Projected Climate and Hydroregime Variability Constrain Ephemeral Wetland-Dependent Amphibian Populations in Simulations of Southern Toads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of Assessment Type and Model Objectives (Phase I)
2.2. Data Compilation (Phase II)
2.3. Model Decision Steps (Phase III)
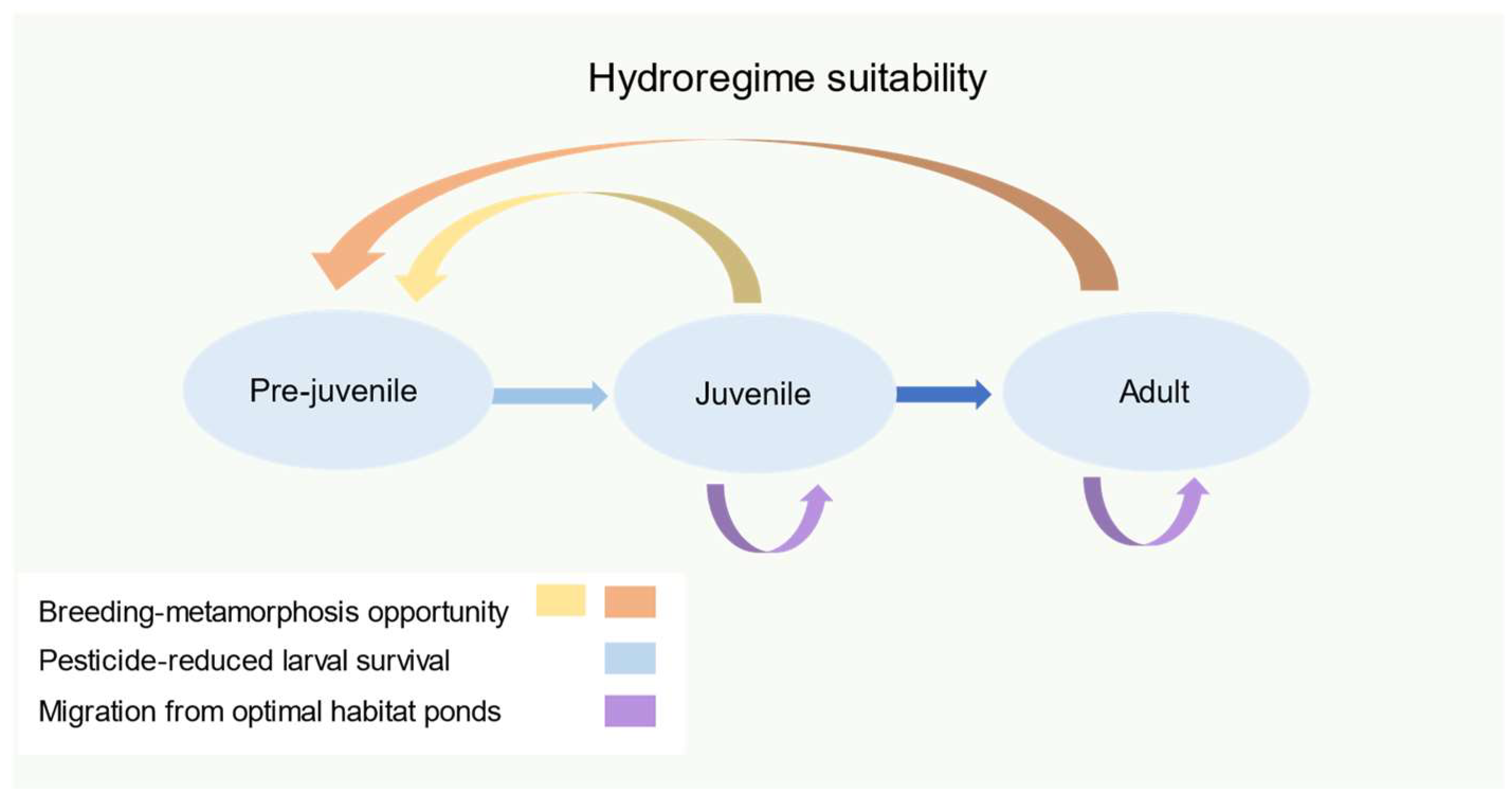
2.4. Conceptual Model (Phase IV)
3. Results
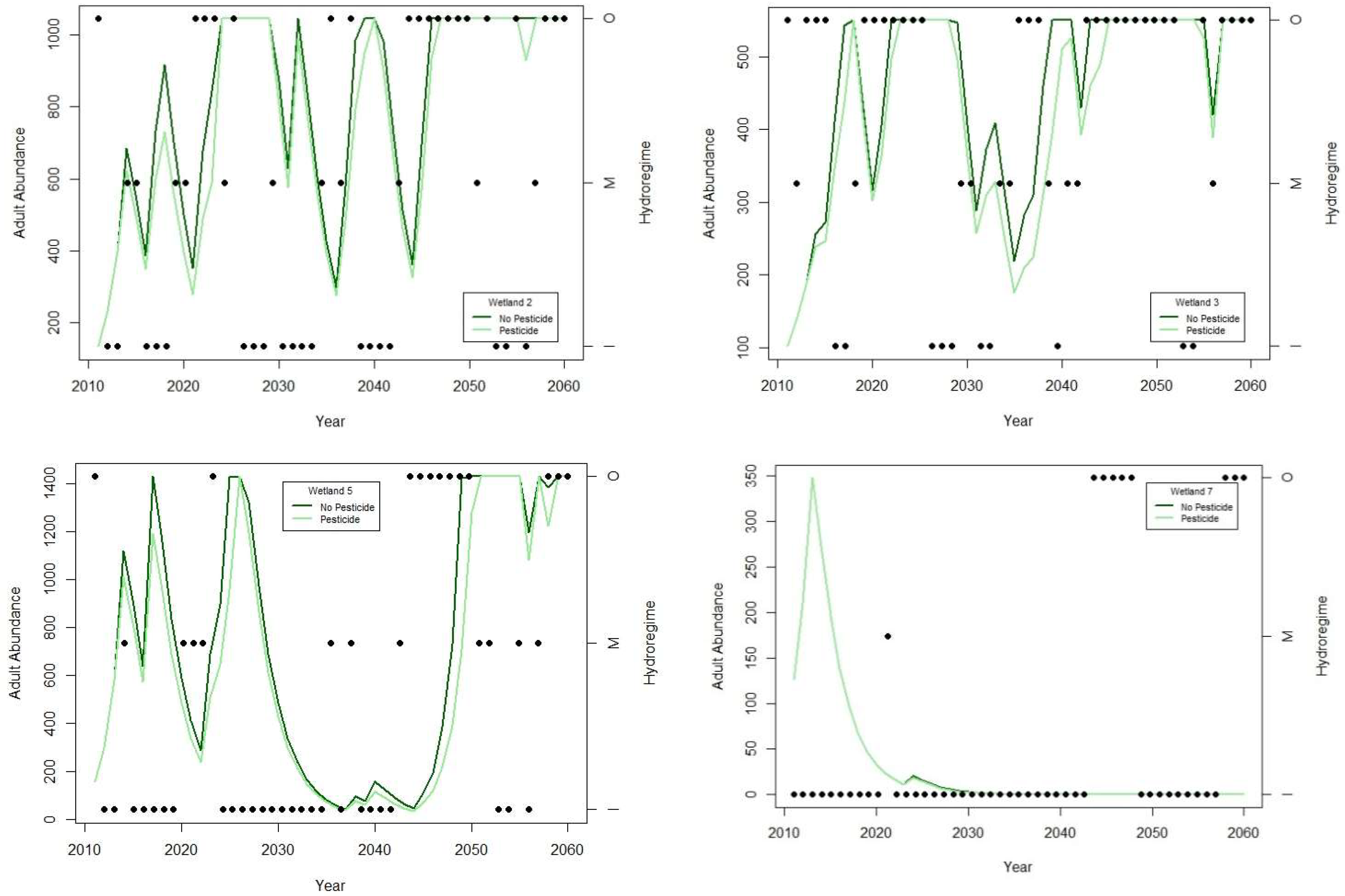
3.1. Spatial Variability in Hydroregime Suitability
3.2. Effects of Aquatic Carbaryl Exposure
3.3. Probabilistic Representation of Suitable Hydroregime Frequency
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sodhi, N.S.; Bickford, D.; Diesmos, A.C.; Lee, T.M.; Koh, L.P.; Brook, B.W.; Sekercioglu, C.H.; Bradshaw, C.J.A. Measuring the Meltdown: Drivers of Global Amphibian Extinction and Decline. PLoS ONE 2008, 3, e1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, E.H.C.; Miller, D.; Schmidt, B.R.; Adams, M.J.; Amburgey, S.M.; Chambert, T.; Cruickshank, S.; Fisher, R.N.; Green, D.M.; Hossack, B.R.; et al. Quantitative evidence for the effects of multiple drivers on continental-scale amphibian declines. Sci. Rep. 2016, 6, 25625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pechmann, J.; Scott, D.; Gibbons, J.W.; Semlitsch, R. Influence of wetland hydroperiod on diversity and abundance of metamorphosing juvenile amphibians. Wetl. Ecol. Manag. 1989, 1, 3–11. [Google Scholar] [CrossRef]
- Todd, B.D.; Scott, D.; Pechmann, J.H.K.; Gibbons, J.W. Climate change correlates with rapid delays and advancements in reproductive timing in an amphibian community. Proc. R. Soc. B Boil. Sci. 2011, 278, 2191–2197. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.A.W.; Grant, E.H.C.; Muths, E.; Amburgey, S.M.; Adams, M.J.; Joseph, M.B.; Waddle, J.H.; Johnson, P.T.J.; Ryan, M.E.; Schmidt, B.R.; et al. Quantifying climate sensitivity and climate-driven change in North American amphibian communities. Nat. Commun. 2018, 9, 3926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberg, C.H.; Zarnoch, S.J.; Austin, J.D. Weather, hydroregime, and breeding effort influence juvenile recruitment of anurans: Implications for climate change. Ecosphere 2017, 8, e01789. [Google Scholar] [CrossRef] [Green Version]
- Blaustein, A.R.; Belden, L.K.; Olson, D.H.; Green, D.M.; Root, T.L.; Kiesecker, J.M. Amphibian Breeding and Climate Change. Conserv. Biol. 2001, 15, 1804–1809. [Google Scholar] [CrossRef]
- Daszak, P.; Scott, D.; Kilpatrick, A.M.; Faggioni, C.; Gibbons, J.W.; Porter, D. Amphibian population declines at Savannah River Site are linked to climate, not chytridiomycosis. Ecology 2005, 86, 3232–3237. [Google Scholar] [CrossRef]
- Fonseca, C.R.; Coutinho, R.M.; Azevedo, F.; Berbert, J.M.; Corso, G.; Kraenkel, R.A. Modeling Habitat Split: Landscape and Life History Traits Determine Amphibian Extinction Thresholds. PLoS ONE 2013, 8, e66806. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, C.; Zarnoch, S.; Austin, J. Long term amphibian monitoring at wetlands lacks power to detect population trends. Biol. Conserv. 2018, 228, 120–131. [Google Scholar] [CrossRef]
- Forbes, V.E.; Calow, P. Population growth rate as a basis for ecological risk assessment of toxic chemicals. Philos. Trans. Biol. Sci. 2002, 357, 1299–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biek, R.; Funk, W.C.; Maxell, B.A.; Mills, L.S. What Is Missing in Amphibian Decline Research: Insights from Ecological Sensitivity Analysis. Conserv. Biol. 2002, 16, 728–734. [Google Scholar] [CrossRef]
- Awkerman, J.; Raimondo, S.; Schmolke, A.; Galic, N.; Rueda-Cediel, P.; Kapo, K.; Accolla, C.; Vaugeois, M.; Forbes, V. Guidance for Developing Amphibian Population Models for Ecological Risk Assessment. Integr. Environ. Assess. Manag. 2020, 16, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Weir, S.M.; Scott, D.E.; Salice, C.J.; Lance, S.L. Integrating copper toxicity and climate change to understand extinction risk to two species of pond-breeding anurans. Ecol. Appl. 2016, 26, 1721–1732. [Google Scholar] [CrossRef]
- Awkerman, J.A.; Raimondo, S. Simulated developmental and reproductive impacts on amphibian populations and implications for assessing long-term effects. Ecotoxicol. Environ. Saf. 2018, 149, 233–240. [Google Scholar] [CrossRef]
- Zylstra, E.R.; Steidl, R.J.; Swann, D.E.; Ratzlaff, K. Hydrologic Variability Governs Population Dynamics of a Vulnerable Amphibian in an Arid Environment. PLoS ONE 2015, 10, e0125670. [Google Scholar] [CrossRef]
- Crawford, B.A.; Maerz, J.C.; Terrell, V.C.; Moore, C.T. Population viability analysis for a pond-breeding amphibian under future drought scenarios in the southeastern United States. Glob. Ecol. Conserv. 2022, 36, e02119. [Google Scholar] [CrossRef]
- Boone, M.D.; Bridges, C.M. Impacts of chemical contaminants on amphibian populations. In Amphibian Conservation; Semlitsch, R.D., Ed.; Smithsonian Press: Washington, DC, USA, 2003; pp. 152–167. [Google Scholar]
- Gilliom, R.J.; Barbash, J.E.; Crawford, C.G.; Hamilton, P.A.; Martin, J.D.; Nakagaki, N.; Nowell, L.H.; Scott, J.C.; Stackelberg, P.E.; Thelin, G.P.; et al. The Quality of Our Nation’s Waters: Pesticides in the Nation’s Streams and Ground Water, 1992–2001; Circular 1291; U.S. Geological Survey: Reston, VA, USA, 2006.
- Boone, M.D.; Semlitsch, R.D.; Fairchild, J.F.; Rothermel, B. Effects of an insecticide on amphibians in large-scale experimental ponds. Ecol. Appl. 2004, 14, 685–691. [Google Scholar] [CrossRef] [Green Version]
- Distel, C.A.; Boone, M.D. Effects of aquatic exposure to the insecticide carbaryl are species-specific across life stages and mediated by heterospecific competitors in anurans. Funct. Ecol. 2010, 24, 1342–1352. [Google Scholar] [CrossRef]
- Wood, L.; Welch, A.M. Assessment of interactive effects of elevated salinity and three pesticides on life history and behavior of southern toad (Anaxyrus terrestris) tadpoles. Environ. Toxicol. Chem. 2015, 34, 667–676. [Google Scholar] [CrossRef]
- Pastorok, R.A.; Bartell, S.M.; Ferson, S.; Ginzburg, L.R. Ecological modeling in risk assessment. Hum. Ecol. Risk Assess. 2002, 9, 939–972. [Google Scholar] [CrossRef]
- Morris, W.F.; Doak, D.F. Quantitative Conservation Biology: Theory and Practice of Population Viability Analysis; Sinauer Associates: Sunderland, MA, USA, 2003; 480p. [Google Scholar]
- Barnthouse, L.W.; Munns, W.R.; Sorenson, M.T. (Eds.) Population-Level Ecological Risk Assessment; Taylor and Francis: Boca Raton, FL, USA, 2008; 376p. [Google Scholar]
- Forbes, V.E.; Calow, P. Developing predictive systems models to address complexity and relevance for ecological risk assessment. Integr. Environ. Assess. Manag. 2013, 9, e75–e80. [Google Scholar]
- Schmolke, A.; Kapo, K.E.; Rueda-Cediel, P.; Thorbek, P.; Brain, R.; Forbes, V. Developing population models: A systematic approach for pesticide risk assessment using herbaceous plants as an example. Sci. Total Environ. 2017, 599–600, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Etterson, M.; Pollesch, N.; Garber, K.; Kanarek, A.; Lehmann, W.; Awkerman, J. A framework for linking population model development with ecological risk assessment objectives. Integr. Environ. Assess. Manag. 2017, 14, 369–380. [Google Scholar] [CrossRef]
- Raimondo, S.; Schmolke, A.; Pollesch, N.; Accolla, C.; Galic, N.; Moore, A.; Vaugeois, M.; Rueda-Cediel, P.; Kanarek, A.; Awkerman, J.; et al. Pop-guide: Population modeling guidance, use, interpretation, and development for ecological risk assessment. Integr. Environ. Assess. Manag. 2021, 17, 767–784. [Google Scholar] [CrossRef]
- Greenberg, C.H.; Goodrick, S.; Austin, J.; Parresol, B.R. Hydroregime Prediction Models for Ephemeral Groundwater-Driven Sinkhole Wetlands: A Planning Tool for Climate Change and Amphibian Conservation. Wetlands 2015, 35, 899–911. [Google Scholar] [CrossRef]
- Jeffrey, S.; Rotstayn, L.; Collier, M.; Dravitzki, S.; Hamalainen, C.; Moeseneder, C.; Wong, K.; Syktus, J. Australia’s CMIP5 submission using the CSIRO-Mk3.6 model. Aust. Meteorol. Oceanogr. J. 2013, 63, 1–13. [Google Scholar] [CrossRef]
- Raju, K.S.; Kumar, D.N. Review of approaches for selection and ensembling of GCMs. J. Water Clim. Chang. 2020, 11, 577–599. [Google Scholar] [CrossRef]
- Wright, A.N.; Schwartz, M.; Hijmans, R.J.; Shaffer, H.B. Advances in climate models from CMIP3 to CMIP5 do not change predictions of future habitat suitability for California reptiles and amphibians. Clim. Chang. 2016, 134, 579–591. [Google Scholar] [CrossRef]
- Levins, R. The strategy of model building in population biology. Am. Sci. 1966, 54, 421–431. [Google Scholar]
- Greenberg, C.H. Spatio-Temporal Dynamics of Pond Use and Recruitment in Florida Gopher Frogs (Rana capito aesopus). South Am. J. Herpetol. 2001, 35, 74. [Google Scholar] [CrossRef]
- Harper, E.B.; Semlitsch, R.D. Density dependence in the terrestrial life history stage of two anurans. Oecologia 2007, 153, 879–889. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 5 June 2022).
- Semlitsch, R.D.; Bodie, J.R. Are small, isolated wetlands expendable? Conserv. Biol. 1998, 12, 1129–1133. [Google Scholar] [CrossRef] [Green Version]
- Amburgey, S.M.; Murphy, M.; Funk, W.C. Phenotypic plasticity in developmental rate is insufficient to offset high tadpole mortality in rapidly drying ponds. Ecosphere 2016, 7, e01386. [Google Scholar] [CrossRef]
- Earl, J.E. Evaluating the assumptions of population projection models used for conservation. Biol. Conserv. 2019, 237, 145–154. [Google Scholar] [CrossRef]
- Walls, S.C.; Barichivich, W.J.; Brown, M.E. Drought, Deluge and Declines: The Impact of Precipitation Extremes on Amphibians in a Changing Climate. Biology 2013, 2, 399–418. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, C.H.; Perry, R.W.; Franzreb, K.E.; Loeb, S.C.; Saenz, D.; Rudolph, D.C.; Winters, E.; Fucik, E.M.; Kwiatkowski, M.A.; Parresol, B.R.; et al. Climate change and wildlife in the southern United States: Potential effects and management options. In Climate Change Adaption and Mitigation Management Options: A Guide for Natural Resource Managers in Southern Forest Ecosystems; CRC Press—Taylor and Francis: Boca Raton, FL, USA, 2014; pp. 379–420. 42p. [Google Scholar]
- Brooks, R.T.; Hayashi, M. Depth-area-volume and hydroperiod relationships of ephemeral (vernal) forest pools in southern New England. Wetlands 2002, 22, 247–255. [Google Scholar] [CrossRef]
- Cartwright, J.M.; Wolfe, W.J. Increasing Hydroperiod in a Karst-depression Wetland Based on 165 Years of Simulated Daily Water Levels. Wetlands 2021, 41, 75. [Google Scholar] [CrossRef]
- Sáenz, D.; Fitzgerald, L.A.; Baum, K.; Conner, R.N. Abiotic correlates of anuran calling phenology: The importance of rain, temperature, and season. Herpetol. Monogr. 2006, 20, 64–82. [Google Scholar] [CrossRef]
- Rowe, C.L.; Dunson, W.A. Impacts of hydroperiod on growth and survival of larval amphibians in temporary ponds of Central Pennsylvania, USA. Oecologia 1995, 102, 397–403. [Google Scholar] [CrossRef]
- Beck, C.W.; Congdon, J.D. Effects of individual variation in age and size at metamorphosis on growth and survivorship of southern toad (Bufo terrestris) metamorphs. Can. J. Zool. 1999, 77, 944–951. [Google Scholar] [CrossRef]
- Schmidt, B.R.; Hödl, W.; Schaub, M. From metamorphosis to maturity in complex life cycles: Equal performance of different juvenile life history pathways. Ecology 2012, 93, 657–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohba, S.-Y. Density-Dependent Effects of Amphibian Prey on the Growth and Survival of an Endangered Giant Water Bug. Insects 2011, 2, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, C.; Johnson, S.; Owen, R.; Storfer, A. Amphibian breeding phenology and reproductive outcome: An examination using terrestrial and aquatic sampling. Can. J. Zool. 2017, 95, 673–684. [Google Scholar] [CrossRef] [Green Version]

| Characteristic | Estimate | Source | |
|---|---|---|---|
| Organism-level characteristics | Life span | 10 years | AnAge database |
| Reproductive season | mid-February to mid-August | [30] | |
| Reproductive frequency | hydroregime dependent | [30] | |
| Clutch size | 3829 first year, 4431 adult years | [14] | |
| Onset of maturation | 3 years, female | AnAge database; [14] | |
| Embryo-larval survival | 0.012 | Based on [14] | |
| Metamorph survival | 0.3 | [14] | |
| Juvenile survival | 0.3 | Based on [14] | |
| Immature transition rate | 0.1 | Based on [14] | |
| Sex ratio | 0.5 | assumed even | |
| Adult survival | 0.7 | Based on [14] | |
| Population and spatial characteristics | Density dependence | in larval development, terrestrial capacity | [31,35] |
| Population size | wetland-specific density | [30] | |
| Spatial structure | small breeding wetlands with minimal migration | [30] | |
| Movement | low percentage of migration between close wetlands | [6] | |
| Habitat features | eight ephemeral breeding wetlands | [30] | |
| Geographical range | US southeast | [30] | |
| Hydroregime suitability | hydroregime sufficient for larval survival | [30] | |
| Extrinsic factors | Predation | no fish predation in temporary wetlands; no aquatic invertebrate predation included here | [30] |
| Competition | not evident | assumption | |
| Environmental conditions | highly variable hydroregime | [30] | |
| Pathogens | many; not identified in breeding range | assumption | |
| Abiotic stressors | habitat degradation; not identified in study area | assumption | |
| Management | not included in model | NA | |
| Indirect effects | not included in model | NA | |
| Stochasticity | environmental | [30] | |
| Exposure and effects characterization | Chemical exposure | carbaryl exposure | simulation |
| Temporal exposure | larval stage | assumption | |
| Exposure across habitat | aquatic habitat | simulation | |
| Toxic effects | reduced survival | assumption | |
| Effects by life stage | reduced larval survival | [20] | |
| Effects by exposure route | not specified | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awkerman, J.A.; Greenberg, C.H. Projected Climate and Hydroregime Variability Constrain Ephemeral Wetland-Dependent Amphibian Populations in Simulations of Southern Toads. Ecologies 2022, 3, 235-248. https://doi.org/10.3390/ecologies3020018
Awkerman JA, Greenberg CH. Projected Climate and Hydroregime Variability Constrain Ephemeral Wetland-Dependent Amphibian Populations in Simulations of Southern Toads. Ecologies. 2022; 3(2):235-248. https://doi.org/10.3390/ecologies3020018
Chicago/Turabian StyleAwkerman, Jill A., and Cathryn H. Greenberg. 2022. "Projected Climate and Hydroregime Variability Constrain Ephemeral Wetland-Dependent Amphibian Populations in Simulations of Southern Toads" Ecologies 3, no. 2: 235-248. https://doi.org/10.3390/ecologies3020018
APA StyleAwkerman, J. A., & Greenberg, C. H. (2022). Projected Climate and Hydroregime Variability Constrain Ephemeral Wetland-Dependent Amphibian Populations in Simulations of Southern Toads. Ecologies, 3(2), 235-248. https://doi.org/10.3390/ecologies3020018





