepared with the Aegean Sea water whose salinity is about 36‰.
Table 1 shows the mycelial growth of Phytophthora species on CA supplemented with NaCl or prepared with sea water presented as percentage of growth on the control medium for each species. The data showed that low levels of salinity (4.5‰ and 9‰ of NaCl) lead to the induction of the mycelial growth of some species compared to the control medium, which is more obvious for members of clade 6, except for P. chlamydospora. Their growth varied from 104% to 123% compared to the growth on the control CA (100%). The same effect was also found for the species P. polonica that was stimulated even by 18‰ NaCl (110%). In contrast, the highest sensitivity to 36‰ NaCl in the medium was monitored for the species P. plurivora and P. gallica, demonstrating 5% and 0% radial growth, respectively, compared to the growth on the control medium.

Table 1.
Mycelial growth of Phytophthora species on CA supplemented with NaCl or prepared with sea water presented as percentage of growth on control media.
On the other hand, a high tolerance to sea water for some of the tested species (P. pseudocryptogea, P. syringae, P. polonica, and P. gallica) was observed, while the growth on CA with corresponding amounts of NaCl was often suppressed (Table 1). A significant stimulating effect of the Black Sea water was measured for P. polonica, P. pseudosyringae, and P. inundata in percentages of 139%, 116%, and 105%, respectively, compared to the growth on the control medium. The Aegean Sea water induced more intensive growth (105%) compared to the control medium (100%) only for P. inundata. Tolerance to sea water was observed even for both species that are most sensitive to 36‰ NaCl (P. plurivora and P. gallica) as they demonstrated 34% growth on the medium prepared with the Aegean Sea water compared to the control.
3.2. Effect of High Salinity on Colony Type, Growth, Formation of Morphological Structures, and Development of Sporangia by Phytophthora Species
In general, the colony type of the studied Phytophthora species cultivated on control media and CA with 18‰ NaCl or the Black Sea water was similar for most of the tested isolates (Table 2). However, some differences in colony type and growth when the medium was supplemented with 36‰ NaCl or prepared with Aegean Sea water were observed (Table 2). Most of the tested species formed compact colonies and the growth of P. plurivora and P. syringae was highly restricted on the medium with 36‰ NaCl. Colony morphology of P. citricola, P. plurivora, P. chlamydospora, P. gonapodyides, P. polonica, and P. honggalleglyana-1 on the medium prepared with the Aegean Sea water was different compared to the control. The species P. bilorbang demonstrated modified colony type in all salt stress conditions compared to the control medium. The other two species, P. lacustris-1 and P. inundata, showed no difference in the colony type for all tested media.

Table 2.
Effect of high salinity on colony type, growth, and formation of morphological structures during prolonged incubation on CA and development of sporangia on V8A.
The effect of high salinity on the growth of Phytophthora species after prolonged incubation (7 weeks) showed different response of tested isolates to salinity obtained by NaCl and sea water (Table 2). All tested isolates tolerate the presence of sea water in the CA and a moderate inhibition on the medium prepared with the Aegean Sea water was observed only for P. citricola, P. syringae, and P. gallica. In contrast, most of the species were sensitive to 36‰ NaCl and a reduction in colony diameter compared to the control was observed, indicating that they stopped growing after rapid initial growth. The inhibition of P. pseudosyringae, P. citricola, P. gonapodyides, and P. honggalleglyana-2 on CA with 36‰ NaCl was moderate, whereas the growth of P. plurivora and P. syringae was more restricted. No growth of P. gallica on CA with 36‰ NaCl was found. However, all isolates restored the growth after the transfer of a mycelial plug from the restricted colonies on fresh carrot agar, demonstrating tolerance to media supplemented with 36‰ NaCl.
The ability of the homothallic Phytophthora species involved in the study to produce oogonia spontaneously at high salinity conditions was proved only for both species from clade 2, P. citricola and P. plurivora (Table 2). In addition to cultivation on CA, these structures for sexual reproduction were observed on the medium prepared with the Black Sea water for the two isolates and on the medium supplemented with 18‰ NaCl only for P. citricola after 7 weeks of cultivation. No oogonia on media supplemented with 36‰ NaCl or prepared with the Aegean Sea water for these isolates were observed. The other two homothallic species, P. pseudosyringae and P. bilorbang, produced oogonia only on the control CA medium for the tested period (Table 2).
Of the species that are characterized by the formation of chlamydospores, these structures for asexual reproduction were produced on saline media only by two of the tested species (Table 2). The development of chlamydospores by all three P. honggalleglyana isolates was monitored on the control medium and CA supplemented with 18‰ and 36‰ NaCl, but not when CA prepared with sea water was used. P. gallica produced chlamydospores on the four saline media, but not on pure CA. In contrast, the formation of chlamydospores by P. chlamydospora was observed only on the control medium, whereas the species was not able to produce these morphological structures on the tested salinity conditions.
A number of Phytophthora isolates among the tested species were able to produce sporangia under salt stress conditions (Table 2). The application of standard protocol for the induction of sporangia, which includes cultivation on V8A and treatment with spring water for 24–48 h, led to the production of sporangia on all tested media by P. plurivora, P. chlamydospora (except the medium with the Aegean Sea water), P. gonapodyides, P. lacustris-1, and P. lacustris-2. P. citricola and P. bilorbang were able to produce sporangia on V8A and V8A supplemented with 18‰ and 36‰ NaCl but not on the two media prepared with sea water. The formation of sporangia by P. pseudosyringae on V8A, the medium with 18‰ NaCl and the Aegean Sea water, was also observed. The species P. inundata produced sporangia on media supplemented with 18‰ and 36‰ NaCl, but not on the control V8A or the media prepared with any of the tested sea waters. P. syringae, P. gallica, and P. honggalleglyana produced sporangia only on the control V8A but failed to form these structures for asexual reproduction during cultivation at salt stress conditions.
The release of zoospores from sporangia by some species was also observed (Table 2). Swimming zoospores produced by P. citricola and P. plurivora cultivated on V8A and V8A supplemented with 18‰ NaCl were monitored, as well as on V8A with 36‰ NaCl only by P. plurivora. The release of zoospores by both P. lacustris isolates was also documented as follows: on V8A and the medium prepared with the Aegean Sea water for P. lacustris-1 and on V8A with 18‰ NaCl and V8A with sea water for P. lacustris-2. Zoospores germinating inside of sporangia were monitored for some species in the tested conditions: P. pseudosyringae on V8A and the medium with 18‰ NaCl; P. gonapodyides on four variants of salt stress media, but not on V8A; P. lacustris-1 on medium supplemented with 18‰ and 36‰ NaCl; and P. lacustris-2 on V8A with 36‰ NaCl and the medium with the Aegean Sea water.
4. Discussion
The study aims to investigate whether Phytophthora species tolerate varying degrees of salinity by adding NaCl or sea water in the culture medium. Observation of the mycelial growth, the type of colonies, and the formation of morphological structures on saline media proved the potential of the tested species to have a maritime lifestyle. These results are fairly unexpected due to the limited information regarding the distribution of the oomycetes in marine environments. On the other hand, members of the genus Phytophthora are characterized by high plasticity because of their ability to use sexual and asexual morphological structures, to carry out interspecific hybridization, and to adapt to new hosts and new biogeographic environments. All isolates included in the study, except for P. syringae and P. polonica, produced one or more structures for reproduction on the tested salty media, demonstrating their potential to reproduce under these conditions. Most of the isolates showed intensive mycelial growth at different salinity levels, sometimes comparable to the growth on the control medium. A number of species including P. pseudosyringae, P. inundata, P. chlamydospora, P. gonapodyides, P. bilorbang, P. lacustris, and P. polonica showed even more intensive growth at low salinity levels than on the control medium. It is interesting to note that some species such as P. pseudosyringae, P. chlamydospora, P. syringae, P. polonica, and P. gallica, which belong to different clades of the genus, demonstrated significantly better mycelial growth when the medium was prepared with the Black Sea water or the Aegean Sea water compared to the medium with corresponding salinity obtained by adding sodium chloride. This result indicates the potential of the species to be found in marine ecosystems and highlights the need of more detailed studies on Phytophthora distribution in diverse habitats. On the other hand, the ability of species for adaptation to different conditions, such as aquatic environments with higher salinity, can lead to the occupation of new habitats. This in turn may pose a threat to populations of marine plants, as some members of the genus Phytophthora are known as aggressive pathogens [10,12]. Their spread to costal zones can cause considerable ecological implications and economic losses in marine ecosystems [5,23,24].
In the present study, Phytophthora species that are members of clade 6 stand out by their ability to grow on media with increased salt content better than on the control medium. These data support the assumption that species from clade 6 had a common marine ancestor because of their phylogenetic relationship to brown algae and adaptation to terrestrial habitats [23]. In addition, this group of the genus Phytophthora is closely related phylogenetically and ecologically to species from the genus Halophytophthora, which are widespread in estuarine and marine habitats [24]. However, the fact that only P. inundata and P. bilorbang from clade 6 were found in the river estuary of the Black Sea in a recent study [20] rather supports the idea for the evolution of a separate cluster from clade 6 [6]. Moreover, these two species have not been previously found in Bulgaria, in contrast to the most widespread terrestrial species from our collection, like P. chlamydospora, P. gonapodyides, and P. lacustris, which, however, were not recovered from the estuary. It is well known that species from clade 6 predominantly have an aquatic lifestyle [15,25,26,27], but further investigations are needed to elucidate the origin, evolution, and distribution of these oomycetes.
The tolerance to high salinity of the two species (P. pseudosyringae and P. polonica) that belong to different clades and have diverse origins in our collection also indicates the potential for adaptation to the marine environment of Phytophthora rather than the marine ancestor. The species P. pseudosyringae is a member of clade 3 and was isolated from a high mountain river [17]. The species P. polonica, which belongs to clade 9, was recently found in soil samples from the coastal zone, located immediately next to the river [19], but not in the river mouth. The distribution of P. pseudosyringae in different geographical conditions, from the coastal forests of California to the Sierra Nevada Mountains in North America, has been previously reported [28] and indicates its great adaptability. Moreover, recently published analyses on the phylogeography and population structure of P. pseudosyringae leads to the assumption of a continental origin for the species and migration from Europe to North America [29]. On the other hand, P. polonica is mainly known as a pathogen on wooden species and has been rarely isolated from water habitats [30,31,32], which also reduces the likelihood of marine origin for this species.
For the species P. lacustris and P. honggalleglyana, more than one isolate were included in the study, as some of them being derived near the sea coast and others were isolated from the inland. No significant differences between isolates from both species in their potential to grow and produce morphological structures for reproduction at tested salinity conditions were observed. This sample set is limited and more isolates, as well as representatives from other clades of the genus Phytophthora, are needed to draw reliable implications. Nevertheless, the experimental data showed that P. lacustris and P. honggalleglyana that belong to clade 6 and clade 9, respectively, are among the oomycetes with elevated tolerance to salty conditions.
The Phytophthora species that have been isolated from sea ecosystems up to now belong to clade 6 (P. gemini, P. inundata, and P. chesapeakensis) [6,8] and clade 9 (P. rhizophorae, P. estuarina, and P. insolita) [1,2,3,4]. The results from the present study proved high salinity tolerance and the potential for a life in marine habitats predominantly of Phytophthora species from the same two clades and are in line with previous reports. These abilities are most likely based on specific morphological and physiological characteristics typical for the two groups or their separate clusters. It has been suggested that the clades of the genus Phytophthora are result of migration and worldwide radiation [16], but there is still a lot to learn about them. Further research on the diversity and distribution of Phytophthora in coastal regions and marine ecosystems is needed to clarify the origin and evolution, as well as to identify new habitats of these oomycete species.
5. Conclusions
This study provides evidence for the ability of Phytophthora species to survive in marine environments. Nevertheless a few species have been isolated from coastal and sea ecosystems up to now, the potential of some members of the genus to exist in saline media is obvious. Therefore, more detailed surveys using classical detection methods, but also the latest molecular methods for the screening of oomycetes, are needed. The results of this study clearly demonstrate that Phytophthora species from clade 6, as well as some representatives from clade 3 and clade 9, are most likely to be found in marine environments due to their tolerance to salt stress conditions and to their aquatic lifestyle. A significant stimulating effect of the Black Sea water on the growth of some species (P. polonica, P. pseudosyringae, and P. inundata) compared to the colony growth on the control medium indicates the potential for adaptability in marine ecosystems but also points to the possibility of a wider distribution of species from the genus Phytophthora than is currently known. Moreover, there is also a significant opportunity to find new species in these unexplored habitats.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in sections of the article.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Marano, A.V.; Jesu, A.L.; Pires-Zottar, C.L.A. Fungal diversity notes 253–366: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016, 78, 194. [Google Scholar] [CrossRef]
- Pires-Zottar, C.L.A.; Jesus, A.L.; Marano, A.V. Fungal diversity notes 253–366: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016, 78, 196. [Google Scholar] [CrossRef]
- Bennett, R.M.; Thines, M. Confirmation that Phytophthora insolita (Peronosporaceae) is present as a marine saprotroph on mangrove leaves and first report of the species for the Philippines. Nova Hedwig. 2017, 105, 185–196. [Google Scholar] [CrossRef]
- Bennett, R.M.; Thines, M. An overview on Philippine estuarine oomycetes. Philipp. J. Syst. Biol. 2020, 14, 1–14. [Google Scholar]
- Govers, L.L.; Man in‘t Veld, W.A.; Meffert, J.P.; Bouma, T.J.; van Rijswick, P.C.J.; Heusinkveld, J.H.T.; Orth, R.J.; van Katwijk, M.M.; van der Heide, T. Marine Phytophthora species can hamper conservation and restoration of vegetated coastal ecosystems. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160812. [Google Scholar] [CrossRef] [PubMed]
- Man in’t Veld, W.A.; Rosendahl, K.; van Rijswick, P.C.J.; Meffert, J.P.; Boer, E.; Westenberg, M.; van der Heide, T.; Govers, L.L. Multiple Halophytophthora spp. and Phytophthora spp. including P. gemini, P. inundata, and P. chesapeakensis sp. nov. isolated from the seagrass Zostera marina in the Northern Hemisphere. Eur. J. Phytopathol. 2019, 153, 341–357. [Google Scholar] [CrossRef]
- Menning, D.M.; Ward, D.H.; Wyllie-Echeverria, S.; Sage, G.K.; Gravley, M.C.; Gravley, H.A.; Talbot, S.L. Are migratory waterfowl vectors of seagrass pathogens? Ecol. Evol. 2020, 10, 2062–2073. [Google Scholar] [CrossRef]
- Menning, D.M.; Gravley, H.A.; Cady, M.N.; Pepin, D.; Wyllie-Echeverria, S.; Ward, D.H.; Talbot, S.L. eDNA metabarcoding shows wide distribution of eelgrass pathogens in the Pacific. Metabarcoding Metagenom. 2021, 5, 35–42. [Google Scholar] [CrossRef]
- Blaker, N.S.; MacDonald, J.D. The effect of soil salinity on formation of sporangia and zoospores by three isolates of Phytophthora. Phytopathology 1985, 75, 270–274. [Google Scholar] [CrossRef]
- Swiecki, T.J.; MacDonald, J.D. Soil salinity enhances Phytophthora Root Rot of tomato but hinders asexual reproduction by Phytophthora parasitica. J. Amer. Soc. Hort. Sci. 1991, 116, 471–477. [Google Scholar] [CrossRef]
- Wilkens, S.; Field, C.D. Effect of varying sea-water salinity on growth kinetics of Phytophthora polymorphica. Mycol. Res. 1993, 97, 1135–1139. [Google Scholar] [CrossRef]
- Preuett, J.; Collins, D.; Luster, D.; Widmer, T. The effects of salinity on the survival, growth, sporulation and infection of Phytophthora ramorum. Fungal Ecol. 2016, 23, 123–130. [Google Scholar] [CrossRef]
- Hamlington, B.D.; Bellas-Manley, A.; Willis, J.K.; Fournier, S.; Vinogradova, N.; Nerem, R.S.; Piecuch, C.G.; Thompson, P.R.; Kopp, R. The rate of global sea level rise doubled during the past three decades. Commun. Earth Environ. 2024, 5, 601. [Google Scholar] [CrossRef]
- Oh, E.; Gryzenhout, M.; Wingfield, B.D.; Wingfield, M.J.; Burgess, T.I. Surveys of soil and water reveal a goldmine of Phytophthora diversity in South African natural ecosystems. IMA Fungus 2013, 4, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Redondo, M.A.; Boberg, J.; Stenlid, J.; Oliva, J. Contrasting distribution patterns between aquatic and terrestrial Phytophthora species along a climatic gradient are linked to functional traits. ISME J. 2018, 12, 2967–2980. [Google Scholar] [CrossRef] [PubMed]
- Brasier, C.; Scanu, B.; Cooke, D.; Jung, T. Phytophthora: An ancient, historic, biologically and structurally cohesive and evolutionarily successful generic concept in need of preservation. IMA Fungus 2022, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Christova, P.K.; Lyubenova, A.B.; Kostov, K.V.; Slavov, S.B. First report of Phytophthora pseudosyringae recovered from aquatic ecosystems in Bulgaria. For. Pathol. 2019, 49, e12505. [Google Scholar] [CrossRef]
- Christova, P.K. Detection of Phytophthora gallica in Bulgaria and co-existence with other Phytophthora species in a small river. J. Plant Dis. Prot. 2022, 129, 1377–1387. [Google Scholar] [CrossRef]
- Christova, P.K. Phytophthora polonica and Phytophthora hydropathica from clade 9 associated with alder decline in Bulgaria. Life 2024, 14, 720. [Google Scholar] [CrossRef]
- Christova, P.K. Fishing for estuarine oomycetes. Diversity 2024, 16, 530. [Google Scholar] [CrossRef]
- Poulos, S.E. Water Masses of the Mediterranean Sea and Black Sea: An Overview. Water 2023, 15, 3194. [Google Scholar] [CrossRef]
- Jung, T.; Stukely, M.J.C.; Hardy, G.E.S.J.; White, D.; Paap, T.; Dunstan., W.A.; Burgess, T.I. Multiple new Phytophthora species from ITS Clade 6 associated with natural ecosystems in Australia: Evolutionary and ecological implications. Persoonia 2011, 26, 13–39. [Google Scholar] [CrossRef]
- Marano, A.V.; Jesus, A.L.; de Souza, J.I.; Jerônimo, G.H.; Gonçalves, D.R.; Boro, M.C.; Rocha, S.C.O.; Pires-Zottarelli, C.L.A. Ecological roles of saprotrophic Peronosporales (Oomycetes, Straminipila) in natural environments. Fungal Ecol. 2016, 19, 77–88. [Google Scholar] [CrossRef]
- Maia, C.; Horta Jung, M.; Carella, G.; Milenković, I.; Janoušek, J.; Tomšovský, M.; Mosca, S.; Schena, L.; Cravador, A.; Moricca, S.; et al. Eight new Halophytophthora species from marine and brackish-water ecosystems in Portugal and an updated phylogeny for the genus. Persoonia 2022, 48, 54–90. [Google Scholar] [CrossRef] [PubMed]
- Nechwatal, J.; Bakonyi, J.; Cacciola, S.O.; Cooke, D.E.L.; Jung, T.; Nagy, Z.A.; Vannini, A.; Vettraino, A.M.; Brasier, C.M. The morphology, behavior and molecular phylogeny of Phytophthora taxon Salixsoil and its redesignation as Phytophthora lacustris sp. nov. Plant Pathol. 2013, 62, 355–369. [Google Scholar] [CrossRef]
- Abad, Z.; Burgess, T.; Bourret, T.; Bensch, K.; Cacciola, S.; Scanu, B.; Mathew, R.; Kasiborski, B.; Srivastava, S.; Kageyama, K.; et al. Phytophthora: Taxonomic and phylogenetic revision of the genus. Stud. Mycol. 2023, 106, 259–348. [Google Scholar] [CrossRef]
- Schoebel, C.N.; Prospero, S.; ·Rigling, D.; Rufner, B. Fishing for Phytophthora in watercourses of the highly urbanized Swiss Plateau. Mycol. Prog. 2024, 23, 17. [Google Scholar] [CrossRef]
- Wickland, A.C.; Jensen, C.E.; Rizzo, D.M. Geographic distribution, disease symptoms and pathogenicity of Phytophthora nemorosa and Phytophthora pseudosyringae in California, USA. For. Pathol. 2008, 38, 288–298. [Google Scholar] [CrossRef]
- Mullett, M.S.; Harris, A.R.; Scanu, B.; Van Poucke, K.; LeBoldus, J.; Stamm, E.; Bourret, T.B.; Christova, P.K.; Oliva, J.; Redondo, M.A.; et al. Phylogeography, origin and population structure of the selffertile emerging plant pathogen Phytophthora pseudosyringae. Mol. Plant Pathol. 2024, 25, e13450. [Google Scholar] [CrossRef]
- Bregant, C.; Rossetto, G.; Meli, L.; Sasso, N.; Montecchio, L.; Brglez, A.; Piškur, B.; Ogris, N.; Maddau, L.; Linaldeddu, B.T. Diversity of Phytophthora Species Involved in New Diseases of Mountain Vegetation in Europe with the Description of Phytophthora pseudogregata sp. nov. Forests 2023, 14, 1515. [Google Scholar] [CrossRef]
- Matsiakh, I.; Kramarets, V.; Cleary, M. Occurrence and diversity of Phytophthora species in declining broadleaf forests in western Ukraine. For. Pathol. 2021, 51, e12662. [Google Scholar] [CrossRef]
- Bregant, C.; Batista, E.; Hilário, S.; Linaldeddu, B.T.; Alves, A. Phytophthora Species Involved in Alnus glutinosa Decline in Portugal. Pathogens 2023, 12, 276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).