Restoring High Mountain Sphagnum Communities in the Central Pyrenees
Abstract
1. Introduction
2. Materials and Methods
2.1. The Restoring Site
2.2. Prior Experiments with Plant Material
2.3. Setting and Monitoring the Restoration of Habitats
2.4. Data Analysis
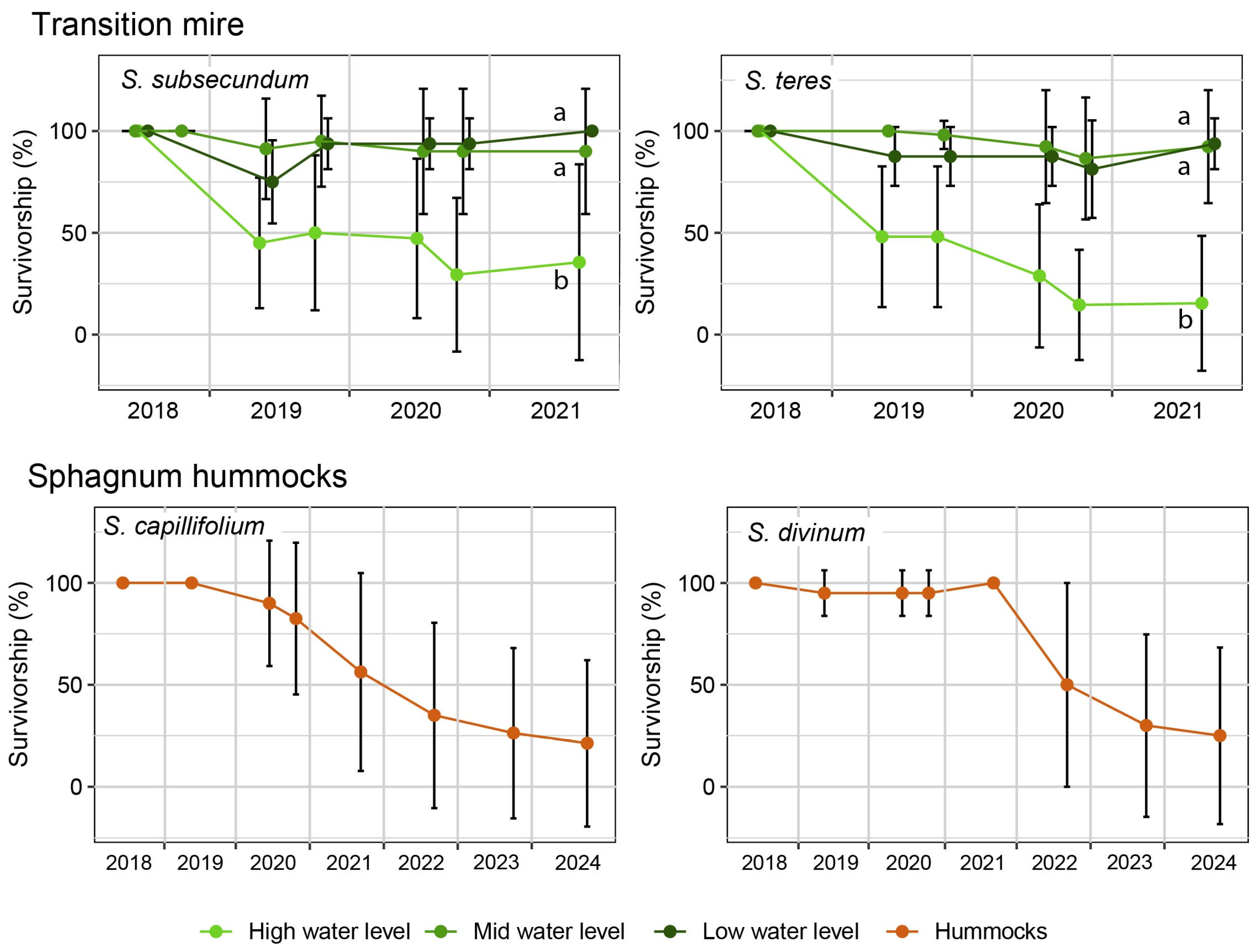
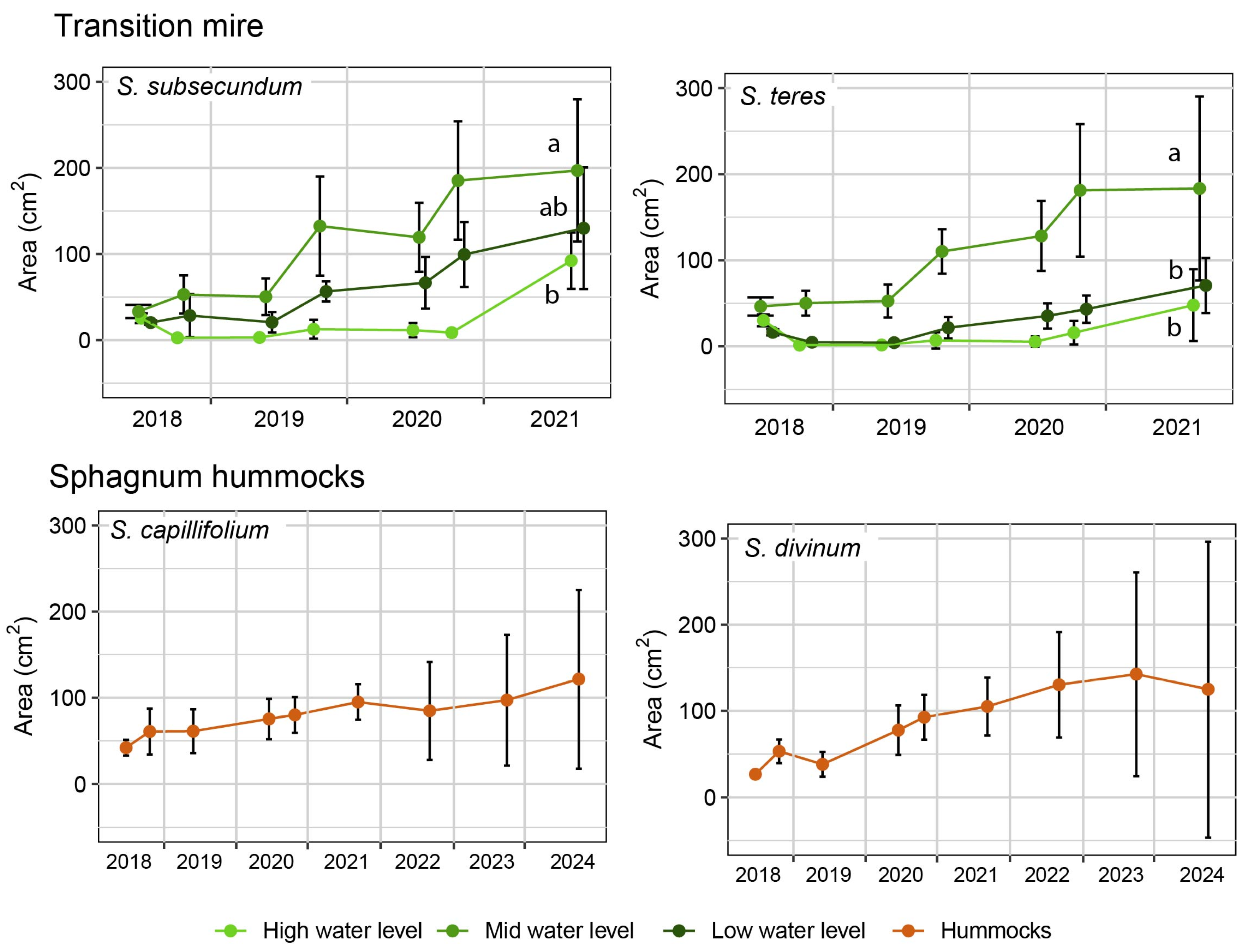
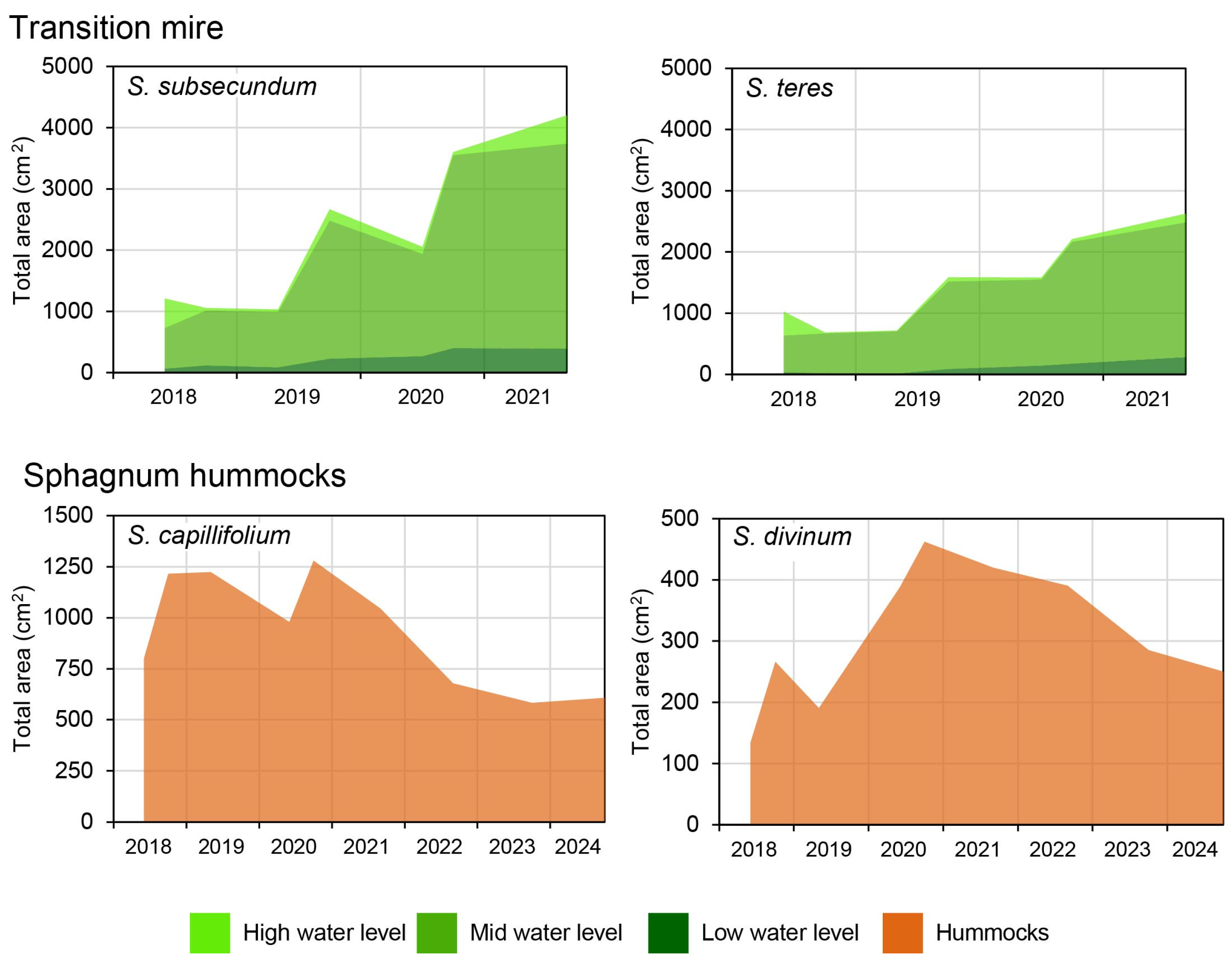
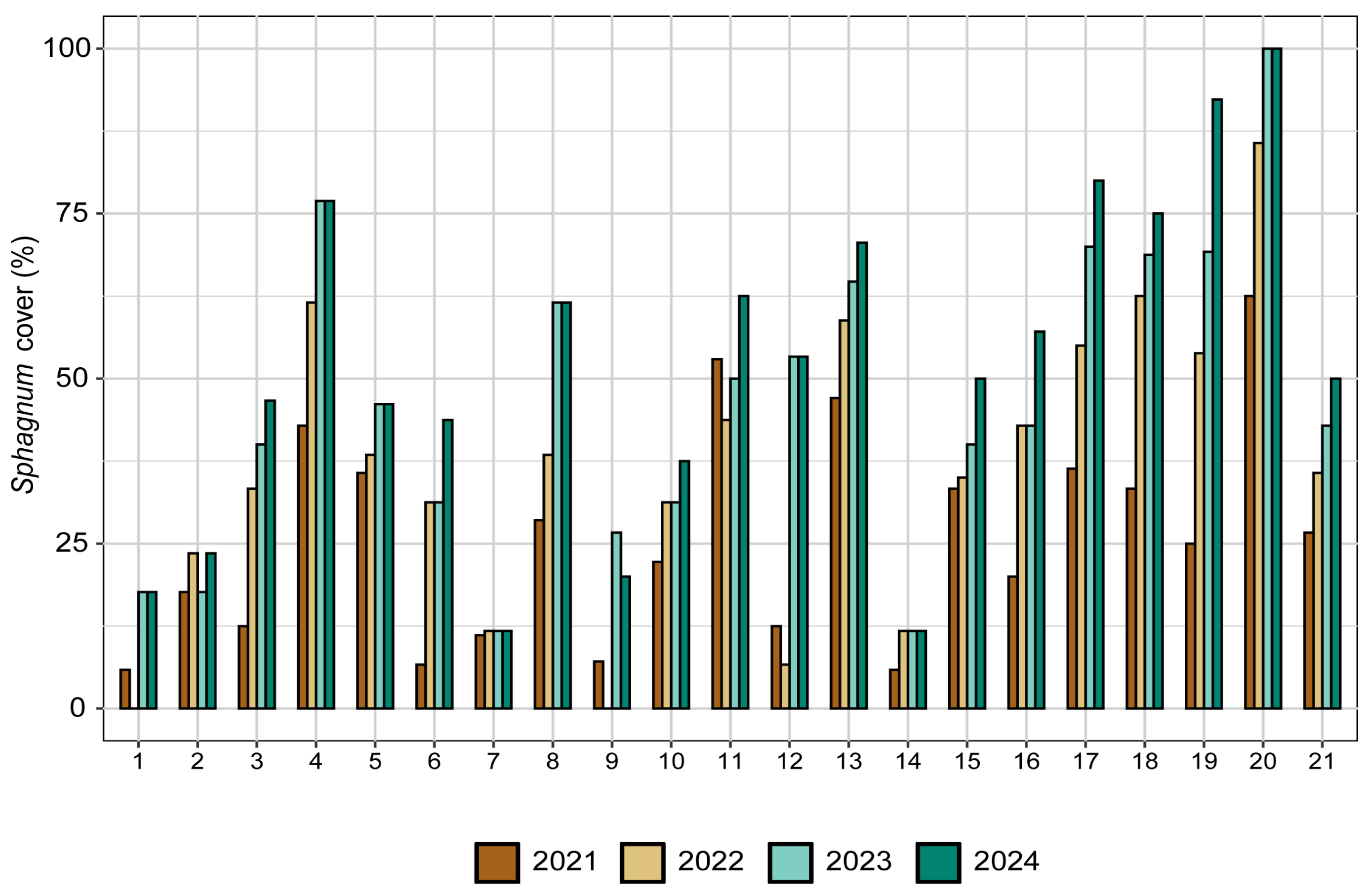
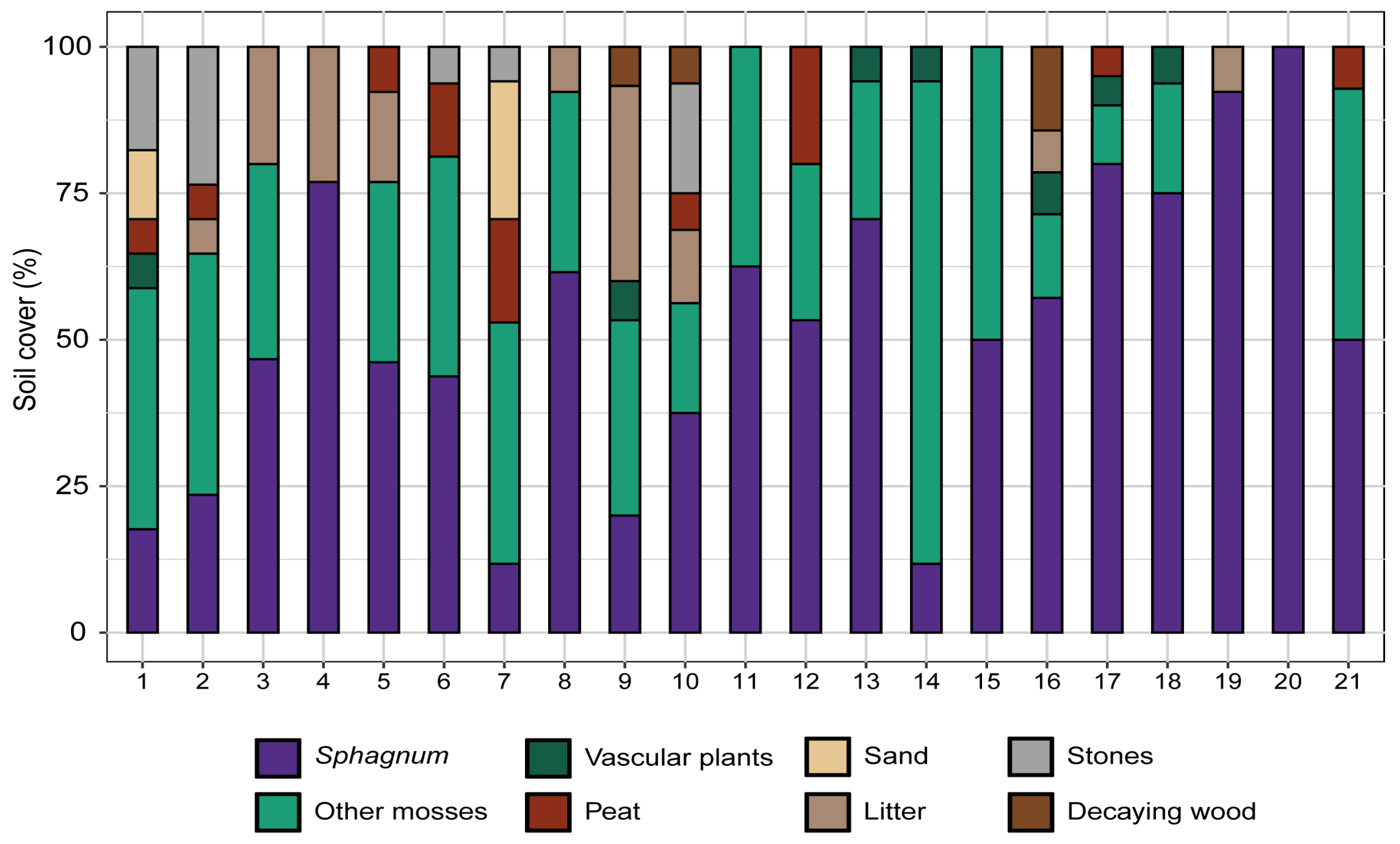
3. Results
4. Discussion
4.1. Plants Response to the Restoration Procedure
4.2. Ecological Succession in the Newly Restored Habitats
4.3. Learning Points
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
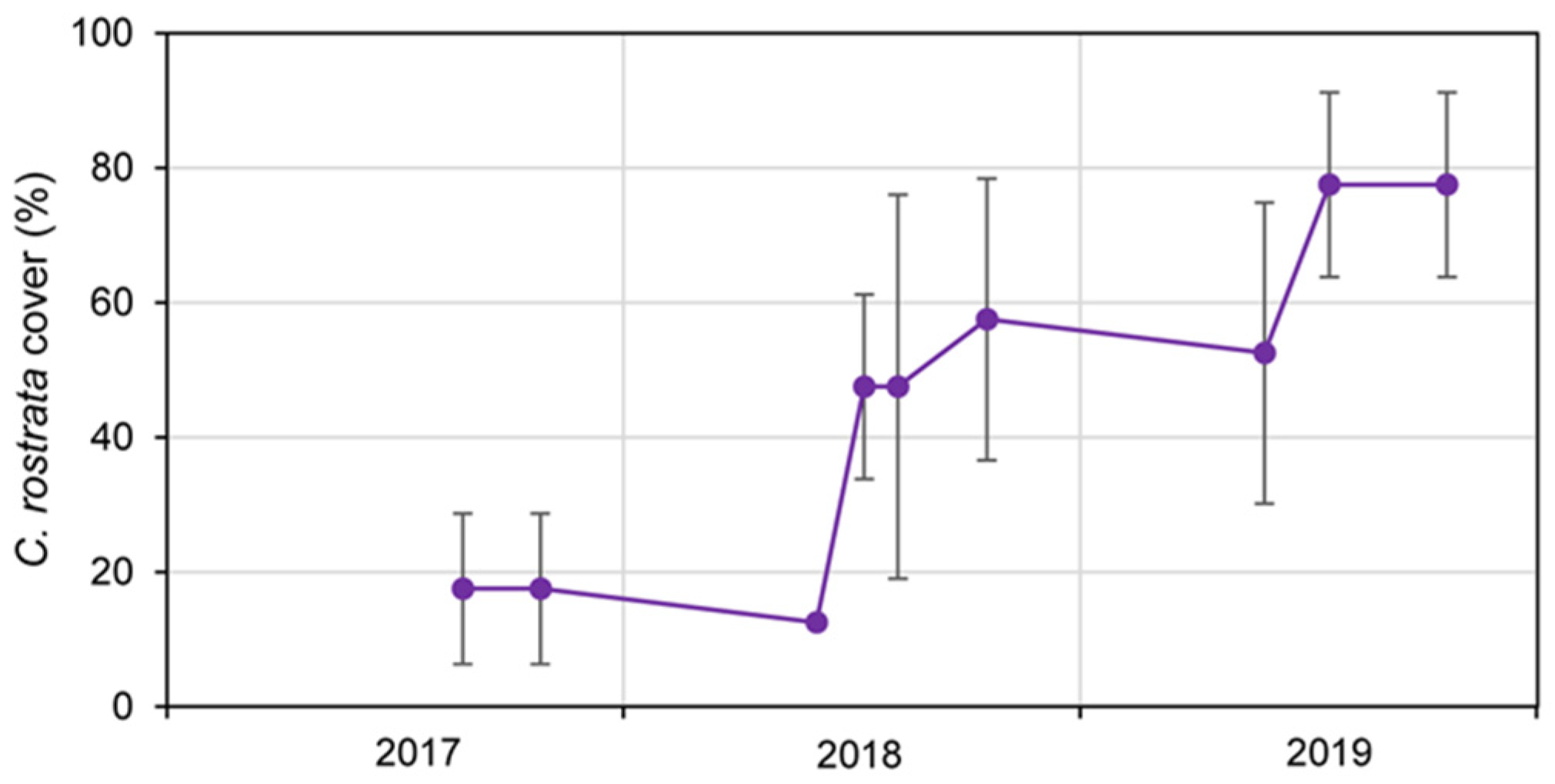
Appendix A. Summer Meteorology and Flooding Dynamics
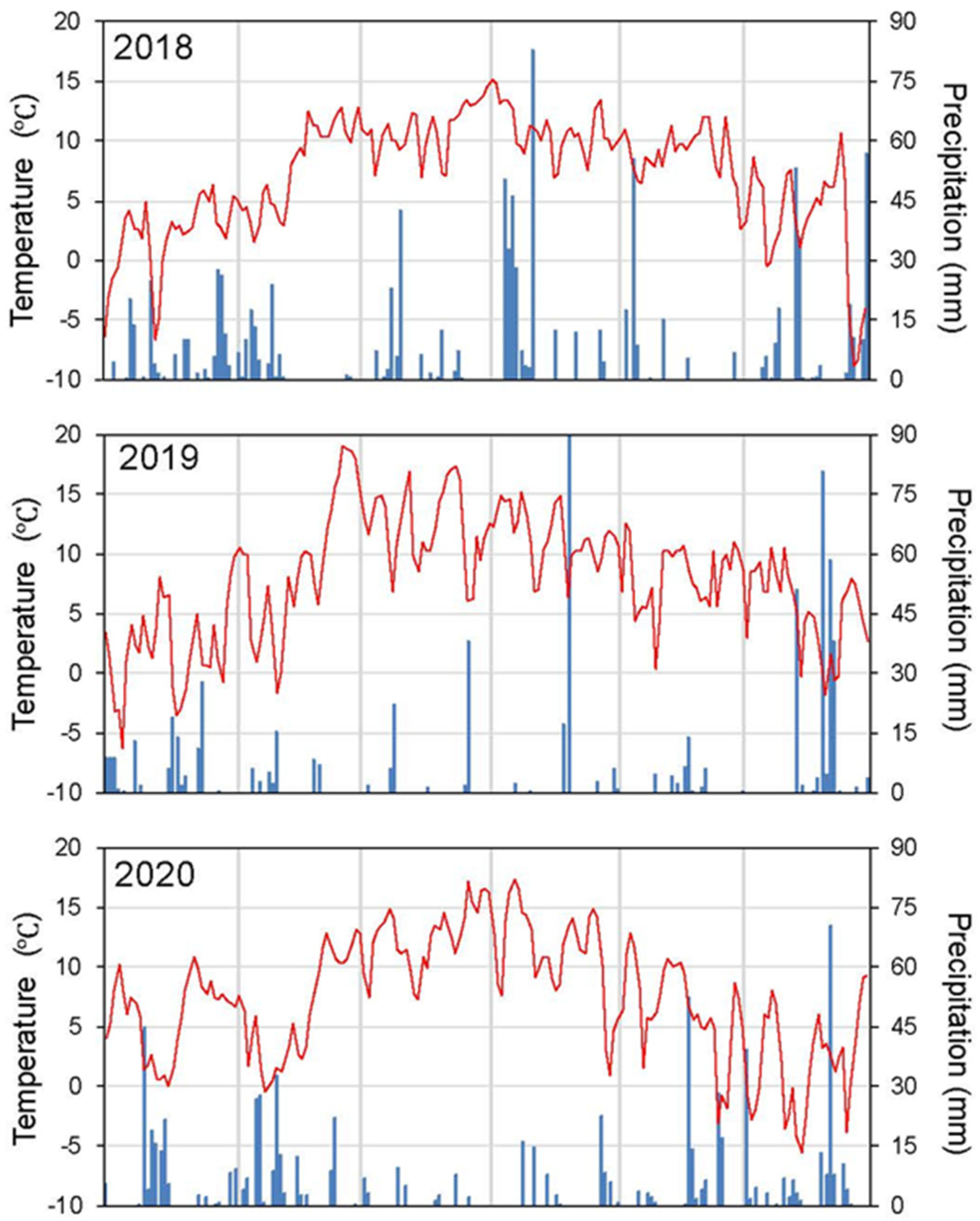
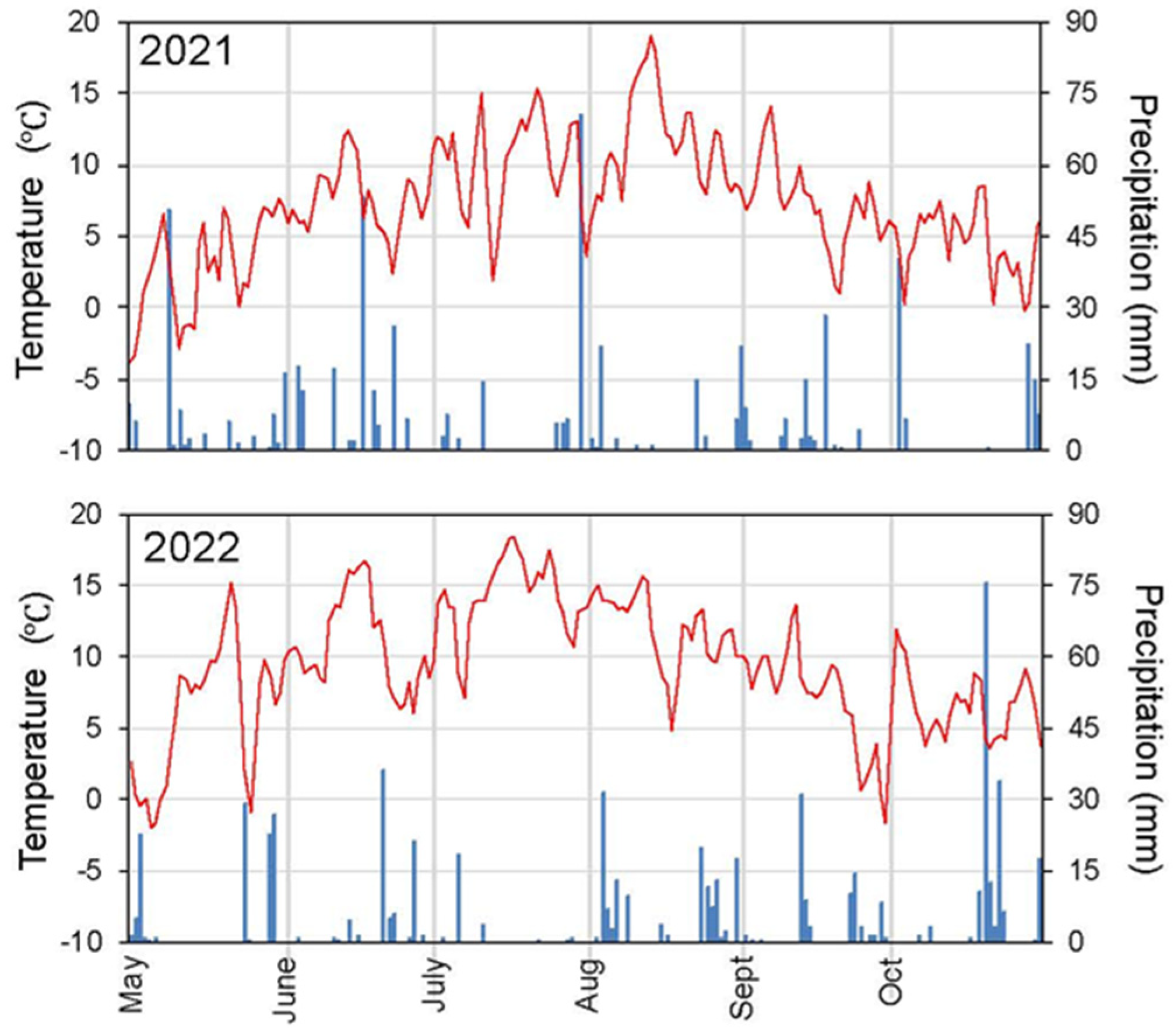
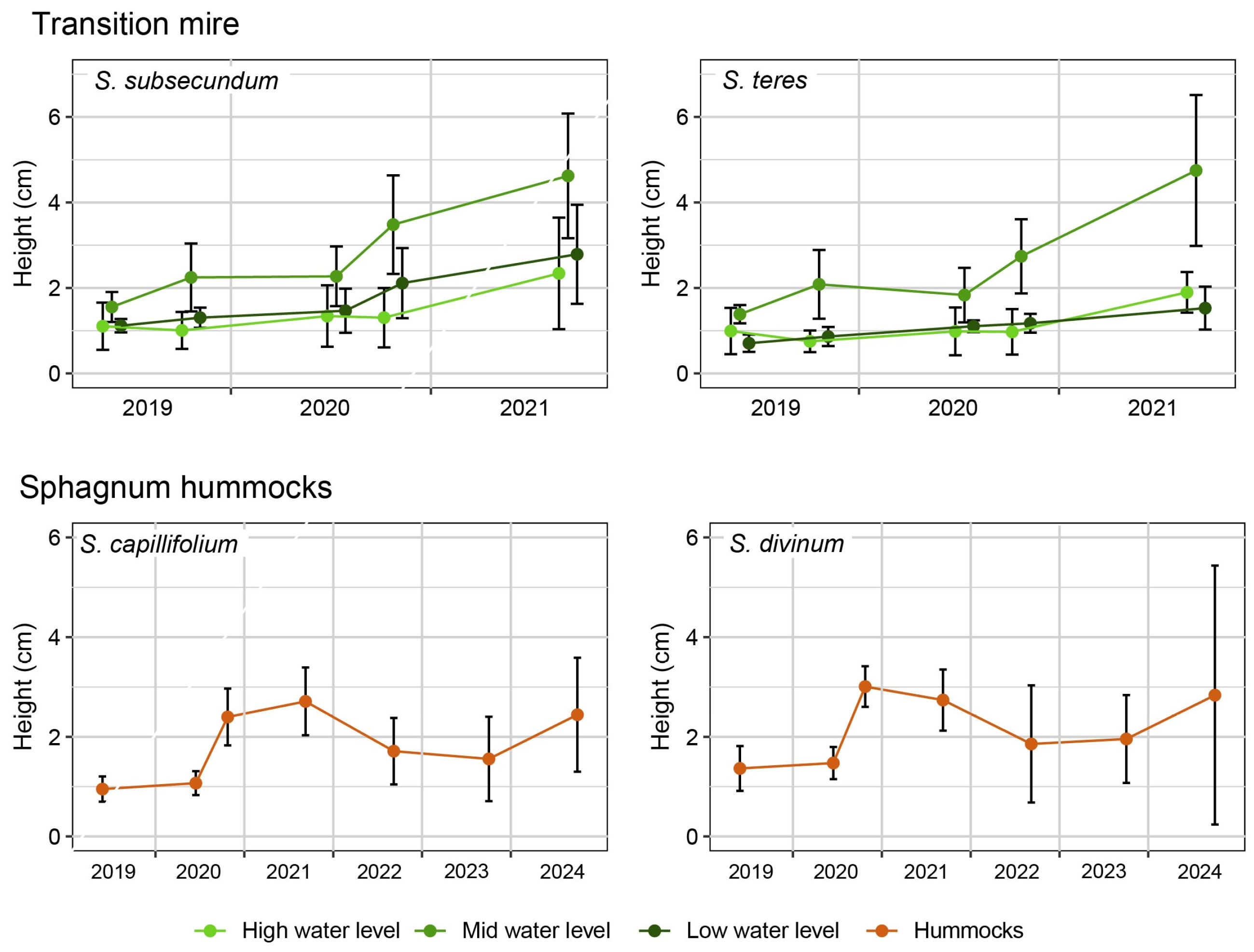
Appendix B. Complementary Results on Sphagnum Restoration
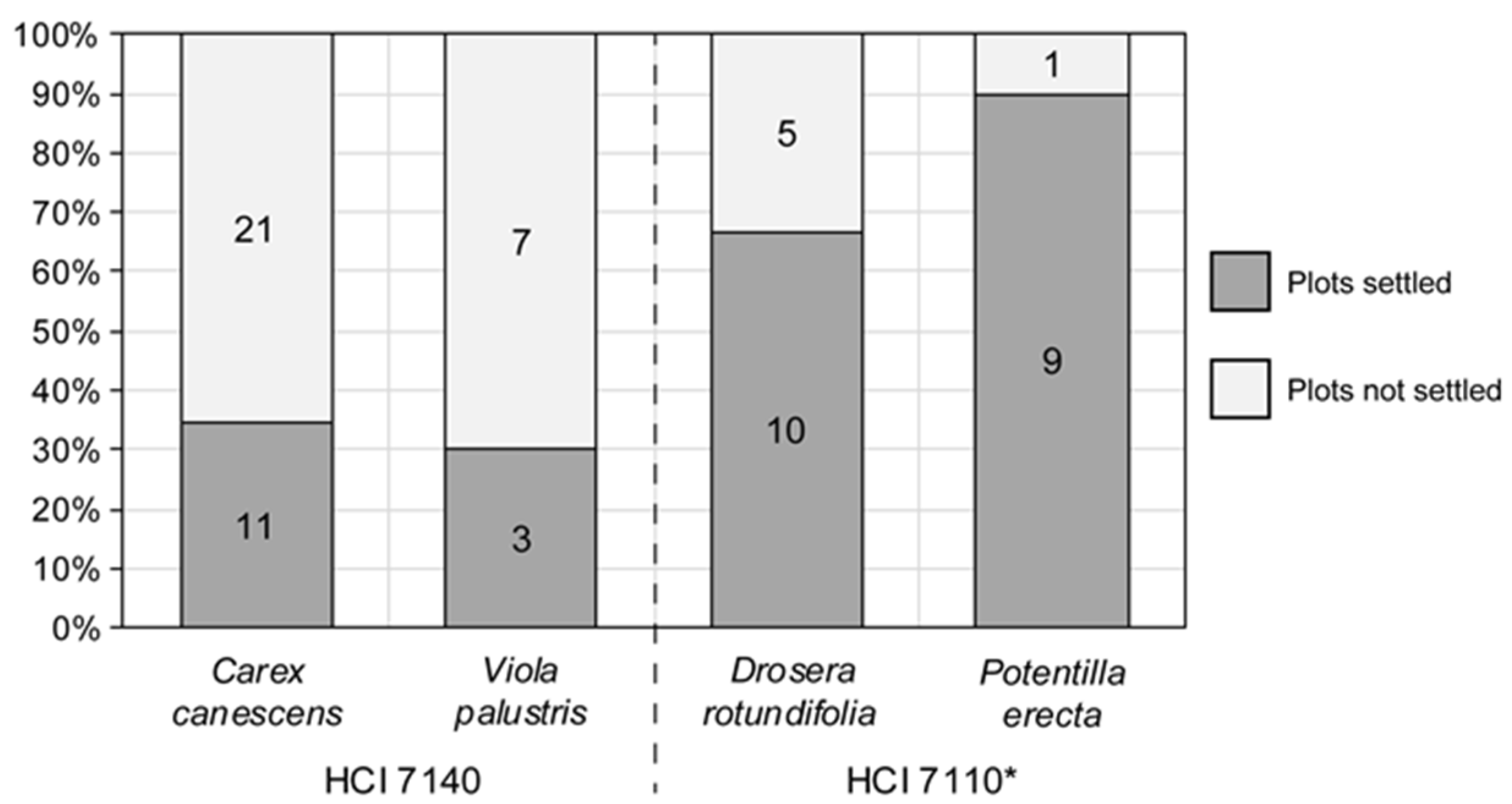
Appendix C. Other Plant Species in the Experimental Sphagnum Plots
| Species | Transition Mire (7140) | Sphagnum Hummocks (7110*) |
|---|---|---|
| Carex rostrata Stokes | 93 | 28 |
| Potentilla erecta (L.) Raeusch. | 20 | 8 |
| P. erecta (seedlings) | 4 | 4 |
| Juncus articulatus L. | 18 | 4 |
| J. articulatus (seedlings) | 4 | |
| Pinus uncinata Ram. ex DC. (seedlings) | 2 | 24 |
| Carex flava L. agg. | 2 | |
| C. flava agg. (seedlings) | 20 | |
| Eleocharis quinqueflora (Hartmann) O. Schwarz | 20 | |
| E. quinqueflora (seedlings) | 4 | |
| Carex flacca Schreb. | 16 | |
| Epilobium sp. | 16 | |
| Prunella vulgaris L. | 13 | |
| Carex sp. (seedlings) | 5 | 4 |
| Succisa pratensis Moench. | 5 | 4 |
| Pleurocarpous mosses | 9 | 4 |
| Pedicularis pyrenaica Gay agg. | 5 | |
| P. pyrenaica agg. (seedlings) | 8 | |
| Sagina cf. saginoides H. Karst. | 9 | |
| Polytrichum sp. | 8 | |
| Trifolium sp. | 7 | |
| Viola palustris L. | 5 | |
| Lotus corniculatus L. | 5 |
Appendix D. Supplementary Images










References
- Costea, G.; Pusch, M.T.; Bănăduc, D.; Cosmoiu, D.; Curtean-Bănăduc, A. A review of hydropower plants in Romania: Distribution, current knowledge, and their effects on fish in headwater streams. Renew. Sustain. Energy Rev. 2001, 145, 111003. [Google Scholar] [CrossRef]
- Rodriguez, J.-F. Hydropower landscapes and tourism development in the Pyrenees: From natural resource to cultural heritage. J. Alpine Res. 2012, 100, 2. [Google Scholar] [CrossRef]
- Schwarz, U. Hydropower Projects in Protected Areas on the Balkans; RiverWatch & EuroNatur: Vienna, Austria, 2015; pp. 1–34. [Google Scholar]
- Catalan, J.; Vilalta, R.; Weitzman, B.; Comas, E.; Pigem, C.; Aiguabella, P. La Indústria Hidràulica en els Pirineus: Avaluació, Correcció i Prevenció dels Impactes Ambientals al Parc Nacional d’Aigüestortes i Estany de Sant Maurici; La Caixa: Barcelona, Spain, 1997. [Google Scholar]
- Catalan, J.; Ninot, J.M.; Aniz, M.M. The high mountain conservation in a changing world. In High Mountain Conservation in a Changing World; Catalan, J., Ninot, J.M., Aniz, M.M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 3–36. [Google Scholar] [CrossRef]
- Lindenmayer, D. Improving restoration programs through greater connection with ecological theory and better monitoring. Front. Ecol. Evol. 2020, 8, 50. [Google Scholar] [CrossRef]
- Dam Removal Europe 2023. Available online: https://damremoval.eu/ (accessed on 1 October 2023).
- Schletterer, M.; Kurz, B.; Schönegger, A.; Egger, G.; Feistmantl, K. Transplantation of an alpine Carex-fen—A mitigation measure related to the construction of a reservoir in the Austrian Alps. In Proceedings of the VI International Scientific Conference “Problems of Industrial Botany of Industrially Developed Regions”, Kemerovo, Russia, 5–7 October 2021. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; Fernández-Pascual, E.; Díaz González, T.E.; Pérez-Haase, A.; Ninot, J.M. Diversity of rich fen vegetation and related plant specialists in mountain refugia of the Iberian peninsula. Folia Geobot. 2021, 47, 403–419. [Google Scholar] [CrossRef]
- Pérez-Haase, A. Patrons Estructurals, Ecològics i Biogeogràfics en Vegetació de Molleres i de Torberes D’esfagnes. Ph.D. Thesis, University of Barcelona, Barcelona, Spain, 2016. [Google Scholar]
- LIFE Limnopirineus. 2019. Available online: http://www.lifelimnopirineus.eu/es (accessed on 10 October 2024).
- Pérez-Haase, A.; Ninot, J.M. Hydrological heterogeneity rather than water chemistry explains the high plant diversity and uniqueness of a Pyrenean mixed mire. Folia Geobot. 2017, 52, 143–160. [Google Scholar] [CrossRef]
- Colomer, J.; Pérez-Haase, A.; Carrillo, E.; Ventura, M.; Ninot, J.M. Fine-scale vegetation mosaics in Pyrenean mires are driven by complex hydrological regimes and threatened by extreme weather events. Ecohydrology 2019, 12, e2070. [Google Scholar] [CrossRef]
- Pouliot, R.; Rochefort, L.; Karofeld, E.; Mercier, C. Initiation of Sphagnum moss hummocks in bogs and the presence of vascular plants: Is there a link? Acta Oecol. 2011, 37, 346–354. [Google Scholar] [CrossRef]
- Moreno-Mateos, D.; Meli, P.; Vara-Rodríguez, M.I.; Aronson, J. Ecosystem response to interventions: Lessons from restored and created wetland ecosystems. J. Appl. Ecol. 2015, 52, 1528–1537. [Google Scholar] [CrossRef]
- Gorham, E.; Rochefort, L. Peatland restoration: A brief assessment with special reference to Sphagnum bogs. Wetl. Ecol. Manag. 2003, 11, 109–119. Available online: https://link.springer.com/article/10.1023/A:1022065723511 (accessed on 10 October 2024). [CrossRef]
- Quinty, F.; Rochefort, L. Peatland Restoration Guide, 2nd ed.; Canadian Sphagnum Peat Moss Association: Fredericton, NB, Canada, 2003. [Google Scholar]
- Rosenburg, A.E. Restoration and Recovery of Sphagnum on Degraded Blanket Bog. Ph.D. Thesis, Manchester Metropolitan University, Manchester, UK, 2015. [Google Scholar]
- Wittram, B.W.; Roberts, G.; Buckler, M.; King, L.; Walker, J.S. A Practitioners Guide to Sphagnum Reintroduction; Moors for the Future Partnership: Edale, UK, 2015; pp. 1–39. [Google Scholar]
- Rochefort, L. Sphagnum—A keystone genus in habitat restoration. Bryologist 2000, 103, 503–508. [Google Scholar] [CrossRef]
- Graf, U.H.; Bergamini, A.; Bedolla, A.; Boch, S.; Küchler, H.; Küchler, M.; Ecker, K. Regeneration potential of a degraded alpine mountain bog: Complex regeneration patterns after grazing cessation and partial rewetting. Mires Peat 2022, 28, 01. [Google Scholar] [CrossRef]
- Servei Meteorològic de Catalunya. Anuaris de les Estacions Meteorològiques; Generalitat de Catalunya, 2023. Available online: https://www.meteo.cat/wpweb/climatologia/dades-i-productes-climatics/anuari-de-dades/estacions-meteorologiques/ (accessed on 20 April 2024).
- Ninot, J.M.; Ferré, A.; Grau, O.; Font, X.; Pérez-Haase, A.; Carrillo, E. Environmental drivers and plant species diversity in the Catalan and Andorran Pyrenees. Lazaroa 2013, 34, 89–105. [Google Scholar] [CrossRef]
- Pérez-Haase, A.; Carrillo, E.; Batriu, E.; Ninot, J.M. Diversitat de comunitats vegetals a les molleres de la Vall d’Aran (Pirineus centrals). Acta Bot. Barcinonensia 2012, 53, 61–112. Available online: https://raco.cat/index.php/ActaBotanica/article/view/252903/339641 (accessed on 1 October 2024).
- Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. Available online: http://data.europa.eu/eli/dir/1992/43/2013-07-01 (accessed on 5 October 2018).
- Garilleti, R.; Albertos, B. Atlas y Libro Rojo de los Briófitos Amenazados de España; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2012; pp. 1–287.
- Generalitat de Catalunya. Resolució AAM/732/2015, de 9 D’abril, per la Qual s’aprova la Catalogació, Descatalogació i Canvi de Categoria D’espècies i Subespècies del Catàleg de Flora Amenaçada de Catalunya; Generalitat de Catalunya: Barcelona, Spain, 2015; Available online: https://portaljuridic.gencat.cat/ca/document-del-pjur/?documentId=509129 (accessed on 15 January 2018).
- Keddy, P.A. Wetland Ecology: Principles and Conservation, 2nd ed.; Cambridge University Press: New York, NY, USA, 2010; pp. 1–497. [Google Scholar]
- Clymo, R.S.; Hayward, P.M. The ecology of Sphagnum. In Bryophyte Ecology; Smith, A.J.E., Ed.; Springer: Dordrecht, The Netherland, 1992; pp. 229–289. [Google Scholar] [CrossRef]
- Granath, G.; Strengbom, J.; Rydin, H. Rapid ecosystem shifts in peatlands: Linking plant physiology and succession. Ecology 2010, 91, 3047–3056. [Google Scholar] [CrossRef] [PubMed]
- Pladevall-Izard, E. Alpine Vegetation Dynamics and Conservation. The Pyrenean Mires. Ph.D. Thesis, University of Barcelona, Barcelona, Spain, 2022. [Google Scholar]
- Espuny, J. Experimentació per a la restauració d’hàbitats dominats per Sphagnum als Pirineus centrals. Botanique 2018, 4, 21–24. [Google Scholar]
- Abràmoff, M.; Magalhães, P.J.; Ram, S.J. Image processing with IMAGEJ. Biophoton. Int. 2004, 11, 36–42. Available online: https://imagescience.org/meijering/publications/download/bio2004.pdf (accessed on 15 November 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.scirp.org/reference/referencespapers?referenceid=3456808 (accessed on 10 October 2024).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Community Ecology Package, R Package Version 2.5-7; 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 December 2024).
- LIFE Tremedal. 2015. Available online: https://lifetremedal.eu/ (accessed on 10 July 2024).
- Martin-Vide, J.; Prohom, M.; Busto, M. Evolució recent de la temperatura, la precipitació i altres variables climàtiques a Catalunya. In Tercer Informe Sobre el Canvi Climàtic a Catalunya; Generalitat de Catalunya & Institut d’Estudis Catalans: Barcelona, Spain, 2016; pp. 93–112. [Google Scholar]
- Greenwood, O.; Mossman, H.L.; Suggitt, A.J.; Curtis, R.J.; Maclean, I.M.D. Using in situ management to conserve biodiversity under climate change. J. Appl. Ecol. 2016, 53, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.; Cooper, D.J.; Jaunatre, R.; Clément, J.-C.; Gaucherand, S. Wetland restoration: Can short-term success criteria predict long-term outcomes. Restor. Ecol. 2024, 32, e14231. [Google Scholar] [CrossRef]
- Ferland, C.; Rochefort, L. Restoration techniques for Sphagnum-dominated peatlands. Can. J. Bot. 1997, 75, 1110–1118. [Google Scholar] [CrossRef]
- Hinde, S.; Rosenburg, A.; Wright, N.; Buckler, M.; Caporn, S.; Fox, L. Sphagnum re-introduction project: A report on research into the re-introduction of Sphagnum mosses to degraded moorland. Moors Future Res. Rep. 2010, 18, 1–31. Available online: https://durham-repository.worktribe.com/output/1630539 (accessed on 10 July 2024).
- Bengtsson, F.; Granath, G.; Rydin, H. Photosynthesis, growth, and decay traits in Sphagnum—A multispecies comparison. Ecol. Evol. 2016, 6, 3325–3341. [Google Scholar] [CrossRef] [PubMed]
- Spencer, F.M.; Taylor, N. Regeneration of three Sphagnum species. Wetl. Ecol. Manag. 2005, 13, 635–645. [Google Scholar] [CrossRef]
- Keddy, P. Wetland restoration: The potential for assembly rules in the service of conservation. Wetlands 1999, 19, 716–732. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pladevall-Izard, E.; Pérez-Haase, A.; Carrillo, E.; Escolà, N.; Ninot, J.M. Restoring High Mountain Sphagnum Communities in the Central Pyrenees. Ecologies 2025, 6, 67. https://doi.org/10.3390/ecologies6040067
Pladevall-Izard E, Pérez-Haase A, Carrillo E, Escolà N, Ninot JM. Restoring High Mountain Sphagnum Communities in the Central Pyrenees. Ecologies. 2025; 6(4):67. https://doi.org/10.3390/ecologies6040067
Chicago/Turabian StylePladevall-Izard, Eulàlia, Aaron Pérez-Haase, Empar Carrillo, Nil Escolà, and Josep M. Ninot. 2025. "Restoring High Mountain Sphagnum Communities in the Central Pyrenees" Ecologies 6, no. 4: 67. https://doi.org/10.3390/ecologies6040067
APA StylePladevall-Izard, E., Pérez-Haase, A., Carrillo, E., Escolà, N., & Ninot, J. M. (2025). Restoring High Mountain Sphagnum Communities in the Central Pyrenees. Ecologies, 6(4), 67. https://doi.org/10.3390/ecologies6040067






