Simulating the Effects of Pesticides on Honey Bee (Apis mellifera L.) Colonies with BeePop+
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Phase 1: Model Objectives
3.1.1. Determining Necessary Level of Realism and Complexity
3.1.2. How Model Outputs Link to Measurement Endpoints and Protection Goals
3.1.3. Temporal and Spatial Considerations
3.1.4. Assumptions and Sources of Uncertainty
3.2. Phase 2: Data Compilation
3.3. Phase 3: Decision Steps
3.4. Phase 4: Conceptual Model
3.5. Phase 5: Model Implementation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Decourtye, A.; Alaux, C.; Le Conte, Y.; Henry, M. Toward the protection of bees and pollination under global change: Present and future perspectives in a challenging applied science. Curr. Opin. Insect Sci. 2019, 35, 123–131. [Google Scholar] [CrossRef]
- Chopra, S.S.; Bakshi, B.R.; Khanna, V. Economic dependence of US industrial sectors on animal-mediated pollination service. Environ. Sci. Technol. 2015, 49, 14441–14451. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Patch, H.M.; Grozinger, C.M.; Khanna, V. Economic Dependence and Vulnerability of United States Agricultural Sector on Insect-Mediated Pollination Service. Environ. Sci. Technol. 2021, 55, 2243–2253. [Google Scholar] [CrossRef]
- Goodrich, B.; Williams, J.C.; Goodhue, R.E. The great bee migration: Supply analysis of honey bee colony shipments into California for almond pollination services. Am. J. Agric. Econ. 2019, 101, 1353–1372. [Google Scholar] [CrossRef]
- Sumner, D.A.; Matthews, W.A.; Medellin-Azuara, J.; Bradley, A. University of California Agricultural Issues Center. The Economic Impacts of the California Almond Industry. 2016. Available online: http://aic.ucdavis.edu/almonds/Economic%20Impacts%20of%20California%20Almond%20Industry_Full%20Report_FinalPDF_v2.pdf (accessed on 15 May 2022).
- De Grandi-Hoffman, G.; Graham, H.; Ahumada, F.; Smart, M.; Ziolkowski, N. The economics of honey bee (Hymenoptera: Apidae) management and overwintering strategies for colonies used to pollinate almonds. J. Econ. Entomol. 2019, 112, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- Klobuchar, A. The Economic Contribution of America’s Farmers and the Importance of Agricultural Exports; Joint Economic Committee, US Congress: Washington, DC, USA, 2013. Available online: https://www.jec.senate.gov/public/_cache/files/266a0bf3-5142-4545-b806-ef9fd78b9c2f/jec-agriculture-report.pdf (accessed on 15 May 2022).
- Seeram, N.P. Berry fruits: Compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J. Agric. Food Chem. 2008, 56, 627–629. [Google Scholar] [CrossRef]
- Ros, E.; Tapsell, C.L.; Sabaté, J. Nuts and berries for heart health. Curr. Atheroscler. Rep. 2010, 12, 397–406. [Google Scholar] [CrossRef]
- Van Engelsdorp, D.; Hayes, J.; Underwood, R.M.; Pettis, J. A survey of honey bee colony losses in the U.S., fall 2007 to spring 2008. PLoS ONE 2008, 3, e4071. [Google Scholar]
- Potts, S.G.; Roberts, S.; Dean, R.; Marris, G.; Brown, M.; Jones, R.; Neumann, P.; Settele, J. Declines of managed honey bees and beekeepers in Europe. J. Apic. Res. 2010, 49, 15–22. [Google Scholar] [CrossRef]
- Brodschneider, R.; Gray, A.; Adjlane, N.; Ballis, A.; Brusbardis, V.; Charrière, J.D.; Chlebo, R.; Coffey, M.F.; Dahle, B.; de Graaf, D.C.; et al. Multi-country loss rates of honey bee colonies during winter 2016/2017 from the COLOSS survey. J. Apic. Res. 2018, 57, 452–457. [Google Scholar] [CrossRef] [Green Version]
- Steinhauer, N.; Aurell, D.; Bruckner, S.; Wilson, M.; Renmich, K.; vanEnglesdorp, D.; Williams, G. United States Honey Bee Colony Losses 2020–2021: Preliminary Results. 2021. Available online: https://beeinformed.org/2021/06/21/united-states-honey-bee-colony-losses-2020-2021-preliminary-results/ (accessed on 15 May 2022).
- Vilsack, T.; McCarthy, G. National Strategy to Promote the Health of Honey Bees and other Pollinators; DIANE Publishing Company: Collingdale, PA, USA, 2015. [Google Scholar]
- Steinhauer, N.; Kulhanek, K.; Antúnez, K.; Human, H.; Chantawannakul, P.; Chauzat, M.P. Drivers of colony losses. Curr. Opin. Insect Sci. 2018, 26, 142–148. [Google Scholar] [CrossRef] [PubMed]
- USDA. 2021 USDA Annual Strategic Pollinator Priorities and Goals Report; USDA: Washington, DC, USA, 2021.
- USEPA; PMRA; CDPR. Guidance for Assessing Pesticide Risks to Bees. Office of Pesticide Programs United States Environmental Protection Agency, Health Canada Pest Management Regulatory Agency (PMRA), California Department of Pesticide Regulation (CDPR). 19 June 2014. Available online: https://www.epa.gov/sites/default/files/2014-06/documents/pollinator_risk_assessment_guidance_06_19_14.pdf (accessed on 15 May 2022).
- USEPA. BeeREX; Version 1.0; Office of Pesticide Programs, United States Environmental Protection Agency: Washington, DC, USA, 2015. Available online: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/models-pesticide-risk-assessment#beerex (accessed on 15 May 2022).
- USEPA. White Paper in Support of the Proposed Risk Assessment Process for Bees; Submitted to the FIFRA Scientific Advisory Panel for Review and Comment 11–14 September 2012. Available online: http://cues.cfans.umn.edu/old/pollinators/pdf-EPA/EAP-SAP-whitepaper.pdf (accessed on 15 May 2022).
- Raimondo, S.; Schmolke, A.; Pollesch, N.; Accolla, C.; Galic, N.; Moore, A.; Vaugeois, M.; Rueda-Cediel, P.; Kanarek, A.; Awkerman, J.; et al. Pop-GUIDE: Population modeling guidance, use, interpretation, and development for ecological risk assessment. Integr. Environ. Assess. Manag. 2020, 17, 767–784. [Google Scholar] [CrossRef] [PubMed]
- DeGrandi-Hoffman, G.; Curry, R. A mathematical model of Varroa mite (Varroa destructor Anderson and Trueman) and honeybee (Apis mellifera L.) population dynamics. Int. J. Acarol. 2004, 30, 259–274. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Roth, S.A.; Loper, G.L.; Erickson, E.H. BEEPOP—A Honeybee Population-Dynamics Simulation-Model. Ecol. Model. 1989, 45, 133–150. [Google Scholar] [CrossRef]
- Fischer, D.; Moriarty, T. Pesticide Risk Assessment for Pollinators; Wiley: New York, NY, USA, 2014; ISBN 978-1-118-85252-1. [Google Scholar]
- Kuan, A.C.; DeGrandi-Hoffman, G.; Curry, R.J.; Garber, K.V.; Kanarek, A.R.; Snyder, M.N.; Wolfe, K.L.; Purucker, S.T. Sensitivity analyses for simulating pesticide impacts on honey bee colonies. Ecol. Model. 2018, 376, 15–27. [Google Scholar] [CrossRef]
- Minucci, J.M.; Curry, R.; DeGrandi-Hoffman, G.; Douglass, C.; Garber, K.; Purucker, S.T. Inferring pesticide toxicity to honey bees from a field-based feeding study using a colony model and Bayesian inference. Ecol. Appl. 2021, 31, e02442. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Guidelines for Ecological Risk Assessment; Federal Register 63: 26846–26924; USEPA: Washington, DC, USA, 1998.
- Lundie, A.E. The Flight Activities of the Honey Bee; U.S. Department of Agriculture: Washington, DC, USA, 1925; 37p.
- Rashad, S.E. Some Factors Effecting Pollen Collection by Honey Bees and Pollen as a Limiting Factor in Brood Rearing and Honey Production. Ph.D. Thesis, Kansas State College, Manhattan, KS, USA, 1957; 88p. [Google Scholar]
- Horn, J.; Becher, M.A.; Kennedy, P.J.; Osborne, J.L.; Grimm, V. Multiple stressors: Using the honeybee model BEEHAVE to explore how spatial and temporal forage stress affects colony resilience. Oikos 2016, 125, 1001–1016. [Google Scholar] [CrossRef] [Green Version]
- Frazier, M.T.; Mullin, C.A.; Frazier, J.L.; Ashcraft, S.A.; Leslie, T.W.; Mussen, E.C.; Drummond, F.A. Assessing Honey Bee (Hymenoptera: Apidae) Foraging Populations and the Potential Impact of Pesticides on Eight U.S. Crops. J. Econ. Entomol. 2015, 108, 2141–2152. [Google Scholar] [CrossRef] [Green Version]
- Colwell, M.J.; Williams, G.R.; Evans, R.C.; Shutler, D. Honey bee-collected pollen in agro-ecosystems reveals diet diversity, diet quality, and pesticide exposure. Ecol. Evol. 2017, 7, 7243–7253. [Google Scholar] [CrossRef]
- MacInnis, C.I.; Keddie, B.A.; Pernal, S.F. Nosema ceranae (Microspora: Nosematidae): A Sweet Surprise? Investigating the Viability and Infectivity of N. ceranae Spores Maintained in Honey and on Beeswax. J. Econ. Entomol. 2020, 113, 2069–2078. [Google Scholar] [CrossRef]
- Nürnberger, F.; Härtel, S.; Steffan-Dewenter, I. Seasonal timing in honey bee colonies: Phenology shifts affect honey stores and varroa infestation levels. Oecologia 2019, 189, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Pettis, J.S.; van Engelsdorp, D.; Johnson, J.; Dively, G. Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften 2012, 99, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doublet, V.; Labarussias, M.; de Miranda, J.R.; Moritz, R.F.; Paxton, R.J. Bees under stress: Sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ. Microbiol. 2015, 17, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Schmolke, A.; Kapo, K.E.; Rueda-Cediel, P.; Thorbek, P.; Brain, R.; Forbes, V. Developing population models: A systematic approach for pesticide risk assessment using herbaceous plants as an example. Sci. Total Environ. 2017, 599–600, 1929–1938. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Botías, C.; Bailón, E.G.; González-Porto, A.V.; Barrios, L.; Del Nozal, M.J.; Bernal, J.L.; Jiménez, J.J.; Palencia, P.G.; et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 2008, 10, 2659–2669. [Google Scholar] [CrossRef]
- Naug, D. Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biol. Conserv. 2009, 142, 2369–2372. [Google Scholar] [CrossRef]
- Van Engelsdorp, D.; Lengerich, E.; Spleen, A.; Dainat, B.; Cresswell, J.; Baylis, K.; Nguyen, B.K.; Soroker, V.; Underwood, R.; Human, H.; et al. Standard epidemiological methods to understand and improve Apis mellifera health. J. Apic. Res. 2013, 52, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Becher, M.A.; Grimm, V.; Thorbek, P.; Horn, J.; Kennedy, P.J.; Osborne, J.L. BEEHAVE: A systems model of honeybee colony dynamics and foraging to explore multifactorial causes of colony failure. J. Appl. Ecol. 2014, 51, 470–482. [Google Scholar] [CrossRef] [Green Version]
- Baer, B.; Collins, J.; Maalaps, K.; den Boer, S.P. Sperm use economy of honeybee (Apis mellifera) queens. Ecol. Evol. 2016, 6, 2877–2885. [Google Scholar] [CrossRef]
- Free, J.B.; Ferguson, A.W.; Simpkins, J.R. The behaviour of queen honeybees and their attendants. Physiol. Entomol. 1992, 17, 43–55. [Google Scholar] [CrossRef]
- Beekman, M.; Ratnieks, F.L.W. Long-range foraging by the honey-bee, Apis mellifera L. Funct. Ecol. 2000, 14, 490–496. [Google Scholar] [CrossRef] [Green Version]
- USEPA. Pesticide in Water Calculator, Version 2.001. Available online: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/models-pesticide-risk-assessment#PWC (accessed on 15 May 2022).
- Tremolada, P.; Bernardinelli, I.; Colombo, M.; Spreafico, M.; Vighi, M. Coumaphos distribution in the hive ecosystem: Case study for modeling applications. Ecotoxicology 2004, 13, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Traynor, K.S.; Pettis, J.S.; Tarpy, D.R.; Mullin, C.A.; Frazier, J.L.; Frazier, M.; Vanengelsdorp, D. In-hive Pesticide Exposome: Assessing risks to migratory honey bees from in-hive pesticide contamination in the Eastern United States. Sci. Rep. 2016, 6, 33207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sammataro, D.; Gerson, U.; Needham, G. Parasitic mites of honey bees: Life history, implications, and impact. Annu. Rev. Entomol. 2000, 45, 519–548. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Yao, J.; Wang, Y. Varroa mite and deformed wing virus infestations interactively make honey bees (Apis mellifera) more susceptible to insecticides. Environ. Pollut. 2022, 292, 118212. [Google Scholar] [CrossRef] [PubMed]
- Pasho, D.J.; Applegate, J.R.; Hopkins, D.I. Diseases and pests of honey bees (Apis mellifera). Vet. Clin. Food Anim. Pract. 2021, 37, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Schmehl, D.R.; Mullin, C.A.; Frazier, J.L. Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PLoS ONE 2014, 9, e77547. [Google Scholar] [CrossRef]
- DeJong, D.; DeJong, P.H. Longevity of Africanized honey bees (Hymenoptera: Apidae) infested by Varroa jacobsoni (Parasitiformes: Varroidae). J. Econ. Entomol. 1983, 76, 766–768. [Google Scholar] [CrossRef]
- Kovac, H.; Crailsheim, K. Lifespan of Apis mellifera carnica Pollm. infested by Varroa jacobsoni Oud. in relation to season and extent of infestation. J. Apic. Res. 1988, 27, 230–238. [Google Scholar] [CrossRef]
- BeePop+ Source Code. 2022. Available online: https://doi.org/10.5281/zenodo.6856426 (accessed on 15 May 2022).
- Varroapy Python Wrapper. 2022. Available online: https://doi.org/10.5281/zenodo.3550199 (accessed on 15 May 2022).
- Laurino, D.; Porporato, M.; Patetta, A.; Manino, A. Toxicity of neonicotinoid insecticides to honey bees: Laboratory tests. Bull. Insectology 2011, 64, 107–113. [Google Scholar]
- USEPA. Preliminary Bee Risk Assessment to Support the Registration Review of Clothianidin and Thiamethoxam. 2017. Available online: https://www.regulations.gov/document/EPA-HQ-OPP-2011-0865-0173 (accessed on 15 May 2022).
- Becher, M.A.; Osborne, J.L.; Thorbek, P.; Kennedy, P.J.; Grimm, V. Towards a systems approach for understanding honeybee decline: A stocktaking and synthesis of existing models. J. Appl. Ecol. 2013, 50, 868–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; DeGrandi-Hoffman, G.; Ratti, V.; Kang, Y. Review on mathematical modeling of honeybee population dynamics. Math. Biosci. Eng. 2021, 18, 9606–9650. [Google Scholar] [CrossRef]
- Southwick, E.E. Allometric relations, metabolism and heat conductance in clusters of honey bees at cool temperatures. J. Comp. Physiol. B 1985, 156, 143–149. [Google Scholar] [CrossRef]
- University of Wyoming Agricultural Experiment Station. Bulletin No. 187—The Metabolism of Honeybees in Winter Cluster. Univ. Wyoming Agricult. Experiment Stat. Bull. 1932, 187, 1–32. [Google Scholar]
- Environmental Fate and Effects Division, Environmental Protection Agency, Washington DC. California Department of Pesticide Regulation. Environmental Assessment Directorate, Pest Management Regulatory Agency, Health Canada. Available online: http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPP-2012-0543-0004 (accessed on 31 March 2022).
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1987. [Google Scholar]


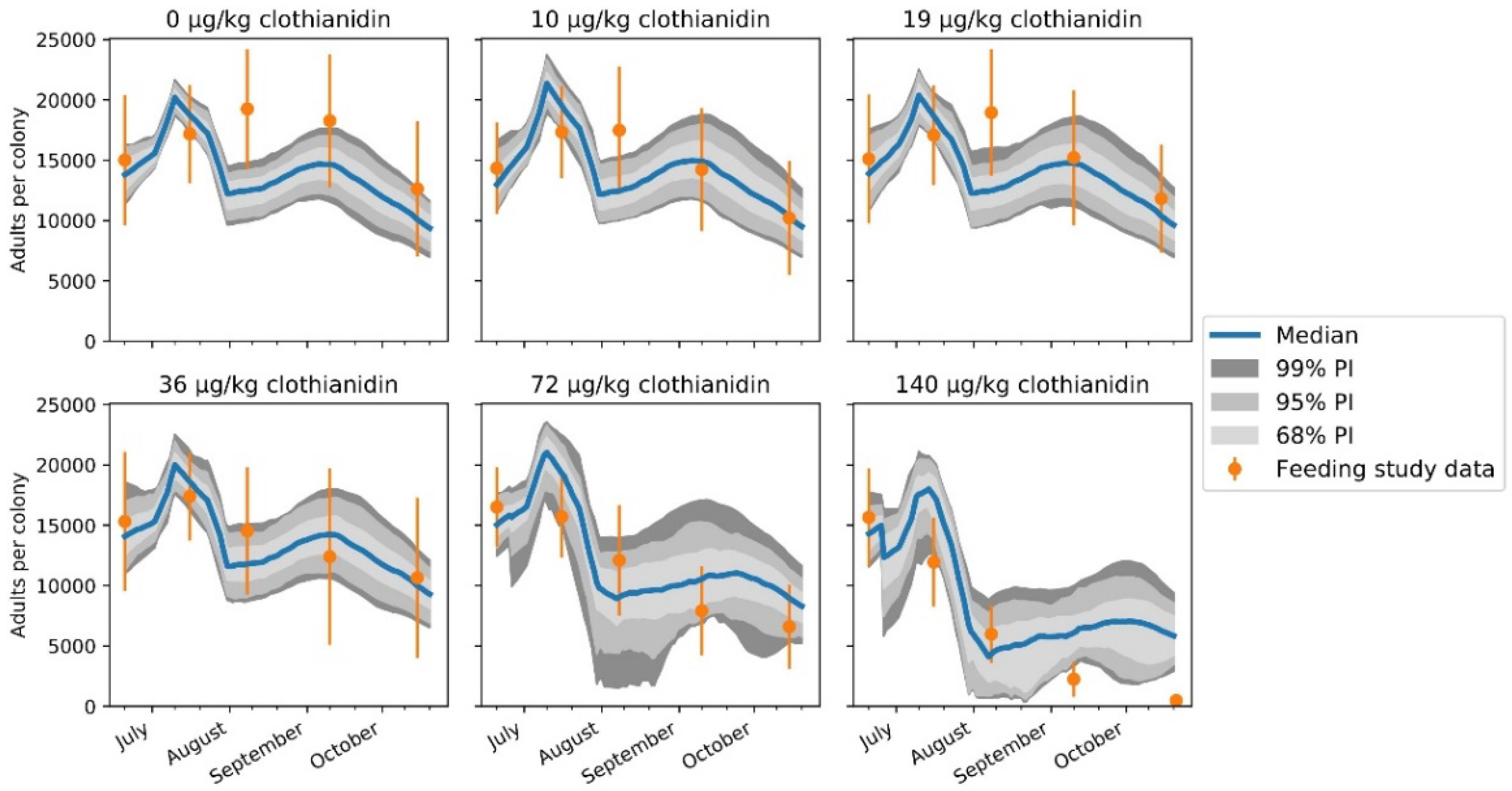
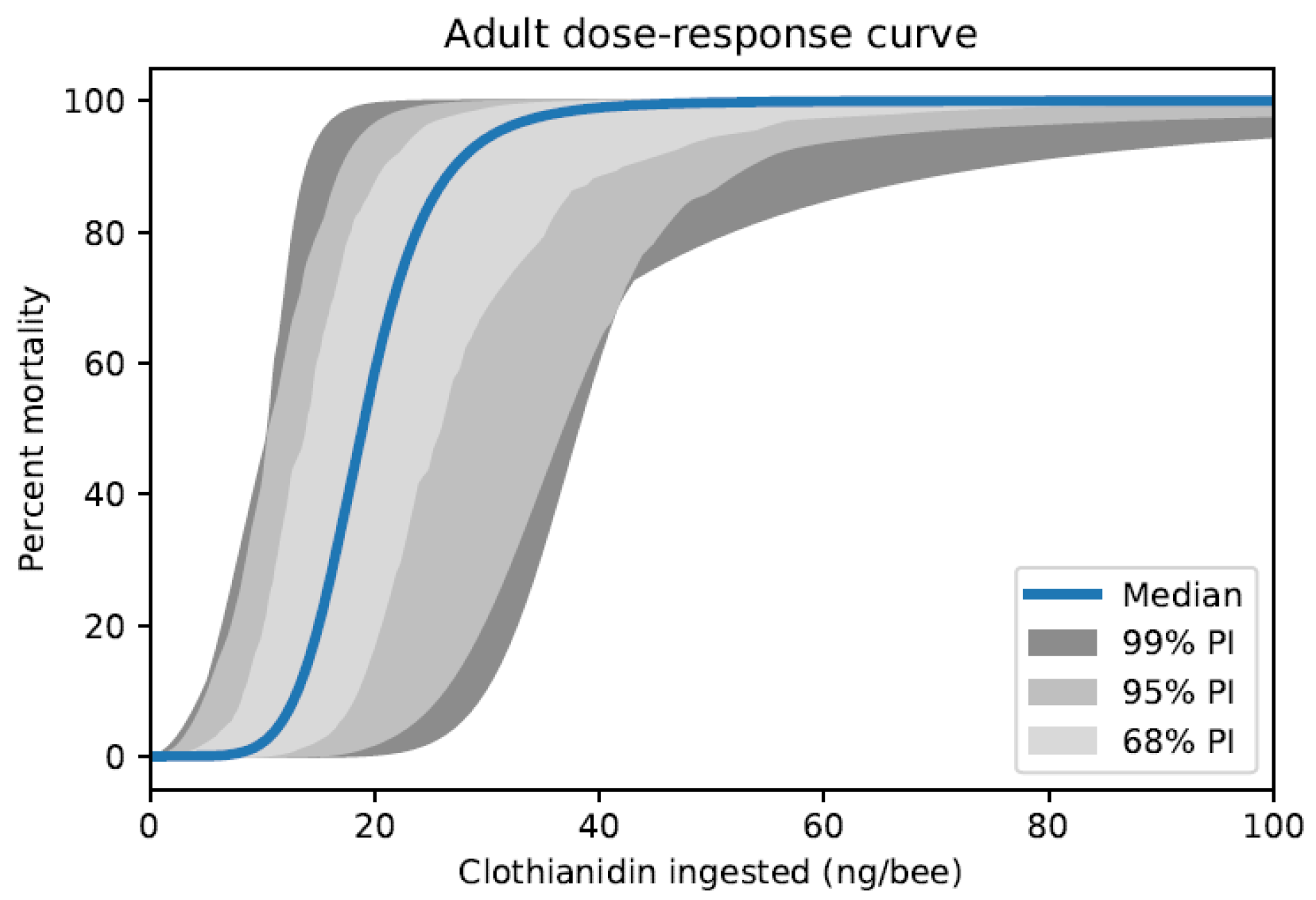
| Protection Goal | Assessment Endpoints | Measurement Endpoints Typically Used in Risk Assessments | |
|---|---|---|---|
| Individual Level | Colony/Population Level | ||
| Provision of pollination services | Numbers of colonies, population size, stability of native bees and commercially managed bees | Adult worker survival; larval survival; pupal survival; adult growth; adult emergence | Number each life stage present over time; presence of queen |
| Production of colony products | Quantity and quality of colony products | Amount of pollen and nectar present in colony; presence of pesticide in colony products | |
| Contribution to bee biodiversity | Species richness and abundance | Species richness and abundance | |
| Characteristic | Ecological Concept | Specification/Parameter (Simplifying Assumptions) |
|---|---|---|
| Stage-level energetics [17] | Different stages (adult, larvae, drones, queens) have specific energetic needs that are met by the consumption of nectar/honey and pollen. | Stage-specific energetic needs are specified directly as model parameters represented by consumption rates. |
| Active season colony-level energetics [17] | Sufficient energy and pollen availabilities are required to raise brood | Nectar and pollen consumption rates for brood stages are included as parameters. |
| Overwintering colony-level energetics [37,38,39] | Sufficient energy reserves and adult members (due to thermoregulation needs) are required to overwinter | Overwinter dynamics are simulated by significantly reduced foraging and egg-laying behavior, as dictated by daylength and temperature. |
| Colony growth * [21,22] | Colony growth is determined by the rate of egg-laying by the queen, the mortality of each stage of bees in the colony, and the density of the colony. | Egg-laying rate is defined as queen strength parameter and is influenced by a function incorporating photoperiod, temperature, and colony size. Natural stage-based mortality rates are included directly as parameters. Mortality also occurs due to pesticide exposure and is modeled via a pesticide exposure module. Queen replacement can be simulated to represent a common beekeeping practice. Queen longevity. Colony size increase due to egg-laying rates, inverse for mortality. |
| Colony population structure and stage-specific rates [22,29,39,40,41] | Stage transition rates and proportion of drones influenced by photoperiod, local climate, colony size, and resource availability. Pesticide tolerance varies by stage and across colonies. | Stage-specific populations (egg, larvae, pupae, adults (drones, workers, foragers) controlled by egg-laying rate and stage-specific development rates and division of duties. Parameter specifying proportion of eggs that become workers and drones influenced by time of year (photoperiod). Parameter specifying proportion of eligible foragers actively foraging. Parameter specifying amount of sperm influences laying of fertilized eggs (lack of sperm results in laying drones). |
| Colony resource collection (by foragers) [22,29,42,43,44] | Empirical data to determine thresholds for foraging activity are limited. Forager activity influenced by temperature, wind velocity, and rainfall. Forage range varies from several hundred meters to 5500 m from colony. Foraging range and time influenced by landscape composition. | Foraging activity limited to threshold as determined by weather parameters (e.g., rainfall, temperature). No explicit spatial component of resources. Constant/unlimited resource availability is assumed. Parameter specifying number of loads and trips made by foragers for pollen and nectar. |
| Seasonal pollen and nectar consumption | Sufficient pollen and nectar resources are required to rear brood, with excess resources collected during the foraging season stored in colony. A colony fails if it runs out of resources over winter. | Specified food consumption rates for different life stages and duties differ by seasons. Minimum energy reserve and adult population (for thermoregulation needs) requirements to survive overwinter are specified. Model allows for simulated supplemental feeding, a common beekeeper practice. |
| Characteristic | Ecological Concept | Specification/Parameter (Simplifying Assumptions) |
|---|---|---|
| Exposure pathway [45] | Consumption of nectar and pollen contaminated with pesticide(s). Contact exposure for foraging bees. | In colony, bees (larvae and adults) have age and stage-specific food consumption rates. Their pesticide dose is a function of food intake rate and pesticide concentration in pollen and nectar. Does not account for in-colony transfer and transformation of pesticide residues. Pollen and nectar (honey) stores are assumed to be well mixed, but they are actually stored in individual cells with variable pesticide concentrations. Does not account for exposure via plant guttation water. |
| Timing of exposures [46] | Contact exposure occurs during application. Dietary exposure happens while crops are blooming. | Migratory bee colonies (for pollination services) may have repeated exposures. Exposure may occur past the growing season when stored nectar and pollen are contaminated with persistent pesticide. |
| Exposure pattern within habitat | Approach assumes that colonies are only feeding on treated crops. | Available information indicates that bees forage on a variety of plants, not just crops. Model can be adjusted to calculate percent of foraging on treated crop needed to impact a colony. |
| Exposure profile by stage [18] | Stage-specific food consumption rates account for different exposure levels among life stages. | BeeREX includes information on stage and age-specific food consumption rates. Food consumption rates can be varied in Monte Carlo simulation. |
| Representation of toxic effects | Standard laboratory-based toxicity information for adult and larval bees used to calculate magnitude of mortality resulting from specific doses. Accounts for exposures and effects of a pesticide active ingredient. | Magnitude of mortality in a cohort corresponds to magnitude of exposure and dose-response curve from standard LD50 study. Does not account for sublethal effects; however, user can adjust adult longevity or decrease foraging activity if toxicity data are available. Does not incorporate queen mortality resulting from pesticide exposure, user can adjust queen longevity/replacement. Queen exposure assumed to be lower than workers because workers consume pollen and nectar and queen consumes jelly. Does not account for effects of multiple pesticide active ingredient exposures. |
| Characteristic | Ecological Concept | Specification/Parameter (Simplifying Assumptions) |
|---|---|---|
| Biological stressors [47,48,49] | Varroa mites impose stress on colony dynamics through the spread of viruses, including deformed wing virus (DWV). Other stressors include bacteria, fungal diseases, and pest insects. | Biological stressors associated with Varroa mites, including viral infection, are accounted for directly by Varroa infestation in pupal cells and indirectly by manipulating adult worker longevity rates. Other stressors are not considered. |
| Beekeeper management practices [50] | The model simulates impacts of miticide treatments on reducing Varroa impacts. Supplemental feeding can be considered. | Interactions between in-colony medications/miticides and pesticides. |
| Environmental stressors [22] | Egg laying rates influenced by temperature and photoperiod Foraging influenced by temperature, wind speed, and rainfall | Weather parameters chosen to represent geographic location of interest. Some days may be partial foraging days. |
| Landscape composition [29] | Assemblage of natural and cultivated plants determine nectar and pollen resource availability, which influences foraging success and distance of foraging. Availability of pollen and nectar varies over time based on phenology of plants. | Landscape composition and corresponding resource availability is temporo-spatially determined. Model assumes that bees forage 100% on treated crop; however, user can evaluate impact of this assumption by calculating % foraging on crop needed to impact colonies. Effect of landscape on forager lifespan and mortality is not considered. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garber, K.; DeGrandi-Hoffman, G.; Curry, R.; Minucci, J.M.; Dawson, D.E.; Douglass, C.; Milone, J.P.; Purucker, S.T. Simulating the Effects of Pesticides on Honey Bee (Apis mellifera L.) Colonies with BeePop+. Ecologies 2022, 3, 275-291. https://doi.org/10.3390/ecologies3030022
Garber K, DeGrandi-Hoffman G, Curry R, Minucci JM, Dawson DE, Douglass C, Milone JP, Purucker ST. Simulating the Effects of Pesticides on Honey Bee (Apis mellifera L.) Colonies with BeePop+. Ecologies. 2022; 3(3):275-291. https://doi.org/10.3390/ecologies3030022
Chicago/Turabian StyleGarber, Kristina, Gloria DeGrandi-Hoffman, Robert Curry, Jeffrey M. Minucci, Daniel E. Dawson, Cameron Douglass, Joseph P. Milone, and S. Thomas Purucker. 2022. "Simulating the Effects of Pesticides on Honey Bee (Apis mellifera L.) Colonies with BeePop+" Ecologies 3, no. 3: 275-291. https://doi.org/10.3390/ecologies3030022
APA StyleGarber, K., DeGrandi-Hoffman, G., Curry, R., Minucci, J. M., Dawson, D. E., Douglass, C., Milone, J. P., & Purucker, S. T. (2022). Simulating the Effects of Pesticides on Honey Bee (Apis mellifera L.) Colonies with BeePop+. Ecologies, 3(3), 275-291. https://doi.org/10.3390/ecologies3030022






