Abstract
In the quest of finding local strains of marine ciliates that can be easily cultured under a broad range of salinity and fed with microalgae, Fabrea salina Henneguy, 1890 and Condylostoma sp. Bory de St. Vincent, 1826 were cultured for 22 days in small volumes at a temperature of 16–18 °C and fed with flagellated microalgae. F. salina presented a clear preference for the salinity of 40 ppt and Condylostoma. sp. for 20 ppt. Rhodomonas salina Hill and Wetherbee, 1989 were the most efficient feeds, resulting in 30 ind./mL in F. salina and 73 ind./mL in Condylostoma. Dunaliella salina Teodoresco, 1905 and Nephroselmis sp. F. Stein, 1878 also resulted in considerable ciliate densities while Isochrysis galbana Parke, 1949 came last with the highest density in Condylostoma. The strain of Tetraselmis sp. F. Stein, 1878 (var. red pappas) which is transformed in immobilized palmelloid cells and the dinoflagellate Amphidinium carterae Hulburt, 1957, which is suspected of toxin production, were inappropriate for both ciliates. These ciliates can be easily cultured and can serve as useful organisms in bioassays and probably as live food in marine fish hatcheries.
Keywords:
Fabrea salina; Condylostoma; growth; salinity; Tetraselmis; Dunaliella; Isochrysis; Rhodomonas; Nephroselmis; Amphidinium 1. Introduction
Ciliates are ubiquitous mainly planktonic protists thriving in all kinds of water bodies and occupy a key niche in the trophic web [1,2,3]. In fact, they comprise the first direct link from primary producers to the upper trophic levels and recycle the bacterial communities along with microalgae [4,5,6]. Their unicellular nature, their rapid reproduction by simple cell division [7] and their mode of nutrient acquisition make them ideal for mass culturing in various containers and environmental conditions, with the only drawback their microscopic size hindering direct observation, unless microscopically examined. Apart from ecological studies concerning their trophic role in natural environments, ciliates are used in water quality assessment studies as they are sensitive to various toxicants of human origin [8,9,10]. They are ideal for evaluation of water quality by in vitro bioassessment techniques, testing the lethal or sublethal effects of substances on them [11,12]. Their microscopic size and their planktonic dispersal in water, coupled with their rapid generation time and ease of culture, permit the confinement of an adequate number of them in multiple small vessels, thus enhancing the range of various conditions for testing and the credibility of the results in statistical evaluation [13,14,15]. Screening the literature, more than 50 species of ciliates are found to be routinely cultured in protozoology and bioassay research [16]. Among them the most common genera [9] are Euplotes, Paramecium, Tetrachymena, Colpoda and Colpidium to name but a few, due to their well understood biology, ecology and physiology [15,16,17]. There are many more species of ciliates candidates for culture but not all of them can be effectively kept under ordinary room conditions in a constantly thriving population [18,19,20,21]. From a screening of the protists in saltworks [22], it was found that among 39 ciliate species tested, only 2 of them (Fabrea salina and Euplotes sp.) exhibited an easily effective increase of their confined population followed by Condylostoma sp. The reason for the cumbersome culture of the majority of ciliates remains unknown. Being unicellular creatures, it is logical to assume that they are greatly affected by any slight alteration of the optima required for their blooming. If these optima are limited in a very narrow range of environmental parameters (e.g., temperature, salinity, etc.), then any attempt to keep them thriving in the laboratory and even more in mass culture is destined to fail. Fabrea salina has been proved as a promising ciliate for mass culture in order to serve as a candidate first live feed for marine finfish larvae [23,24,25,26,27]. In this respect however, it cannot compete with the rotifer Brachionus plicatilis (the universal live feed) in terms of population increase. Nevertheless, it emerges as its only competitor due to its similar size (~250 μm), dispersal in the water column, brownish coloration, slow movement and filtration of various food particles. Therefore, in the quest for finding easily cultured ciliates either for toxicological studies, bioassay research or for live food perspectives, in the present study two locally isolated ciliates, one (Fabrea salina) from the saltworks of Messolonghi (W. Greece) and the other (Condylostoma sp.) from the Patraikos Gulf (N. Peloponisos, Greece), were examined concerning their growth as affected by different salinities and microalgal food. In contrast to the existing literature for the culture of F. salina (e.g., [28,29,30,31,32,33]), very limited information is available on the culture of Condylostoma [34]. In this respect the aim of the present study is to examine whether this local strain of Condylostoma can be effectively cultured and to clarify whether there are differences among various easily cultured microalgal taxa used as feeds for Condylostoma and F. salina in combination with salinity on the growth of both of them. Furthermore, it aims to supply more data on the culture potential of F. salina to supplement existing research. Overall, this study contributes to the quest for finding marine ciliates that can effectively be grown in a broad range of salinity, fed with commonly cultured microalgae, in order to be used as suitable species for bioassays and probably as live feeds for larval fish.
2. Materials and Methods
Both species of ciliates (Figure 1) originated from local strains and were kept in clonal monoxenic cultures. Fabrea salina (of 120–210 μm size, Video S1) was isolated from a water sample ~20 cm under the surface of a pond of 90 ppt salinity of Messolonghi saltworks (38°23′37.61′′ N, 21°24′29.68′′ E) and then acclimatized to a stock culture in the laboratory in 50 mL conical glass Erlenmeyer vials containing saltwater of 50 ppt, under dim light of 350 lux at room temperature during winter months (16–18 °C). Condylostoma sp. (of 240–295 μm size, Video S2) was isolated from a coastal 40 ppt seawater sample ~20 cm under the surface of Patraikos Gulf (38°17′34.12′′ N, 21°46′01.21′′ E) which was screened for zooplankton. After its population was well established in the laboratory, Condylostoma was kept under the same conditions as F. salina above, with the only exception the salinity, which was 40 ppt. Salinity manipulation in order to attain 20 ppt was accomplished by the proper dilution of autoclaved (121 °C, 1.2 bar, 20 min) seawater of 40 ppt with sterilized distilled water. Higher salinities of 50, 60 and 100 ppt were made by diluting the proper amount of sterilized artificial salt (InstantOcean®) in sterilized 40 ppt seawater. Both ciliates were fed periodically with a mixture of microalgae (Dunaliella + Rhodomonas + Isochrysis) in small quantities by imparting water to achieve a slight coloration. Under these conditions and by frequent re-inoculations in fresh media, the density of the stock cultures of F. salina was kept at 10–15 ind./mL and that of Condylostoma at 15–20 ind./mL.
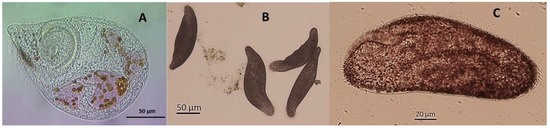
Figure 1.
Microphotographs of (A) Fabrea salina and (B,C) Condylostoma sp. (at different magifications) used in the experimentation. Inside F. salina many digestive vacuoles are prominent, filled with partly digested cells of Rhodomonas salina that have released their saps, imparting reddish coloration on the vacuoles. This particular strain of Condylostoma had a characteristic orange-brownish coloration that made it almost impossible to discern cell details.
Using 12-well multi-well plates (CytoOne), each well of the assigned plate was filled with 3 mL of sterilized water enriched with Walne’s nutrients with the appropriate salinity. Three individuals of either F. salina or Condylostoma were introduced to each cell. By this procedure 12 replicates were created for every plate and each plate represented a combination of salinity and microalgae. In total, using 3 salinities and 6 species of microalgae for each ciliate, 18 plates were assigned to F. salina and 18 to Condylostoma. The salinities were 20, 40 and 60 ppt for Condylostoma and 40, 60 and 100 ppt for F. salina (from preliminary experiments F. salina was found not able to cope with salinities lower than 35 ppt). The microalgae used were taken from cultures in the exponential phase at 40 ppt salinity and were Tetraselmis sp. (var. red pappas) (Video S3), Dunaliella salina (Video S4), Rhodomonas salina (Video S5), Isochrysis galbana (Video S5), Nephroselmis sp. (Video S6) and Amphidinium carterae (Video S7). All microalgae used in the experiment originated from separate cultures. They were cultured in 2 L Erlenmeyer flasks under 8000 lux, 16 hL:8 hD, 18 °C, salinity 40 ppt, supply of filtered air and Walne’s nutrient medium. The progress of each culture was monitored by counting cells with a Fuchs–Rosenthal haematocytometer and a quantity of culture was collected from the middle of the exponential phase. This culture was centrifuged (3000 rpm, 3 min) and the algal mass was concentrated appropriately to create a 90–100 × 105 cells/mL stock culture. The next day, after the introduction of the ciliates in the wells, a 0.1 mL drop of the concentrated algal stock culture of 90–100 × 105 cells/mL of the specific microalgae was poured in each well of the appropriate plate and 0.1 mL of distilled water was also introduced where necessary to minimize the salinity change. The density of the algae in the wells was estimated to be 3.0–3.3 × 105 cells/mL using the haematocytometer. All plates were kept under low illumination of 350–450 lux. As the plates were covered and the room temperature was quite low (16–18 °C), evaporation did not have any remarkable effect on the water salinity and this was corroborated in a preliminary blank trial (no ciliates, no microalgae) with wells filled with the intended salinities and left for 2 weeks. The counting of individuals was performed directly in the wells by placing the plate on the illuminated surface of a dissecting microscope. When the number of organisms observed was more than ~15 ind./mL, a 1 mL sample (in triplicate) was drawn using a 1 mL glass pipette after a slight stirring of the well’s water in order for the cells to be dispersed uniformly. Then the cells were counted along the length of the pipette under a dissecting microscope. After each counting the content of the pipette was poured back into its well.
The maximum specific growth rate (SGR as doublings day−1) was estimated during the culture period between definite days using the equation:
where C1 and C2 stand for cells/mL at days t1 and t2, respectively (t2 > t1).
SGR = (lnC2 − lnC1)/(t2 − t1)
From the above equation the generation time (Tg) of the population was calculated as days required for duplication using the formula:
Tg = 0.6931/SGR
Statistical treatment was made by using ANOVA and then pair-wise Tukey’s test comparison of the means using the free PAST3 software.
3. Results
3.1. Fabrea Salina
In all treatments the elevated values of F. salina density occurred after the first week and were under 30 ind./mL (Figure 2). Great differences were observed among the microalgae used, thus creating two categories. The first category includes the species Tetraselmis sp. (var. red pappas), Isochrysis galbana and Amphidinium carterae for which in all three salinities used (40, 60 and 100 ppt) population growth was negative (Tetraselmis) (Figure 2A), nil (A. carterae) (Figure 2F) or mediocre (I. galbana) (Figure 2C). In the second category there are the species which gave a remarkable response on the growth of F. salina in both population density and specific growth rate (Table 1). These are D. salina, R. salina and Nephroselmis sp. In all 3 of these species the maximum values were recorded at 40 ppt salinity significantly higher (p < 0.05) than the relevant values of the other two salinities (60 and 100 ppt) at all days of recording. D. salina gave the highest density on the 22nd day (29.5 ± 0.88 ind./mL), followed by R. salina (25.5 ± 1.55) and Nephroselmis sp. (17.2 ± 1.42). In all latter species growth became more prominent after the first week and steadily kept increasing until the final day of the experimentation. The densities of the 14th and the 22nd day at 40 ppt were significantly different (p < 0.05) in D. salina and Nephroselmis sp. but not (p > 0.05) in R. salina, while in all three species, the relevant densities between the 7th and the 14th day were significantly different (p < 0.05). The data of densities are proportionally reflected on the specific growth rate (SGR in doubl./day) with zero values at all salinities for Tetraselmis and A. carterae and the higher values at the salinity of 40 ppt for D. salina (0.18), R. salina (0.26), Nephroselmis (0.26) and 0.11 for I. galbana (Table 1). Only in the case of I. galbana (Figure 2C) were the densities statistically equal (p > 0.05) for both 40 and 60 ppt salinities during the whole culture period. In all other cases with the exception of Nephroselmis on the 22nd day (Figure 2E), they were different (p < 0.05). The highest salinity of 100 ppt presented considerable densities only in D. salina (Figure 2B) and Nephroselmis (Figure 2E) exhibiting a steady increase during the culture period with a maximum density of (22.7 ± 4.11 ind./mL) on the 22nd day and SGR of 0.16 doubl./day in D. salina and (10.2 ± 1.49) and 0.17 in Nephroselmis, respectively (Figure 2B,E and Table 1).
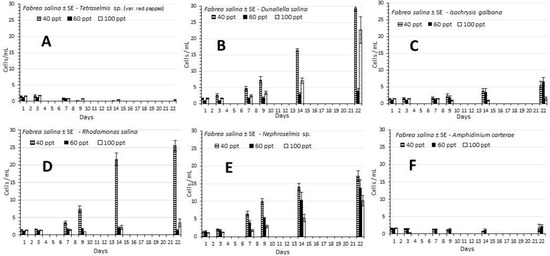
Figure 2.
The development of the density ± SE of F. salina (cells/mL) in the different treatments of microalgae used at the salinities of 40, 60 and 100 ppt. (A) Tetraselmis sp. (var. red pappas), (B) Dunaliella salina, (C) Isochrysis galbana, (D) Rhodomonas salina, (E) Nephroselmis sp., (F) Amphidinium carterae.

Table 1.
Specific growth rate (SGR) of F. salina and Condylostoma sp. fed different microalgae at different salinities.
3.2. Condylostoma sp.
In contrast to F. salina, the culture densities of Condylostoma sp., except for Tetraselmis sp. and A. carterae, in which there was zero growth (Figure 3A,F), exhibited a smoother and larger increase during the culture period than the remaining 4 microalgae (D. salina, I. galbana, R. salina and Nephroselmis sp., Figure 3B–E, respectively). A common feature to all was the zero growth at the highest salinity of 60 ppt. Overall maximum densities attained on the 22nd day were in the region of 50–80 ind./mL almost double or triple of the equivalent values for F. salina. Comparing the densities on the 22nd day, there are significant higher values (p < 0.05) recorded in the salinity of 20 ppt than the salinity of 40 ppt in D. salina, R. salina and Nephroselmis sp., (55 vs. 38.6, 73 vs. 60.5 and 58.7 vs. 42.6 ind./mL, respectively). In I. galbana both values 48.2 and 41.9 ind./mL were statistically equal (p > 0.05). The above situation as presented on the 22nd day was also evident the days before, when on the 14th day the values started to ascend in comparison with the previous time, while until the 9th day growth remained low and this was reflected on the SGR and Tg (Table 1) where, comparing growth between 1st and 7th day and between 7th and 14th day, in the latter case the values almost doubled in SGR and halved in Tg.
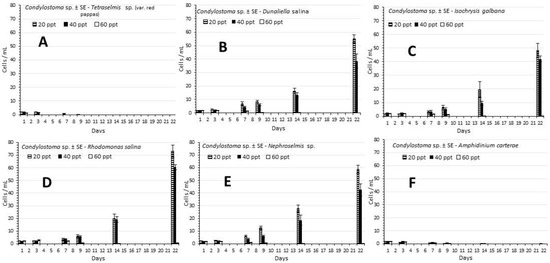
Figure 3.
The development of the density ± SE of Condylostoma sp. (cells/mL) in the different treatments of microalgae used at the salinities of 20, 40 and 60 ppt. (A) Tetraselmis sp. (var. red pappas), (B) Dunaliella salina, (C) Isochrysis galbana, (D) Rhodomonas salina, (E) Nephroselmis sp., (F) Amphidinium carterae.
4. Discussion
The two species cultured in the present study differ in their mode of life as Condylostoma spp. are benthic ciliates [10,34] while F. salina are basically pelagic [25]. However as observed in the present work and from previous routine cultures, this distinction is not absolute as sometimes (for unknown reasons) a great proportion of the Condylostoma population suspends (without aeration) in the water column and F. salina swim around the bottom and frequently are encysted. In this respect it seems logical to assume that both species can exploit food particles, being suspended or sedimented, although to a somehow different degree as Condylostoma is predominantly a voracious grazer [35,36]. All 6 species of microalgae used were motile of roughly two size groups. The smaller one comprised of I. galbana, Nephroselmis sp. and D. salina had a size along the long cell axis of roughly 6–8 μm and the other comprised by Tetraselmis sp., R. salina and A. carterae of 12–14 μm [37]. Among them the Tetraselmis strain was peculiar in the sense that the majority of its cells without aeration (from observation of its stock culture) fell into palmelloid stage on the bottom of the vessel. This seems to be the reason why both ciliates were unable to consume it and grow. Because of this, we can infer that only suspended feeds can be of value for consumption by these ciliates contrary to what was mentioned above about the benthic nature of Condylostoma. The case with A. carterae which proved also inappropriate for the growth of both ciliates may be different in causality. Although A. carterae is planktonic, its negative effect on the growth of ciliates can probably be attributed to its presumably toxic nature as it produces various bioactive compounds [38,39] associated with antialgal activity [40] or even with fish deaths [41]. In this context, apart from the rest of the issues of food quality on the growth of ciliates [42], toxicity or inhibition of growth by using as food dinoflagellates (e.g., A. carterae) cannot be excluded based on similar findings on several species of ciliates [43,44]. However, as was evidenced in the present work, toxicity probably is manifested differently among species of ciliates as A. carterae affected lethally Cobdylostoma by eliminating totally its presence after 7 days, while in F. salina acted as an inhibitory factor with its population surviving but not reproducing during the whole experimentation period (22 days). In this respect, Condylostoma, being more sensitive, seems more suitable than F. salina in certain toxicological studies concerning dinoflagellate poisoning.
Salinity seems to greatly affect growth of both ciliates. To the best of our knowledge, this is the first study on the effect of salinity on growth of Condylostoma spp. and clearly indicates towards a beneficial effect of a brackish water medium (20 ppt), evidenced by a higher SGR in the majority of algae treatments compared to 40 ppt and a devastating one of salinities higher than 60 ppt (zero SGR). F. salina on the other hand is notoriously known as the dominant protist in hypersaline water bodies, able to osmoregulate and adapt to high salt waters [22,45,46], with no mortality occurring even with abrupt population transfer from a salinity of 25 ppt to 50, 75, 100, 125 or 150 ppt [31]. However, based on our long experience with this ciliate, the salinity of 25 ppt is totally unacceptable by F. salina. It is well documented that F. salina can grow at salinities a little lower or well over that of ~40 ppt which is typical of seawater [31,33,47], although it is not clear whether raising the salinity from 35 to 60 ppt would increase the density of F. salina as indicated in [31]. Our results, corroborating those previously reported [33], clearly show that the seawater salinity (35–40 ppt) can be the most effective factor on the growth of its population, as was clearly indicated by using D. salina, I. galbana, R. salina and Nephroselmis sp. microalgal feeds. Another issue, that of retarded population growth of F. salina in small or unaerated vessels [31,48] due to settling of food particles, or the presence of dwarf forms due to intracellular autophagy from starvation [49] and even cannibalistic giant forms [31] similar to those of Blepharisma spp. [50,51], are not corroborated by our work as this ciliate exhibited a smooth and continuous population increase for an extended period of 22 days in very small unaerated vessels. Indeed, in our cultures under rather low temperatures (16–18 °C), there was not a negative impact on growth on F. salina (and Condylostoma also) as reported elsewhere [30,47] due to the low temperature of 18 °C as stated in their work. On the contrary, our low temperatures supported a continuous long lasting (22 days) increase of both ciliates and not a peak on the 5th day and decline afterwards as reported for F. salina cultures under 28 °C [31]. It is well documented that higher temperatures than ours, in the region of 25–30 °C [52,53], can positively influence metabolism and ingestion rate of ciliates resulting in increased reproduction [47], so it is logical to assume that both F. salina and Condylostoma can attain much higher specific growth rates and densities if higher than 18 °C temperatures are used.
Considering the differences in growth of the two ciliate species as affected by the various microalgal feeds used, putting aside the influence of salinity and the negative effect of Tetraselmis and A. carterae explained previously, it is probable that the morphological characteristics and the size of the algae are significant factors for their preference and rate of ingestion by the ciliates [54,55,56,57,58]. From the limited existing data, it was recorded that Condylostoma can more efficiently consume large microalgae of 15–40 μm than smaller of 5–7 μm [54] but in our study such a differentiation was not prominent. R. salina, the biggest microalgae (12–14 μm), resulted in the highest densities of both ciliates, but the other smaller (6–8 μm) microalgae (D. salina, Nephroselmis and I. galbana) also produced remarkable high densities, although I. galbana much less in F. salina compared to Condylostoma. The issue of preference by ciliates of a certain microalgal feed over another may be related to the swimming mode of the algal cells [59], the difficulty of digestion of cell walled species like Chlorella or Nannochloropsis [60,61], the bacterial community of the medium [62,63,64] or even to various substances excreted by the algae [65]. Solely or in combination, these factors seem to influence the acceptability and ingestion of various microalgae by ciliates and in the present study it was clearly recorded that both F. salina and Condylostoma grow best with R. salina and equally well with the other flagellated species D. salina, Nephroselmis sp. and I. galbana. Similar findings strengthening the credibility of the present ones were also recorded in [33].
5. Conclusions
Fabrea salina and Condylostoma sp. can grow efficiently and keep multiplying for a long period even in very small vessels and low temperatures (around 18 °C), feeding on a variety of flagellated algae of small (6–8 μm) or medium size (12–14 μm). Rhodomonas salina, Nephroselmis sp. and Dunaliella salina proved very efficient feeds for both ciliates while Isochrysis galbana was more suitable for Condylostoma. The dinoflagellate Amphidinium carterae and the chlorophyte strain of Tetraselmis sp. (var. red pappas), which soon precipitates, are inappropriate. Based on the characteristics of this experimentation, both ciliates are excellent candidates for use in toxicological studies and bioassays in general and probably as live feed in marine finfish hatcheries due to their tolerance in a wide range of salinity and their high growth potential using certain easily cultured microalgae.
Supplementary Materials
The following are available online at the following URL. Video S1. Fabrea salina. https://www.youtube.com/watch?v=QGQSKP2ZGxw. Video S2. Condylostoma sp. https://www.youtube.com/watch?v=qqI9goQGmb8. Video S3. Tetraselmis sp. (var. red pappas) https://www.youtube.com/watch?v=3Vew3G9IRUE. Video S4. Dunaliella salina https://www.youtube.com/watch?v=a7X_0walwRQ. Video S5. Rhodomonas salina and Isochrysis galbana https://www.youtube.com/watch?v=HqWxnhv-Ka0. Video S6. Nephroselmis sp. https://www.youtube.com/watch?v=giZ430t15Sc. Video S7. Amphidinium carterae https://www.youtube.com/watch?v=R8ue4H6zuYQ.
Author Contributions
Conceptualization, G.N.H.; methodology, G.N.H. and I.T.; validation, G.N.H. and I.T.; formal analysis, G.N.H.; investigation, G.N.H. and I.T.; resources, G.N.H.; data curation, G.N.H. and I.T.; writing—original draft preparation, G.N.H.; writing—review and editing, G.N.H.; visualization, G.N.H.; supervision, G.N.H.; project administration, G.N.H.; funding acquisition, G.N.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the research program “ALGAVISION: Isolation and culture of local phytoplankton species aiming to mass production of antibacterial substances, fatty acids, pigments and antioxidants” (MIS 5048496), funded by the General Secretariat of Research and Technology of the Greek Government.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the technical staff of the laboratory Despoina Avramidou for her help in maintaining the algal cultures.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Porter, K.G.; Sherr, E.B.; Sherr, B.E.; Pace, M.; Sanders, R.W. Protozoa in planktonic food webs. J. Protoz. 1985, 32, 409–415. [Google Scholar] [CrossRef]
- Fenchel, T. Ecology of Protozoa. The Biology of Free-Living Phagotrophic Protists; Springer-Verlag: Berlin, Germany, 1987; 197p. [Google Scholar]
- Kamiyama, T. The impact of grazing by microzooplankton in northern Hiroshima Bay, the Seto Inland Sea, Japan. Mar. Biol. 1994, 119, 77–88. [Google Scholar] [CrossRef]
- Pace, M.L.; Orcutt, J.D., Jr. The relative importance of protozoans, rotifers, and crustaceans in a freshwater zooplankton community. Limnol. Ocean. 1981, 26, 822–830. [Google Scholar] [CrossRef]
- Pierce, R.W.; Turner, J.T. Ecology of planktonic ciliates in marine food webs. Rev. Aquat. Sci. 1992, 6, 139–181. [Google Scholar]
- Liu, H.B.; Dagg, M.J.; Wu, C.-J.; Chiang, K.-P. Mesozooplankton consumption of microplankton in the Mississippi River plume, with special emphasis on planktonic ciliates. Mar. Ecol. Prog. 2005, 286, 133–144. [Google Scholar] [CrossRef][Green Version]
- Siegel, R.W. Mating types in Oxytricha and the significance of mating type systems in ciliates. Biol. Bull. 1956, 110, 352–357. [Google Scholar] [CrossRef]
- Lynn, D.H.; Gilron, G.L. A brief review of approaches using ciliated protists to assess aquatic ecosystem health. J. Aq. Ecos. Health 1992, 1, 263–270. [Google Scholar] [CrossRef]
- Gilron, G.L.; Lynn, D.H. Ciliated protozoa as test organisms in toxicity assessments. In Microscale Testing in Aquatic Toxicology: Advances, Techniques, and Practice; Wells, P.G., Lee, K., Blaise, C., Eds.; CRC Press: Boca Raton, FL, USA, 1998; pp. 323–336. [Google Scholar]
- Madoni, P. Benthic ciliates in Adriatic Sea lagoons. Eur. J. Protistol. 2006, 42, 165–173. [Google Scholar] [CrossRef]
- Sladecek, V. System of Water Quality from the Biological Point of View; Lubrecht & Cramer Ltd.: Devon, UK, 1973; pp. 1–218. [Google Scholar]
- Logar, R.M.; Vodovnik, M. The Applications of Microbes in Environmental Monitoring. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.555.7498&rep=rep1&type=pdf (accessed on 10 May 2022).
- Dias, N.; Mortara, R.A.; Lima, N. Morphological and physiological changes in Tetrahymena pyriformis for the in vitro cytotoxicity assessment of Triton X-100. Toxicol. Vitr. 2003, 17, 357–366. [Google Scholar] [CrossRef]
- Nałecz-Jawecki, G. Spirotox-Spirostomum ambiguum acute toxicity test-10 years of experience. Environ. Toxicol. 2004, 19, 359–364. [Google Scholar] [CrossRef]
- Rao, J.V.; Srikanth, K.; Arepalli, S.K.; Gunda, V.G. Toxic effects of acephate on Paramecium caudatum with special emphasis on morphology, behaviour, and generation time. Pestic. Bioch. Physiol. 2006, 86, 131–137. [Google Scholar] [CrossRef]
- Vilas-Boas, J.A.; Senra, M.V.X.; Dias, R.J.P. Ciliates in ecotoxicological studies: A minireview. Acta Limnol. Brasil. 2020, 32, e202. [Google Scholar] [CrossRef]
- Mansano, A.S.; Moreira, R.A.; Pierozzi, M.; Oliveira, T.M.A.; Vieira, E.M.; Rocha, O.; Regali–Selechim, M.H. Effects of diuron and carbofuran pesticides in their pure and commercial forms on Paramecium caudatum: The use of protozoan in ecotoxicology. Environ. Poll. 2016, 213, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Soldo, A.T.; Brickson, S.A. Isolation, cloning, and axenic cultivation of marine ciliates. In Handbook of Methods in Aquatic Microbial Ecology; Kemp, P.F., Cole, J.J., Sherr, B.F., Sherr, E.B., Eds.; CRC Press: Boca Raton, FL, USA, 1993; p. 97. [Google Scholar]
- Duff, R.J.; Ball, H.; Lavrentyev, P.J. Application of combined morphological-molecular approaches to the identification of planktonic protists from environ-mental samples. J. Eukaryot. Microbiol. 2008, 55, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Carney, L.T.; Wilkenfeld, J.S.; Lane, P.D.; Solberg, O.D.; Fuqua, Z.B.; Cornelius, N.G.; Gillespie, S.; Williams, K.P.; Samocha, T.M.; Lane, T.W.; et al. Pond crash forensics: Presumptive identification of pond crash agents by next generation sequencing in replicate raceway mass cultures of Nannochloropsis salina. Algal Res. 2016, 17, 341–347. [Google Scholar] [CrossRef]
- Hue, N.; Kim, T.; Khang, D.T.; Men, T.T.; Vanoverberghe, I.; Callens, M.; Muylaert, K. Isolation and identification of herbivorous ciliates from contaminated microalgal cultures. Europ. J. Protist. 2020, 76, 125743. [Google Scholar] [CrossRef]
- Hotos, G.N. A preliminary survey on the planktonic biota in a hypersaline pond of Messolonghi saltworks (W. Greece). Diversity 2021, 13, 270. [Google Scholar] [CrossRef]
- Renè, F. Rearing of gilt-head (Sparus aurata). In The Early Life History of Fish; Blaxter, J.H.S., Ed.; Springer-Verlag: Berlin, Germany, 1974; p. 747. [Google Scholar]
- Harvey, H.R.; Ederington, M.C.; MacManus, G.B. Lipid composition of the marine ciliates Pleuronema sp. and Fabrea salina: Shifts in response to changes in diet. J. Euk. Microb. 1997, 44, 189–193. [Google Scholar] [CrossRef]
- Pandey, B.D.; Yeragi, S.G.; Pal, A.K. Nutritional value of a heterotrichous ciliate, Fabrea salina with emphasis on its fatty acid profile. Asian-Austrailian J. An. Sci. 2004, 17, 995–999. [Google Scholar] [CrossRef]
- Rhodes, M.A.; Phelps, R.P. Ciliated protozoans as alternative live food for first feeding red snapper Lutjanus campechanus larvae. Proc. Gulf Caribb. Fish. Inst. 2006, 57, 963–974. [Google Scholar]
- Rhodes, A.M.; Phelps, P.R. Evaluation of the Ciliated Protozoa, Fabrea salina as a First Food for Larval Red Snapper, Lutjanus campechanus in a Large Scale Rearing Experiment. J. Appl. Aquac. 2008, 20, 120–133. [Google Scholar] [CrossRef]
- Repak, A.J. The suitability of selected marine algae on the growth of Fabrea salina. J. Protozool. 1983, 30, 52–54. [Google Scholar] [CrossRef]
- Brown, M.R.; Jeffrey, S.W.; Volkman, J.K.; Dunstan, G.A. Nutritional properties of microalgae for mariculture. Aquaculture 1997, 151, 315–331. [Google Scholar] [CrossRef]
- Rattan, P.; Ansari, Z.A.; Chatterji, A. Studies on experimental culture of a marine ciliate Fabrea salina. J. Aquat. Trop. 1999, 14, 299–308. [Google Scholar]
- Pandey, B.D.; Yeragi, S.G. Preliminary and mass culture experiments on a heterotrichous ciliate, Fabrea salina. Aquaculture 2004, 232, 241–254. [Google Scholar] [CrossRef]
- Guermazi, W.; Elloumi, J.; Ayadi, H.; Bouain, A.; Aleya, L. Rearing of Fabrea salina Henneguy (Ciliophora, Heterotrichida) with three unicellular feeds. CR Biol. 2008, 331, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Hotos, G.N. Feeding with various microalgae the salt “loving” ciliate Fabrea salina in normal salinity of 35 ppt. Intern. J. Food Sci. Agric. 2019, 3, 150–152. [Google Scholar] [CrossRef]
- Li, C.; Xu, K.; Lei, Y. Growth and grazing responses to temperature and prey concentration of Condylostoma spatiosum, a large benthic ciliate, feeding on Oxyrrhis marina. Aquat. Microb. Ecol. 2011, 64, 97–104. [Google Scholar] [CrossRef]
- Fenchel, T. The ecology of marine microbenthos II. The food of marine benthic ciliates. Ophelia 1968, 5, 73–121. [Google Scholar] [CrossRef]
- Lei, Y.; Stumm, K.; Volkenborn, N.; Wickham, S.; Berninger, U.G. Impact of Arenicola marina (Polychaeta) on the microbial assemblages and meiobenthos in a marine intertidal flat. Mar. Biol. 2010, 157, 1271–1282. [Google Scholar] [CrossRef]
- Hotos, G.N.; Avramidou, D. The effect of various salinities and light intensities on the growth performance of five locally isolated microalgae [Amphidinium carterae, Nephroselmis sp., Tetraselmis sp. (var. red pappas), Asteromonas gracilis and Dunaliella sp.] in laboratory batch cultures. J. Mar. Sci. Eng. 2021, 9, 1275. [Google Scholar] [CrossRef]
- Pagliara, P.; Caroppo, C. Toxicity assessment of Amphidinium carterae, Coolia cfr. monotis and Ostreopsis cfr. ovata (Dinophyta) isolated from the northern Ionian Sea (Mediterranean Sea). Toxicon Off. J. Int. Soc. Toxinol. 2012, 60, 1203–1214. [Google Scholar] [CrossRef]
- Moreira-González, Á.R.; Fernandes, L.F.; Uchida, H.; Uesugi, A.; Suzuki, T.; Chomérat, N.; Bilien, G.; Pereira, A.T.; Mafra, L.L. Morphology, growth, toxin production, and toxicity of cultured marine benthic dinoflagellates from Brazil and Cuba. J. Appl. Phyc. 2019, 31, 3699–3719. [Google Scholar] [CrossRef]
- Kong, X.; Han, X.; Gao, M.; Su, R.; Wang, K.; Li, X.; Lu, W. Antialgal and antilarval activities of bioactive compounds extracted from the marine dinoflagellate Amphidinium carterae. J. Ocean. Univ. China 2016, 15, 1014–1020. [Google Scholar] [CrossRef]
- Murray, S.A.; Kohli, G.S.; Farrell, H.; Spiers, Z.B.; Place, A.R.; Dorantes-Aranda, J.J.; Ruszczyk, J. A fish kill associated with a bloom of Amphidinium carterae in a coastal lagoon in Sydney, Australia. Harmful Algae 2015, 49, 19–28. [Google Scholar] [CrossRef]
- Chen, B.Z.; Liu, H.B.; Lau, M.T.S. Grazing and growth responses of a marine oligotrichous ciliate fed with two nanoplankton: Does food quality matter for micrograzers? Aquat. Ecol. 2010, 44, 113–119. [Google Scholar] [CrossRef]
- Hansen, P.J. Growth and grazing responses of a ciliate feeding on the red-tide dinoflagellate Gyrodinium aureolum in monoculture and in mixture with a non-toxic alga. Mar. Ecol. Prog. Ser. 1995, 121, 65–72. [Google Scholar] [CrossRef]
- Buskey, E.J.; Hyatt, C.J. Effects of the Texas (USA) brown tide alga on planktonic grazers. J. Plankton Res. 1995, 126, 285–292. [Google Scholar] [CrossRef]
- Javor, B.J. Hypersaline Environments; Springer-Verlag: Berlin, Germany, 1989; pp. 159–162. [Google Scholar]
- Gunde-Cimerman, N.; Oren, A.; Plemenitas, A. Adaptation to Life at High Salt Concentrations in Archaea, Bacteria and Eukarya; Springer: Berlin/Heidelberg, Germany, 2005; pp. 517–541. [Google Scholar] [CrossRef]
- De Winter, F.; Persoone, G. Preliminary experiments with the ciliate Fabrea salina as a potential live food for mariculture purposes. In Proceedings of the 10th European Symposium on Marine Biology, Osterid, Belgium, 17–23 September 1975; Research in Mariculture at Laboratory and Pilot Scale; Persoone, G., Jaspers, E., Eds.; IZWO: Wetteren, Belgium, 1975; pp. 37–48. [Google Scholar]
- De Winter, F.; Persoone, G.; Benijts-Claus, C. Fabrea salina A Promising Live Food for Mariculture Purposes. World Maric. Soc. 1975, 6, 429–439. [Google Scholar] [CrossRef]
- Fenchel, T. Ecology of heterotrophic microflagellates: III. Adaptations to heterogenous environments. Mar. Ecol. Prog. Ser. 1982, 9, 25–33. [Google Scholar] [CrossRef]
- Pierce, E.; Isquith, I.R.; Repak, A.J. Quantitative study of cannibal-gigantism in Blepharisma. Acta Protozool. 1978, 17, 493–501. [Google Scholar]
- Giffered, D.J. Laboratory culture of marine planktonic oligotrichs (Ciliophora, Oligotrichida). Mar. Ecol. Prog. Ser. 1985, 23, 257–267. [Google Scholar] [CrossRef]
- Marangoni, R.; Batistini, A.; Puntoni, S.; Colombetti, G. Temperature effects on motion parameters and phototactic reaction of the marine ciliate Fabrea salina. J. Photochem. Photobiol. B Biol. 1995, 30, 123–127. [Google Scholar] [CrossRef]
- Henrique Cezar, A.; Javaroti, D.C.D.; Ferreira, R.J.; Seleghim, H.R.M. Optimized culture and growth curves of two ciliated protozoan strains of Paramecium caudatum Ehrenberg, 1833 to use in ecotoxicologycal assays. Rev. Brasil. Zooc. 2016, 17, 77–90. [Google Scholar]
- Takamura, N.; Yasuno, M. Food selection of the ciliated protozoa, Condylostoma vorticella (Ehrenberg) in Lake Kasumigaura. Jap. J. Limnol. 1983, 44, 184–189. [Google Scholar] [CrossRef]
- Fenchel, T. Suspension feeding in ciliated protozoa: Functional response and particle size selection. Microb. Ecol. 1980, 6, 1–11. [Google Scholar] [CrossRef]
- Fenchel, T. Suspension feeding in ciliated protozoa: Feeding rates and their ecological significance. Microb. Ecol. 1980, 6, 13–25. [Google Scholar] [CrossRef]
- Fenchel, T. Suspension feeding in ciliated protozoa: Structure and function of feeding organelles. Arch. Protistenk. 1980, 123, 230–260. [Google Scholar] [CrossRef]
- Fenchel, T. Relation between particle size selection and clearance in suspension feeding ciliates. Limnol. Oceanogr. 1980, 25, 733–738. [Google Scholar] [CrossRef]
- Yang, J.P.; Loder, M.G.J.; Boersma, M.; Wiltshire, K.H. Factors influencing the grazing response of the marine oligotrichous ciliate Strombidium cf. sulcatum. Aquat. Microb. Ecol. 2015, 74, 59–71. [Google Scholar] [CrossRef]
- Cheng, S.H.; Aoki, S.; Maeda, M.; Hino, A. Competition between the rotifer Brachionus rotundiformis and the ciliate Euplotes vannus fed on two different algae. Aquaculture 2004, 241, 331–343. [Google Scholar] [CrossRef]
- Guilherme de Freitas, C.; Tsuzuki, Y.M.; Costa Melo, M.E. Monoculture of the ciliate protozoan Euplotes sp. (Ciliophora; Hypotrichia) fed with different diets. Acta Scientiarum. Biol. Sci. 2013, 35, 15–19. [Google Scholar] [CrossRef][Green Version]
- Sherr, E.B.; Sherr, B.F. High rates of consumption of bacteria by pelagic ciliates. Nature 1987, 325, 710–711. [Google Scholar] [CrossRef]
- Day, J.G.; Thomas, N.J.; Achilles-Day, U.E.M.; Leakey, R.J.G. Early detection of protozoan grazers inalgal biofuel cultures. Bioresour. Technol. 2012, 114, 715–719. [Google Scholar] [CrossRef]
- Erkelens, M.; Ball, A.S.; Lewis, D.M. The influence of protozoa with a filtered and non-filtered seawater culture of Tetraselmis sp., and effects to the bacterial and algal com-munities over 10 days. Bioresour. Technol. 2014, 173, 361–366. [Google Scholar] [CrossRef]
- Zhuang, L.-L.; Wu, Y.-H.; Espinosa, D.M.V.; Zhang, T.-Y.; Dao, G.-H.; Hu, H.-Y. Soluble Algal Products (SAPs) in large scale cultivation of microalgae for biomass/bioenergy production: A review. Renew. Sustain. Energy Rev. 2016, 59, 141–148. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).