Abstract
Fungal lytic polysaccharide monooxygenases (LPMOs) have revolutionized the field of biomass degradation by introducing an oxidative mechanism that complements traditional hydrolytic enzymes. These copper-dependent enzymes catalyze the cleavage of glycosidic bonds in recalcitrant polysaccharides such as cellulose, hemicellulose, and chitin, through the activation of molecular oxygen (O2) or hydrogen peroxide (H2O2). Their catalytic versatility is intricately modulated by structural features, including the histidine brace active site, surface-binding loops, and, in some cases, appended carbohydrate-binding modules (CBMs). The oxidation pattern, whether at the C1, C4, or both positions, is dictated by subtle variations in loop architecture, amino acid microenvironments, and substrate interactions. LPMOs are embedded in a highly synergistic fungal enzymatic system, working alongside cellulases, hemicellulases, lignin-modifying enzymes, and oxidoreductases to enable efficient lignocellulose decomposition. Industrial applications of fungal LPMOs are rapidly expanding, with key roles in second-generation biofuels, biorefineries, textile processing, food and feed industries, and the development of sustainable biomaterials. Recent advances in genome mining, protein engineering, and heterologous expression are accelerating the discovery of novel LPMOs with improved functionalities. Understanding the balance between O2- and H2O2-driven mechanisms remains critical for optimizing their catalytic efficiency while mitigating oxidative inactivation. As the demand for sustainable biotechnological solutions grows, this narrative review highlights how fungal LPMOs function as indispensable biocatalysts for the future of the Circular Bioeconomy and green industrial processes.
1. Introduction
The efficient degradation of recalcitrant polysaccharides such as cellulose, hemicellulose, and chitin represents one of the most significant biochemical challenges in natural ecosystems and industrial biotechnology [1,2,3]. For decades, the canonical understanding of polysaccharide depolymerization was predominantly centered around the concerted action of hydrolytic enzymes, including glycoside hydrolases (GHs) and carbohydrate esterases (CEs), collectively classified under the Carbohydrate-Active enZYmes (CAZy) framework (https://www.cazy.org/) [4,5,6]. However, the discovery of lytic polysaccharide monooxygenases (LPMOs) has fundamentally reshaped this paradigm, introducing a powerful oxidative dimension to polysaccharide breakdown that complements hydrolytic mechanisms with remarkable efficiency [7,8].
Originally misannotated as GH family members due to their superficial sequence similarities, LPMOs were only correctly identified in the late 2000s as a distinct class of copper-dependent monooxygenases capable of oxidatively cleaving glycosidic bonds in crystalline polysaccharides [9]. Their ability to introduce chain breaks through oxidation at the C1 and/or C4 positions of glycosidic linkages enables synergistic collaboration with hydrolases, significantly enhancing the deconstruction of highly ordered polymeric substrates that are otherwise resistant to enzymatic attack [9,10].
The catalytic mechanism of LPMOs is particularly intriguing [9,11,12]. It relies on a highly conserved histidine-brace copper active site, which coordinates molecular oxygen or hydrogen peroxide to drive oxidative cleavage [9,13]. This discovery resolved long-standing questions about biomass recalcitrance and opened novel avenues in biocatalysis, renewable energy, and materials science [14].
While LPMOs are widespread across bacteria, archaea, viruses, and fungi [15,16,17,18], the fungal LPMOs exhibit unique structural diversity, functional specialization, and evolutionary trajectories that are tightly linked to the ecological roles and lifestyles of fungi [19,20]. Fungi occupy many ecological niches, from saprotrophic decomposers of plant and insect biomass to symbiotic partners and aggressive pathogens [21,22]. Their unparalleled enzymatic repertoire reflects these diverse ecological strategies, and LPMOs are no exception [23,24].
LPMOs are categorized into eight Auxiliary Activity (AA) families within the CAZy database, AA9–AA11 and AA13–AA17. Fungal LPMOs are predominantly AA9 (formerly GH61), AA10, AA11, AA13, AA14, AA15, and AA16. Among these, AA9 is exclusive to fungi and represents the most extensively studied group, primarily involved in cellulose oxidation. Conversely, AA13 targets starch, AA11 acts on chitin, while AA14 exhibits activity on xylan, highlighting the substrate versatility that fungi have evolved [25].
Despite the rapidly growing body of knowledge surrounding bacterial LPMOs, the fungal counterparts remain relatively underexplored outside of a few model species, such as Trichoderma reesei, Neurospora crassa, Thermothelomyces thermophilus (formerly Myceliophthora thermophila), and Thermothielavioides terrestris (formerly Thielavia terrestris) [23,26,27,28]. Nevertheless, genomic analyses reveal that a vast, largely untapped diversity of LPMO genes exists across fungi, especially in lineages adapted to extreme environments, specialized ecological niches, or unusual substrates [29,30,31]. This points to an enormous, uncharacterized reservoir of enzymatic diversity with potential biotechnological value.
The expansion and diversification of LPMO gene families within the fungal kingdom appear tightly coupled to evolutionary pressures related to substrate complexity, competition, and habitat specialization [8,32,33]. For example, white-rot fungi, capable of fully degrading lignin, often exhibit an expanded arsenal of AA9 LPMOs, synergizing with ligninolytic enzymes such as laccases and peroxidases [34]. In contrast, brown-rot fungi, which selectively degrade cellulose and hemicellulose while leaving lignin largely intact, display distinct LPMO complements with potential adaptations to their unique decay strategies [35].
Furthermore, fungi that form mutualistic associations, such as mycorrhizal species, possess LPMO repertoires that are significantly reduced or specialized, reflecting a shift away from aggressive polysaccharide degradation toward more subtle modifications of plant cell walls [21]. Similarly, pathogenic fungi leverage LPMOs not only for nutrient acquisition but also for breaching host barriers, suggesting a dual role in both metabolism and virulence [36].
These evolutionary patterns underscore the importance of examining fungal LPMOs not merely as isolated biocatalysts but as dynamic components of larger metabolic and ecological networks [19,37]. Understanding these connections could provide critical insights into the fundamental biology of fungi and inform the rational design of enzyme cocktails for industrial processes [38].
Fungal LPMOs are of paramount interest to the biotechnology sector, particularly in biorefineries, biofuel production, textile processing, and functional materials development [36,39,40,41]. Their capacity to disrupt crystalline cellulose and hemicellulose’s crystalline regions drastically improves the efficiency of saccharification processes, reducing enzyme loadings and process costs [42]. Moreover, generating oxidized oligosaccharides with unique chemical functionalities opens new avenues in synthesizing bio-based materials, fine chemicals, and nutraceuticals [43,44,45].
Nevertheless, most industrial applications rely on a narrow set of LPMOs derived from thermophilic filamentous fungi, leaving the broader functional potential of the fungal kingdom vastly underutilized [46]. Emerging genomic and metagenomic resources, coupled with advances in structural biology and enzyme engineering, now offer unprecedented opportunities to explore and exploit this diversity [47].
This review aims to provide a comprehensive overview of the diversity, evolution, mechanisms, and biotechnological potential of fungal LPMOs. We will explore how these enzymes are distributed across fungal phylogeny, how they correlate with different ecological strategies, and how their biochemical properties can be leveraged in industrial applications. By focusing exclusively on the fungal kingdom, this work seeks to fill a critical gap in the current literature, offering new perspectives on the role of LPMOs beyond the confines of standard model systems.
2. Classification of Fungal LPMO Families
The classification of LPMOs is based on the CAZy database, which organizes enzymes involved in the assembly, modification, and degradation of complex carbohydrates. LPMOs are categorized within the Auxiliary Activities (AA) class, reflecting their oxidative mechanism that assists traditional hydrolytic enzymes in biomass deconstruction [25].
Fungal LPMOs exhibit remarkable diversity in substrate specificity, structural features, oxidation patterns, and ecological roles [8,29,32,48]. This diversity results from evolutionary adaptations that have enabled fungi to access and degrade a wide range of recalcitrant polysaccharides present in plant and animal biomass [19]. While the AA9 family is the most studied due to its leading role in cellulose degradation, other families such as AA10, AA11, AA13, AA14, AA15, and AA16 represent functionally specialized enzymes with distinct substrate preferences, often tied to specific ecological niches or metabolic requirements [25]. Table 1 synthesizes current knowledge on these seven fungal LPMO families, providing a comparative framework highlighting their biochemical properties, structural distinctions, and ecological relevance.

Table 1.
Comparative classification of fungal lytic polysaccharide monooxygenases (LPMO) families.
The presence of AA15 family members in fungi is notably rare and, when identified, appears to reflect possible events of horizontal gene transfer or highly specialized niche adaptations [72]. Unlike its widespread distribution in arthropods, where AA15 LPMOs play roles in chitin and cellulose degradation, the occurrence in fungi may be linked to ecological pressures requiring the utilization of substrates derived from insect biomass or other specific environmental conditions [72,73]. The functional role of AA15 in fungi remains largely unexplored and warrants further investigation.
A particularly intriguing aspect of the AA14 family is its strict functional requirement for the co-localization of xylan with cellulose microfibrils to exhibit enzymatic activity. This dependency is unique among LPMOs and highlights a specialization toward modifying the structural heterogeneity of plant cell walls [62,64]. Rather than acting on soluble xylan, AA14 LPMOs facilitate the loosening of the hemicellulose-cellulose matrix, enabling more efficient access for other hydrolytic enzymes involved in lignocellulose degradation [61,62].
The AA13 family exemplifies an evolutionary adaptation that diverges from the typical β-glucan-targeting paradigm of most fungal LPMOs [74]. Instead, AA13 enzymes are specialized for the oxidative cleavage of retrograded (gelatinized) starch, an α-glucan, thereby allowing fungi to exploit starch-rich substrates that become recalcitrant after physical or thermal treatment [75,76]. This functional shift represents a significant expansion of the LPMO enzymatic repertoire beyond plant cell wall polysaccharides.
In contrast, the AA11 family represents an evolutionary solution tailored to the degradation of chitin, a highly crystalline β-1,4-linked polysaccharide found in fungal cell walls and arthropod exoskeletons [56,77]. While functionally analogous to the bacterial AA10 family of chitin-active LPMOs, fungal AA11s exhibit distinct structural adaptations and biochemical properties that reflect the unique evolutionary pressures and ecological contexts faced by fungi [56,78]. This specialization underscores the diversification of LPMOs in fungal lineages to accommodate different structural polysaccharides beyond cellulose.
3. Phylogenetic Distribution of Fungal LPMOs
The phylogenetic distribution of LPMOs within the fungal kingdom reveals intricate evolutionary patterns that are tightly linked to ecological strategies, substrate availability, and the diversification of fungal lineages [19,32,48,79,80]. Among the eight currently classified LPMO families (AA9–AA17), seven are present in fungi, AA9, AA10, AA11, AA13, AA14, AA15, and AA16 [25]. Their distribution is far from homogeneous across the two largest fungal phyla: Ascomycota and Basidiomycota [81].
The AA9 family is by far the most widespread, abundant, and phylogenetically diverse group of LPMOs in fungi [82]. It is extensively represented in both Ascomycota and Basidiomycota, reflecting its leading role in lignocellulose degradation [19]. Phylogenetic studies indicate that AA9 enzymes likely originated alongside the emergence of filamentous fungi capable of exploiting plant biomass, particularly during the colonization of terrestrial ecosystems [83,84]. In Ascomycota, AA9 genes are highly represented in saprotrophic species such as N. crassa, Aspergillus spp., and T. thermophilus, as well as in some plant pathogens [27,50,85,86]. These enzymes often show specialization towards crystalline cellulose, but display expanded activity towards hemicelluloses such as xyloglucan in specific lineages [87,88]. In Basidiomycota, AA9s are equally abundant, particularly in white-rot fungi like Pycnoporus sanguineus and Pleurotus ostreatus, which are capable of complete lignin mineralization [53,89]. Brown-rot fungi, though lacking extensive ligninolytic systems, also retain AA9 LPMOs, emphasizing the universal role of oxidative cellulose cleavage across divergent fungal lifestyles [35,90].
The AA10 family, originally characterized in bacterial systems (formerly known as CBM33), targets both chitin and crystalline cellulose through C1 and/or C4 oxidative cleavage [15,91,92]. Phylogenomic analyses suggest that fungal AA10 genes likely originated via horizontal gene transfer from bacterial sources [56]. Functionally, fungal AA10s may provide complementary oxidative activity alongside AA9 enzymes, particularly in mixed polysaccharide substrates or environments rich in chitinous and cellulosic materials [55,93,94].
The AA11 family is predominantly found in filamentous ascomycetes such as Aspergillus fumigatus, N. crassa, and Trichoderma spp. [31,56,57]. Despite being broadly distributed taxonomically, AA11 exhibits low diversification relative to AA9, possibly reflecting functional constraints tied to its narrow substrate specificity, crystalline chitin [31,95].
Similar to the AA11 family, the AA13 family is found exclusively in fungi [69]. It is particularly prevalent in species of the genera Aspergillus and Neurospora, which are known for their saprotrophic or opportunistic lifestyles [27,74].
The evolutionary emergence of AA14 is hypothesized to be relatively recent compared to AA9, representing a lineage highly specialized for acting on xylan that is physically associated with cellulose microfibrils [62,96]. This strict substrate constraint likely limits the ecological spread of AA14, confining it to fungi that occupy specific ecological niches requiring synergistic hemicellulose-cellulose deconstruction [23,62].
The AA15 family majority is found in arthropods, which play essential roles in chitin and cellulose degradation [66,97,98]. When detected, fungal AA15 genes show strong phylogenetic affinity to arthropod AA15 sequences, suggesting that horizontal gene transfer events are the most plausible explanation for their occurrence [69,83,99].
The ecological significance of AA15 in fungi remains largely speculative. Hypotheses include roles in niche-specific degradation of insect-derived biomass, symbiotic interactions, or opportunistic metabolic adaptations. However, the rarity and limited functional characterization of fungal AA15 enzymes prevent robust information about their evolutionary trajectory within the fungal kingdom [69,83,99].
The AA16 family represents one of the most recent additions to the CAZy LPMO classification, being initially identified in Aspergillus aculeatus [69]. Phylogenetic analyses indicate that AA16 likely evolved from a duplication and divergence event within fungal AA9 ancestors, acquiring distinct structural motifs that confer substrate specificity and potentially different redox partner interactions [100,101]. Their limited distribution suggests a specialization linked to specific ecological niches, potentially those involving highly recalcitrant plant biomass or environments with fluctuating oxidative conditions [77].
4. Relationship Between LPMOs and Fungal Lifestyle
LPMOs are widespread among lignocellulolytic fungi [18,23,46,102]. The capacity of fungi to degrade lignocellulosic biomass represents a cornerstone of carbon cycling in terrestrial ecosystems [103,104,105]. This ability has driven the evolution of highly specialized enzymatic systems capable of degrading the complex matrix of cellulose, hemicellulose, and lignin found in plant cell walls [106,107,108]. The functional deployment of LPMOs is tightly linked to the physicochemical challenges posed by lignocellulose, especially the crystalline regions of cellulose and tightly bound hemicelluloses that are highly recalcitrant to hydrolytic enzymes alone [42,109]. Then, LPMOs are recognized as central components of the lignocellulolytic machinery in fungi, providing oxidative cleavage mechanisms that complement classical hydrolytic pathways [52,110].
In fungal lignocellulolytic systems, LPMOs function as molecular oxidizers capable of introducing chain breaks in crystalline polysaccharides through the oxidative cleavage of glycosidic bonds [77]. This mechanism differs fundamentally from that of glycoside hydrolases, as it relies on the activation of molecular oxygen (or hydrogen peroxide) to hydroxylate carbon atoms at C1 and/or C4 positions, leading to destabilization and fragmentation of the polysaccharide chains [111]. This oxidative mode of action enhances the accessibility of cellulose microfibrils and hemicellulose fractions to hydrolases [43]. As a result, LPMOs serve as synergistic partners that significantly boost the efficiency of fungal lignocellulose degradation, particularly in complex plant materials such as wood, leaf litter, and agricultural residues [112,113,114].
Among the LPMO families, AA9 is the most prevalent in fungi involved in plant biomass degradation [56]. While AA14, though less common, is specialized for targeting xylan when it is physically associated with cellulose, a feature frequently encountered in hardwoods and grasses [27,78]. This specialization enhances the deconstruction of complex plant cell walls by disrupting the hemicellulose-cellulose interface [62].
The efficiency of LPMOs in lignocellulolytic fungi is further enhanced by their integration with other oxidative enzymes and redox systems [8]. Many fungi co-produce extracellular oxidoreductases such as cellobiose dehydrogenase (CDH), glucose-methanol-choline (GMC) oxidoreductases, or copper radical oxidases, which supply the electrons necessary for LPMO catalytic cycles [25,115]. Additionally, the generation of hydrogen peroxide, either enzymatically or through Fenton-type reactions, can serve as an alternative co-substrate, highlighting a flexible oxidative toolkit that complements hydrolytic enzymology [116].
The presence and functional roles of LPMOs in phytopathogenic and endophytic fungi have emerged as an intriguing area of study that bridges plant-fungal interactions, microbial ecology, and enzymatic evolution [83,117]. While LPMOs were initially characterized for their role in saprotrophic lignocellulose degradation [118], mounting evidence demonstrates that these oxidative enzymes are also employed by fungi engaged in intimate relationships with living plants, whether antagonistic (pathogenic) or mutualistic (endophytic) [83,118].
Phytopathogenic fungi leverage LPMOs as part of their arsenal to breach and colonize plant tissues [117,119]. The plant cell wall constitutes the primary defensive barrier, composed of complex networks of cellulose microfibrils embedded within matrices of hemicellulose, pectin, and lignin [120]. While classical hydrolytic enzymes (e.g., cellulases, pectinases, xylanases) have long been recognized as virulence factors [121], LPMOs provide a complementary oxidative mechanism that enhances cell wall degradation, particularly in crystalline regions that are otherwise resistant to hydrolysis [122].
AA9 LPMOs are the most widespread among plant pathogenic fungi, including species such as Magnaporthe oryzae, Botrytis cinerea, Fusarium graminearum, and Verticillium dahlia [83,123,124,125]. These enzymes facilitate the oxidative cleavage of cellulose, generating chain breaks that weaken cell wall integrity and release oligosaccharides that can serve as nutrients for the pathogen [126]. Importantly, the action of LPMOs may help create physical entry points or exacerbate mechanical damage during host colonization [127,128].
Moreover, some phytopathogens possess AA11 LPMOs, which are chitin-active [95,129]. Although chitin is absent from plant cell walls, AA11 enzymes may participate in self-cell wall remodelling or play roles in inter-microbial competition, particularly in the complex rhizosphere or phyllosphere environments [81,130].
A critical observation is that the expression of LPMO genes in phytopathogenic fungi is often highly regulated and induced during host infection stages [119,126]. Transcriptomic and proteomic studies have shown that LPMOs are upregulated during appressorium formation, tissue penetration, and necrotrophic growth, suggesting that these enzymes are integral to the infection process [83,86,119,131].
Endophytic fungi establish symbiotic or commensal relationships with plants, inhabiting internal tissues without causing visible disease symptoms [132]. Unlike phytopathogens, endophytes generally avoid eliciting strong plant defense responses, which raises questions about the presence and roles of LPMOs in these organisms [133].
Genome analyses reveal that many endophytic fungi encode AA9 LPMOs, albeit often in reduced numbers compared to saprotrophic or pathogenic relatives. This suggests a selective retention of LPMOs for functions that do not compromise host viability [81,129]. For endophytes, LPMOs may be involved in subtle modifications of the plant cell wall that facilitate colonization of intercellular spaces without triggering defense mechanisms [134,135]. Rather than aggressive degradation, LPMOs in this context may loosen cell wall structures to allow hyphal penetration or movement [115].
Mycorrhizal fungi, including both ectomycorrhizal (ECM) and arbuscular mycorrhizal (AM) species, are generally considered poor decomposers of lignocellulosic material compared to saprotrophs [136]. Nevertheless, genomic studies have revealed that certain ECM fungi, such as Laccaria bicolor, Hebeloma cylindrosporum, Paxillus involutus, and Suillus luteus, retain genes encoding AA9-type LPMOs [115,118,137,138]. These LPMOs are typically present in reduced numbers relative to saprotrophic relatives, reflecting an evolutionary transition towards a symbiotic lifestyle with diminished reliance on extensive plant biomass degradation [136].
Unlike their saprotrophic counterparts that deploy LPMOs for large-scale cellulose depolymerization, mycorrhizal fungi may use LPMOs for subtler modifications of plant cell walls [139]. During the establishment of symbiosis, fungi must penetrate the root epidermis and navigate the apoplastic space without triggering strong plant defense responses [133,140]. LPMOs may facilitate this process by loosening cell wall components, particularly cellulose microfibrils and hemicellulose, to allow hyphal ingress and the formation of fungal structures such as the Hartig net in ECM associations [141].
5. Catalytic Pathways and Specific Modulations of Fungal LPMOs
LPMOs are oxidative enzymes that revolutionized the understanding of polysaccharide depolymerization [7,25,142]. Unlike hydrolytic GHs that cleave glycosidic bonds via hydrolysis, LPMOs utilize an oxidative mechanism to break the crystalline regions of polysaccharides such as cellulose, chitin, starch, and hemicelluloses [25]. This oxidative cleavage introduces chain breaks that dramatically increase biomass accessibility to hydrolases [10]. The mechanistic versatility of fungal LPMOs is intimately connected to their substrate preferences, cofactor requirements, and synergistic interactions with complementary enzymes in complex enzyme cocktails.
5.1. Catalytic Mechanism Overview
At the heart of LPMO catalysis is a conserved active site architecture featuring a mononuclear copper center coordinated by the histidine brace, where the N-terminal histidine coordinates the copper via both its terminal amine and the imidazole side chain, and a second histidine provides the other imidazole ligand [13]. This highly conserved motif enables LPMOs to activate molecular oxygen (O2) or hydrogen peroxide (H2O2) to perform oxidative cleavage of glycosidic bonds [143].
The catalytic cycle can follow two primary oxidative routes:
- Oxygen-Driven (O2) Mechanism: Traditionally, it was believed that LPMOs functioned as monooxygenases, employing molecular oxygen (O2) as the oxygen source [144]. In this pathway, the copper in the resting Cu(II) state must first be reduced to Cu(I) via an external electron donor (e.g., cellobiose dehydrogenase—CDH or phenolic compounds). The reduced Cu(I) center reacts with molecular oxygen to form a reactive oxygen species, which may be a Cu(II)-superoxide or Cu(II)-peroxide intermediate. This intermediate performs hydrogen atom abstraction (HAA) from the C–H bond of the polysaccharide substrate. The subsequent rebound of the hydroxyl group to the carbon leads to cleavage of the glycosidic bond, typically at the C1 or C4 position, depending on the enzyme’s regioselectivity. Although well-characterized in vitro, this O2-driven pathway is kinetically slow, as it requires two sequential electron transfers per catalytic event, one for reducing Cu(II) to Cu(I) and another to complete the O2 reduction [31,116,145].
- Peroxide-Driven (H2O2) Mechanism: In this route, the Cu(I) center reacts directly with hydrogen peroxide (H2O2), forming a highly reactive Cu(II)-oxyl species or a related high-valent intermediate. This pathway is significantly faster than the O2 route because only one electron is required to reduce Cu(II) to Cu(I) for catalytic turnover, and the reaction with H2O2 produces a potent oxidant capable of immediate hydrogen abstraction and glycosidic bond cleavage. It is important to highlight that in the H2O2-driven mechanism, LPMO stays in the reduced state (Cu(I)) at the end of the catalytical cycle, then multiple cycles are possible without additional reductant being necessary. However, this route carries inherent risks. Excessive or uncontrolled H2O2 levels can lead to enzyme self-inactivation through oxidative damage to the histidine brace or other nearby residues. Consequently, fungi have evolved intricate systems to regulate H2O2 production, including tight coupling with oxidoreductases (like CDH, glucose oxidase, and aryl-alcohol oxidases) and enzymes that scavenge excess H2O2 (such as catalases and peroxidases) [116,142,146,147,148,149].
This dual mechanism provides LPMOs with remarkable flexibility under varying environmental conditions or within different enzymatic systems. Table 2 summarizes the key differences between oxygen-driven (O2) and hydrogen peroxide-driven (H2O2) catalytic mechanisms in LPMOs, highlighting their biochemical, ecological, and industrial implications.

Table 2.
Differences between oxygen-driven (O2) and hydrogen peroxide-driven (H2O2) catalytic mechanisms in lytic polysaccharide monooxygenases (LPMOs) [31,116,142,145,146].
5.2. Cofactor Requirements and Electron Donors
LPMOs are strictly copper-dependent enzymes, with their catalytic mechanism fundamentally relying on the redox cycle of a mononuclear copper center [115]. The copper ion within the active site must transition from its oxidized Cu(II) resting state to the reduced Cu(I) form prior to each catalytic turnover, enabling the activation of molecular oxygen or hydrogen peroxide for the oxidative cleavage of polysaccharide substrates [143]. This redox cycle necessitates a continuous supply of external electrons, and the nature of these electron donors varies considerably depending on the ecological niche and metabolic capabilities of the fungus [122,150].
One of the most prominent and well-studied electron donors in fungal pathways is cellobiose dehydrogenase (CDH), a flavocytochrome enzyme co-secreted alongside LPMOs, particularly in wood-decaying fungi [151]. CDH possesses both a flavin domain, which oxidizes cellobiose or other sugars, and a cytochrome domain, which directly transfers electrons to the copper center of LPMOs [152]. This creates an exceptionally efficient and synergistic enzymatic partnership, where CDH facilitates LPMO catalysis and couples lignocellulose oxidation with sugar metabolism [25].
In addition to CDH, pyrroloquinoline-quinone-dependent pyranose dehydrogenases (PQQ-PDHs) have also emerged as potential fungal redox partners for LPMOs. These enzymes catalyze the oxidation of a wide range of aldopyranoses and transfer electrons via PQQ cofactors, contributing to LPMO activation in certain fungal systems. While less characterized than CDH, evidence of fungal expression of PQQ-PDHs and their redox activity suggests they may play an auxiliary role in sustaining oxidative polysaccharide degradation under specific ecological or developmental conditions [153].
Beyond CDH and PPQ-PDH, a variety of small phenolic compounds (e.g., caffeic acid, vanillic acid, catechol, guaiacol, among others) generated during lignin degradation serve as natural, diffusible redox mediators capable of donating electrons to LPMOs [154]. These phenolics arise as by-products of the oxidative action of lignin-modifying enzymes such as laccases and peroxidases [155]. Their role extends beyond mere electron donation; they contribute to an intricate redox network within the fungal extracellular space, linking lignin depolymerization with polysaccharide oxidation [156]. This crosstalk between lignin breakdown and carbohydrate degradation is a hallmark of fungal biomass deconstruction strategies [157].
In addition to enzymatic and phenolic electron donors, fungi can exploit simpler small-molecule reductants such as ascorbic acid, gallic acid, and reduced glutathione. While these molecules are often employed in vitro to study LPMO activity, they are also present in fungal microenvironments under certain physiological conditions [43]. These reductants provide flexible electron supply options, particularly when dedicated enzymes like CDH are absent or less active [158,159].
Remarkably, recent studies have uncovered that some fungi utilize light-driven photoredox systems to sustain LPMO activity [84,113,160]. In these systems, photoactive pigments, such as melanin-like compounds or quinone-containing molecules, absorb visible light and generate excited states capable of transferring electrons to LPMOs [161]. This photochemical strategy allows fungi to harness environmental light as an auxiliary energy source to drive oxidative degradation, an adaptation particularly relevant in surface-exposed or shallow-soil fungal habitats [84,113,160,161]. In addition to serving as electron donors, these light-activated systems have also been shown to produce hydrogen peroxide (H2O2), which can act as a direct co-substrate for LPMO catalysis. This dual function, electron donation and H2O2 generation, provides a powerful mechanism for enhancing LPMO activity under light-exposed conditions, potentially enabling multiple oxidative cycles without the need for continuous external reductant supply. Such findings expand our understanding of how photochemical processes are integrated into fungal redox metabolism and polysaccharide degradation [85].
5.3. Substrate Specificity
Fungal LPMOs are classified into multiple Auxiliary Activity (AA) families [25], each with distinct substrate specificities:
- AA9: the most studied in fungi, primarily targets cellulose, with some members also acting on hemicelluloses like xyloglucan, glucomannan, and mixed-linkage β-glucans [25]. Oxidation patterns vary between C1, C4, and mixed C1/C4, depending on the enzyme [162]. This broad substrate range is largely attributed to the flexible architecture of AA9 surface loops (especially L2, L3, LC, and LS), which adapt the conserved LPMO β-sandwich fold to accommodate both crystalline and amorphous substrates [163,164].
- AA10 (rare in fungi): Fungal AA10s exhibit activity on both crystalline cellulose and chitin. They are capable of C1, C4, or mixed oxidation, similar to AA9 [25]. This dual specificity stems from a structurally open substrate-binding surface and conserved residues in the flat catalytic plane of the typical LPMO fold, allowing alignment with both β-1,4-glucans and β-1,4-N-acetylglucosamine chains [165,166].
- AA11: specializes in chitin, particularly in the modification of crystalline chitin [95], with oxidative cleavage at the C1 position being most common [31]. This specificity is associated with a narrower substrate-binding groove and distinct electrostatic surface potentials, tailored to interact with the acetylated chitin chains rather than the more hydrophobic cellulose [31,95].
- AA13: targets starch (α-1,4-glucans), specifically disrupting the crystalline regions of amylose and amylopectin [167]. Its substrate preference reflects adaptations in the catalytic interface that favor the helical conformation of starch polysaccharides, diverging structurally from other LPMOs by modifications to loops around the active site cleft [58,168].
- AA14: exhibits activity on xylan but uniquely requires the presence of cellulose for activity, suggesting that it acts on xylan tightly bound to cellulose fibrils within the plant cell wall. The specificity of AA14 is hypothesized to rely on the positioning of the substrate-binding loops and electrostatic complementarity that favor xylan–cellulose complexes, consistent with the LPMO β-sandwich fold but with added surface constraints [62].
- AA15 (rare in fungi): demonstrates broad activity on both chitin and cellulose, with evidence of oxidation on both C1 and C4 carbons [25]. This functional versatility may reflect its evolutionary origin from arthropods and a more flexible binding surface, superimposed onto the typical LPMO fold, that tolerates multiple polysaccharide geometries [67,98].
- AA16: acts predominantly on cellulose, with oxidation typically at the C1 position. It may be complementary to AA9 enzymes, especially in fungal species specialized in degrading highly recalcitrant plant biomass [25]. The substrate preference of AA16 is linked to specific loop configurations and redox partner preferences that distinguish it from AA9, despite sharing the core β-sandwich fold and catalytic mechanism [70,71].
The substrate specificity of fungal LPMOs is not solely determined by the nature of their catalytic copper center. However, it is profoundly influenced by a series of structural features that fine-tune their interaction with complex polysaccharide substrates [48]. One of the key determinants involves the configuration of surface-binding loops that surround the copper active site [169]. These flexible loops, commonly designated L2, L3, LS (short loop), L8, and LC (C-terminal loop) in AA9 enzymes, play a pivotal role in defining the shape and chemistry of the substrate-binding surface [32,170]. The loop nomenclature is based on their relative sequence positions and structural location in the enzyme: for instance, LC refers to a C-terminal loop that often contributes to substrate positioning, while LS denotes a short loop located near the active site cleft. Variations in the length, amino acid profile, and conformation of these loops dictate how the enzyme aligns with crystalline polysaccharide surfaces, thereby affecting both substrate recognition and oxidation regioselectivity (i.e., C1, C4, or mixed oxidation) [95]. For instance, enzymes with extended loops often display enhanced binding to rough or heterogeneous surfaces, such as amorphous regions or hemicellulose-decorated cellulose fibrils [23,171,172].
In addition to the loop architecture, several LPMOs incorporate carbohydrate-binding modules (CBMs), which are non-catalytic domains specialized in targeting specific polysaccharides. CBMs function as molecular anchors that tether the catalytic domain to the substrate surface, significantly enhancing the local concentration of the enzyme on the target polymer [173]. The presence of CBMs not only increases catalytic efficiency but can also shift substrate preference [174,175]. For example, some AA9 LPMOs with CBM1 domains exhibit improved action on crystalline cellulose, whereas those lacking CBMs may preferentially act on soluble oligosaccharides or less crystalline regions [51,87,176].
Furthermore, the electrostatic surface properties of LPMOs exert a subtle yet critical influence on substrate binding [32]. The distribution of charged residues around the active site can either attract or repel specific polysaccharide surfaces, depending on the chemical environment of the substrate [172]. Cellulose, being relatively hydrophobic and neutral, tends to favor LPMOs with hydrophobic or neutral surfaces in the binding region [177]. In contrast, chitin, which carries acetyl groups and occasional charges, may favor different charge distributions [178].
These structural features do not act in isolation but synergistically modulate the affinity of enzymes, orientation, and reactivity toward diverse carbohydrate substrates [178]. Moreover, subtle point mutations or natural sequence variations in these regions can profoundly alter the catalytic performance, oxidation pattern, and even tolerance to oxidative stress, underscoring the evolutionary plasticity of fungal LPMOs in adapting to specific ecological niches and substrates [179,180,181]. As a result, understanding these structural determinants is essential for deciphering fungal biomass degradation strategies and guiding the engineering of LPMOs with tailored properties for industrial applications [178,182].
5.4. Oxidation Pattern Modulation (C1, C4, Mixed)
The regioselectivity of oxidation in fungal LPMOs is a defining biochemical feature that greatly influences both the mechanism of polysaccharide cleavage and the types of degradation products generated [32,111]. This oxidation can occur at different carbon positions of the glycosidic unit, primarily at the C1 position, the C4 position, or, in some enzymes, at both (mixed C1/C4 oxidation) [183]. Each oxidation mode has distinct biochemical consequences and ecological functions [184].
C1 oxidation generates a terminal lactone that spontaneously hydrolyzes under aqueous conditions to yield an aldonic acid [185]. This process effectively opens the sugar ring, destabilizes the glycosidic bond, and facilitates hydrolysis by exo- or endo-acting enzymes [180,186,187]. C1-oxidizing LPMOs are exceptionally efficient in synergizing with β-glucosidases or cellobiohydrolases that target the resulting oxidized chain ends [42].
C4 oxidation, conversely, introduces a keto group at the C4 position of the glucose unit [178]. This oxidation creates a ketoaldose at the non-reducing end, which also destabilizes the glycosidic linkage but differs chemically from the products of C1 oxidation [188]. The keto functional group can influence downstream enzymatic processing differently, sometimes resulting in slower hydrolysis by standard hydrolases due to altered ring chemistry [178].
Mixed C1/C4 oxidation endows certain LPMOs with enhanced flexibility to act on structurally heterogeneous or highly recalcitrant substrates [32]. These enzymes can introduce oxidative breaks at multiple sites within the same polysaccharide chain, improving accessibility for hydrolytic enzymes and promoting more efficient biomass turnover [162,176].
The determinants of the oxidation pattern in fungal LPMOs are deeply rooted in the structural architecture of the enzyme, particularly in the geometry of the active site relative to the substrate plane [189]. One of the primary factors influencing regioselectivity is the configuration of surface-exposed loops [88]. These loops form the structural framework surrounding the catalytic copper center and critically define how the enzyme orients itself over the polysaccharide chain [164,190].
Substrate surface properties further modulate oxidation patterns. The physical and chemical characteristics of the polysaccharide, whether it is crystalline, amorphous, highly ordered, or chemically modified, directly impact how the LPMO interacts with it [191]. C1 oxidation tends to dominate when the enzyme engages with tightly packed, crystalline cellulose surfaces, where the enzyme binds in a planar orientation that aligns the active site for C1 targeting [186]. In contrast, more flexible, amorphous regions may allow alternative binding modes that favor C4 oxidation or enable mixed C1/C4 cleavage [169]. Furthermore, the presence of accessory plant cell wall components such as hemicelluloses, lignin fragments, or acetylated sugars can influence the binding surface and, consequently, the oxidative outcome [122,192].
Electron transfer dynamics also play a subtle but significant role in oxidation pattern modulation. The nature, availability, and redox potential of electron donors, including cellobiose dehydrogenase (CDH), small aromatic compounds, and even photoexcited systems, can impact how efficiently electrons are delivered to the LPMO’s copper center [78,122,142].
5.5. Synergy with Other Enzymes
LPMOs do not function as standalone depolymerization agents but rather operate in tight synergy with a complex ensemble of hydrolytic and oxidative enzymes present in the fungal secretome (Figure 1). This orchestrated cooperation is one of the key factors underlying the remarkable efficiency of fungi in deconstructing plant biomass, particularly recalcitrant lignocellulosic substrates [187,193].
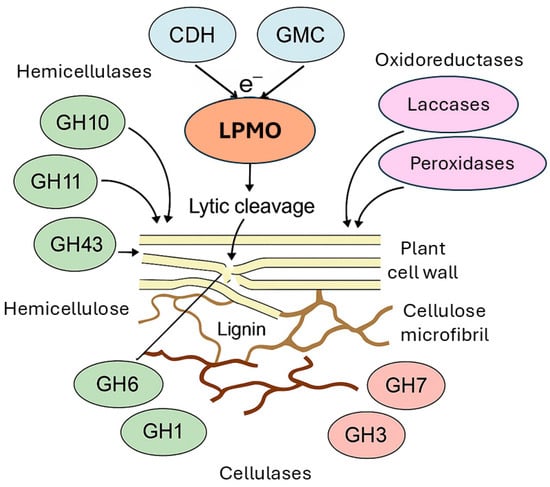
Figure 1.
Schematic representation of the enzymatic synergistic network involved in plant cell wall (represented in yellow) degradation. LPMOs act in conjunction with classical glycoside hydrolases, including exoglucanases and endoglucanases (represented by GH1, GH3, GH6, and GH7), to enhance the depolymerization of cellulose (represented in beige with greater thickness) by introducing oxidative cleavage points. Hemicellulose (shown represented in beige with a smaller thickness) is targeted by hemicellulases (e.g., xylanases, mannanases—represented by GH10, GH11, GH43) gain improved access to hemicellulose domains because of cellulose disruption. Lignin (depicted in brown) is degraded by lignin-modifying enzymes, such as laccases and class II peroxidases (MnP, LiP), which oxidatively degrade lignin, releasing phenolic compounds that serve as electron donors for LPMO catalysis. Auxiliary oxidoreductases like cellobiose dehydrogenase (CDH) and glucose-methanol-choline (GMC) oxidoreductases provide electrons to LPMOs (via mediators), sustaining their catalytic cycle via activation of O2 or H2O2. This coordinated enzymatic interplay ensures efficient deconstruction of the complex plant cell wall matrix, forming the biochemical foundation for both natural fungal decay and industrial biomass valorization.
A vital component of this synergy involves classical cellulases, particularly members of glycoside hydrolase families such as GH6 and GH7. These enzymes catalyze the hydrolytic cleavage of β-1,4-glycosidic bonds in cellulose. However, their efficiency is severely hampered when cellulose is in its native, highly crystalline form. Here, LPMOs play a transformative role by introducing oxidative cleavage at specific positions within the crystalline cellulose microfibrils; LPMOs create novel chain ends and disrupt the tight packing of cellulose chains. This structural loosening dramatically increases the accessibility of the cellulose to exo- and endo-cellulases, thereby enhancing their catalytic turnover and accelerating overall cellulose hydrolysis [19,193,194].
This synergistic effect extends beyond cellulose to hemicellulose components of the plant cell wall. Hemicellulases, including xylanases (GH10, GH11) and arabinofuranosidases or mannanases (GH43 and others), benefit indirectly from LPMO activity. The oxidative disruption of the cellulose scaffold by LPMOs exposes previously inaccessible hemicellulose domains embedded within or strongly associated with cellulose fibrils. As a result, the action of hemicellulases becomes more effective, facilitating a more comprehensive deconstruction of plant polysaccharides [72,194,195].
The synergy is not confined to carbohydrate-active enzymes but extends to lignin-degrading oxidative systems. Fungi produce a suite of lignin-modifying enzymes, such as laccases and class II peroxidases (including manganese peroxidase and lignin peroxidase), which initiate oxidative cleavage of the complex lignin polymer. A key by-product of lignin degradation is the generation of low-molecular-weight phenolic compounds, which can function as effective electron donors for LPMOs. This biochemical crosstalk creates a functional linkage between lignin depolymerization and polysaccharide oxidation, integrating oxidative degradation pathways across different components of the plant cell wall matrix [196,197,198,199].
Additionally, oxidoreductases such as CDH and members of the GMC oxidoreductase family are vital for sustaining the LPMO catalytic cycle. These enzymes facilitate the continuous reduction of the copper active site in LPMOs, delivering electrons necessary for the activation of molecular oxygen or hydrogen peroxide. By maintaining the redox balance, these oxidoreductases ensure that LPMO activity is sustained over prolonged periods during biomass conversion [25,200].
Altogether, the synergistic interplay among LPMOs, glycoside hydrolases, lignin-modifying oxidoreductases, and auxiliary redox enzymes constitutes a highly integrated fungal biomass-deconstruction system. This cooperative enzymatic network is significantly more effective than hydrolytic processes operating alone, particularly when targeting the most recalcitrant forms of plant biomass. Such synergy not only enhances the biological efficiency of fungal decomposition in natural ecosystems but also represents a fundamental principle leveraged in industrial biorefineries for the sustainable conversion of lignocellulosic feedstocks into biofuels and biochemicals [187,193,201].
5.6. Biological Modulation and Regulation
The biological modulation and regulation of LPMOs in fungi is a highly sophisticated and tightly controlled process, reflecting the delicate balance between the energetic benefits of biomass degradation and the potential cellular risks associated with oxidative chemistry [86]. Fungal deployment of LPMOs is primarily governed by substrate availability [32]. The presence of specific polysaccharides, particularly crystalline cellulose or hemicellulose, acts as a potent inducer of LPMO gene expression. This substrate-driven regulation ensures that fungi invest metabolic resources into producing LPMOs only when the appropriate target polysaccharides are present in the environment, thereby maximizing enzymatic efficiency while minimizing unnecessary energy expenditure [202,203].
An additional layer of regulation stems from the intrinsic risks associated with the oxidative nature of LPMO catalysis. Since LPMOs can utilize both molecular oxygen and hydrogen peroxide (H2O2) as co-substrates, their activity inherently generates reactive oxygen species (ROS). Unchecked ROS production can be highly detrimental to fungal cells, leading to oxidative stress and potential cellular damage. As a result, fungi have evolved mechanisms to carefully modulate LPMO activity, balancing the need for efficient biomass degradation with the imperative to maintain redox homeostasis and protect cellular integrity. This modulation likely involves transcriptional regulation and post-transcriptional controls, including feedback from redox sensors and the availability of electron donors [86,204,205].
Transcriptomic and proteomic studies across several fungal species, including N. crassa, T. reesei, T. thermophilus, and P. chrysosporium, consistently reveal that LPMO genes are co-expressed alongside genes encoding cellulases, hemicellulases, CDH, and lignin-modifying enzymes such as laccases and peroxidases. This coordinated expression pattern underscores the integrated role of LPMOs within the broader fungal secretome and highlights their functional interdependence with both hydrolytic and oxidative enzymes. Such regulatory coherence ensures that LPMO deployment is synchronized with the overall strategy for plant biomass decomposition, reflecting a finely tuned adaptation to ecological pressures and resource availability [18,206,207,208].
6. Fungal LPMOs with Industrial Applications
Fungal LPMOs have emerged as pivotal biocatalysts in several industrial sectors due to their unparalleled ability to oxidatively cleave recalcitrant polysaccharides, such as crystalline cellulose and hemicelluloses. Their capacity to disrupt the crystalline architecture of lignocellulosic biomass positions them as indispensable components in modern enzyme formulations designed for biomass valorization. Unlike classical glycoside hydrolases, which rely solely on hydrolytic mechanisms, LPMOs introduce oxidative cleavage at specific carbons, generating chain breaks that dramatically improve the accessibility of polysaccharides to other enzymes [10,77,182].
The most significant industrial application of fungal LPMOs is in the biorefinery sector, particularly in the enzymatic hydrolysis of plant biomass to produce second-generation biofuels (cellulosic ethanol) and biochemicals [10,184]. LPMOs, especially those belonging to the AA9 family, are widely incorporated into commercial cellulase cocktails produced by leading biotechnology companies [46]. Their inclusion has been shown to boost saccharification yields, particularly when processing highly crystalline biomass like pretreated corn stover, sugarcane bagasse, or wheat straw [46,201,209].
Beyond biofuels, LPMOs play an emerging role in the pulp and paper industry, where they facilitate fiber modification, enhancing delignification and improving paper quality with reduced chemical inputs. Their oxidative action disrupts cellulose crystallinity, making fibers more amenable to subsequent chemical or enzymatic treatments, thereby contributing to more sustainable and energy-efficient processing [112,210].
The textile industry also benefits from LPMOs, particularly in cotton processing and denim finishing. Here, LPMOs contribute to fiber modification, offering a greener alternative to harsh chemical treatments. Their ability to weaken cellulose fibers in a controlled manner enables more sustainable bio-polishing, improving fabric softness and appearance while reducing water and chemical usage [211,212].
A rapidly growing frontier for LPMO applications is in the food and feed industries. For example, LPMOs assist in the degradation of plant-based feedstocks, improving digestibility and nutrient accessibility in animal feed formulations [213,214]. Additionally, in the production of plant-based proteins and functional food ingredients, LPMOs contribute to the extraction and modification of polysaccharides from complex biomass [215,216].
From an industrial enzyme engineering perspective, fungal LPMOs have become targets for protein engineering to enhance stability, catalytic efficiency, and oxidative robustness under harsh industrial conditions, including elevated temperatures, variable pH, and oxidative stress. Advances in understanding the H2O2-driven catalytic mechanism have led to optimized process designs where controlled peroxide dosing maximizes LPMO activity while minimizing enzyme inactivation [217,218].
Furthermore, integrated biorefineries are beginning to exploit LPMOs in conjunction with oxidative lignin-modifying enzymes, such as laccases and peroxidases. This synergy improves carbohydrate conversion and opens pathways for lignin valorization into biopolymers, resins, and aromatic compounds, reinforcing the Circular Bioeconomy model [122,161].
In the context of emerging green technologies, LPMOs are being explored for novel applications such as bioplastic degradation, enzymatic textile dye bleaching, and even in the field of carbon capture via engineered fungal systems that degrade plant biomass into stable soil organic carbon [178,219,220].
In summary, fungal LPMOs have transitioned from being a fundamental enzymatic curiosity to becoming a cornerstone of industrial biotechnology. Their incorporation into commercial processes is a direct consequence of their unique catalytic capabilities, offering sustainable solutions for biomass conversion, material processing, and the development of bio-based products. Continued research into their mechanisms, regulation, and interaction with electron donors will further expand their applicability across diverse industrial landscapes.
7. Future Perspectives
The growing industrial relevance of LPMOs continues to drive a surge of interest in unlocking their full potential beyond current applications. As the demand for efficient and sustainable biotechnological processes escalates, future perspectives for fungal LPMOs revolve around three interconnected pillars: (i) the exploration of underexplored fungal genomes, (ii) the engineering of strains and heterologous expression systems, and (iii) the expansion of their applications in biorefineries and biomaterials [7,19,202].
Despite considerable progress in characterizing LPMOs from well-studied fungi, the fungal kingdom harbours an enormous untapped reservoir of enzymatic diversity [24]. Numerous fungal taxa, particularly those adapted to extreme environments (e.g., thermophilic, halophilic, acidophilic fungi) or specialized ecological niches (e.g., marine fungi, endophytes, and soil saprobes), remain largely unexplored for their oxidative enzyme repertoires [221]. Advances in next-generation sequencing and metagenomics are revealing a vast array of novel LPMO genes from these underexplored lineages [47]. Comparative genomics combined with machine-learning-guided enzyme mining is expected to uncover LPMOs with unique properties such as higher thermal stability, broader substrate specificity (e.g., activity on non-cellulosic polysaccharides like chitin or pectin), and enhanced oxidative resistance [222]. These novel biocatalysts could significantly broaden the applicability of LPMOs in industrial contexts that demand more robust enzymes. Furthermore, the exploration of fungal microbiomes associated with decaying lignocellulosic biomass, symbiotic relationships (e.g., lichens, mycorrhizae), or polluted environments may yield LPMOs adapted to challenging chemical conditions, offering new functionalities for biotechnology [223].
A significant bottleneck in the industrial deployment of LPMOs lies in their production yield, oxidative stability, and control over their activity. Future research is increasingly focused on synthetic biology and strain engineering strategies to overcome these limitations [224]. Optimizing heterologous expression systems, such as Komagataella pastoris (formerly Pichia pastoris), Aspergillus niger, and Saccharomyces cerevisiae, is essential for scalable production of tailored LPMO variants. Codon optimization, promoter engineering, and secretory pathway enhancement are being combined to boost yields and ensure proper copper loading and folding [219,225,226]. Additionally, protein engineering approaches targeting improvements in LPMO stability under oxidative stress, tuning their regioselectivity (C1 vs. C4 oxidation), and modifying their interaction with hydrogen peroxide to minimize self-inactivation [227]. Directed evolution, structure-guided mutagenesis, and computational enzyme design are expected to yield next-generation LPMOs with superior catalytic profiles tailored to specific industrial processes. Coupling LPMOs with engineered redox partners, such as optimized CDHs or artificial electron transfer systems, is another frontier poised to enhance operational stability and efficiency in biocatalytic systems [228,229,230].
As the bioeconomy shifts toward zero-waste, circular models, LPMOs are poised to play an increasingly vital role in integrated biorefineries. Their oxidative cleavage ability synergizes with both hydrolytic and lignin-modifying enzymes, enabling comprehensive biomass deconstruction and valorization. Future biorefinery models will leverage LPMOs not only for the saccharification of lignocellulose into fermentable sugars but also for the selective extraction of functional oligosaccharides, nanocellulose, and lignin-derived aromatic compounds. These high-value intermediates are key feedstocks to produce bio-based chemicals, bioplastics, resins, and advanced biomaterials. In parallel, LPMOs are gaining attention for applications in biomaterial processing, such as enhancing fiber properties in textiles, facilitating the production of biodegradable composites, and even contributing to enzymatic recycling strategies for composite materials that blend natural fibers with synthetic polymers. Emerging applications are also exploring the role of LPMOs in the biodegradation of environmental pollutants, including complex plastics, where LPMO-driven oxidative mechanisms may aid in breaking down polymeric structures when coupled with other oxidative enzymes [10,46,122,161,178,184,201,209,210,211,212,213,214,215,216,217,218,219,220].
8. Conclusions
Fungal lytic polysaccharide monooxygenases (LPMOs) have emerged as pivotal enzymes in the global carbon cycle and in industrial biotechnology, owing to their unique oxidative mechanism that complements classical hydrolytic enzymes in lignocellulose degradation. Their ability to cleave recalcitrant polysaccharides such as crystalline cellulose, hemicellulose, and chitin through oxidative reactions has fundamentally reshaped our understanding of fungal biomass conversion. The structural sophistication of these enzymes, particularly the histidine brace copper active site, combined with substrate-binding loops and CBMs, orchestrates a highly selective and efficient catalytic process. Furthermore, the capacity of LPMOs to utilize both molecular oxygen (O2) and hydrogen peroxide (H2O2) as co-substrates adds a remarkable degree of flexibility to their function. However, it necessitates precise cellular regulation to mitigate oxidative stress. Their activity is deeply intertwined with that of other fungal enzymes, including cellulases, hemicellulases, oxidoreductases, and lignin-modifying enzymes, forming an integrated enzymatic arsenal that facilitates effective deconstruction of lignocellulosic biomass in both natural ecosystems and industrial processes.
Looking forward, the biotechnological potential of fungal LPMOs continues to expand. Advances in genome mining, transcriptomics, and metagenomics are uncovering a wealth of untapped fungal diversity, particularly from ecological niches that remain poorly explored. This opens avenues for the discovery of LPMOs with novel substrate specificities, enhanced thermal or chemical stability, and improved oxidative performance. Concurrently, protein engineering and synthetic biology approaches are enabling the development of tailored LPMOs and optimized microbial hosts for heterologous expression, facilitating their integration into enzyme cocktails for biorefineries, biofuel production, and emerging sectors such as sustainable biomaterials and green chemistry. As mechanistic understanding deepens, particularly regarding the balance between O2- and H2O2-driven catalysis, electron transfer dynamics, and enzyme stabilization, fungal LPMOs are poised to play an increasingly significant role in advancing the Circular Bioeconomy and sustainable biotechnological solutions for the future.
Author Contributions
A.G.C.: Conceptualization, Investigation, Formal Analysis, Visualization, Writing—original draft, Funding acquisition, Project administration. C.A.C.-J.: Funding acquisition, Project administration, Writing—Review, and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support provided by the Fundação Carlos Chagas de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Brazil—grant numbers [E-26/210.537/2025], [E-26/202.101/2025], [E-26/203.745/2024], and [E-26/200.891/2021]; the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil—grant numbers [2017/25862-6], [2021/07066-3], and [2023/08824-4]; the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil—grant number [313119/2020-1]; and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil—Finance Code 001.
Data Availability Statement
This narrative review is based on a comprehensive analysis of previously published studies and does not involve original data collection.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Contato, A.G.; Vici, A.C.; Pinheiro, V.E.; de Oliveira, T.B.; Ortolan, G.G.; de Freitas, E.N.; Buckeridge, M.S.; Polizeli, M.L.T.M. Thermothelomyces thermophilus cultivated with residues from the fruit pulp industry: Enzyme immobilization on ionic supports of a crude cocktail with enhanced production of lichenase. Folia Microbiol. 2025, 70, 619–629. [Google Scholar] [CrossRef]
- Weimer, P.J. Degradation of cellulose and hemicellulose by ruminal microorganisms. Microorganisms 2022, 10, 2345. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.; Sharma, M.; Salama, E.S.; Ling, Z.; Li, X. Applications of chitin and chitosan as natural biopolymer: Potential sources, pretreatments, and degradation pathways. Biomass Convers. Biorefinery 2024, 14, 4567–4581. [Google Scholar] [CrossRef]
- Contato, A.G.; Borelli, T.C.; de Carvalho, A.K.F.; Bento, H.B.S.; Buckeridge, M.S.; Rogers, J.; Hartson, S.; Prade, R.A.; Polizeli, M.L.T.M. Comparative analysis of CAZymes from Trichoderma longibrachiatum LMBC 172 cultured with three different carbon sources: Sugarcane bagasse, tamarind seeds, and hemicellulose simulation. Clean Technol. 2024, 6, 994–1010. [Google Scholar] [CrossRef]
- Hage, H.; Rosso, M.N. Evolution of fungal carbohydrate-active enzyme portfolios and adaptation to plant cell-wall polymers. J. Fungi 2021, 7, 185. [Google Scholar] [CrossRef]
- Tingley, J.P.; Low, K.E.; Xing, X.; Abbott, D.W. Combined whole cell wall analysis and streamlined in silico carbohydrate-active enzyme discovery to improve biocatalytic conversion of agricultural crop residues. Biotechnol. Biofuels 2021, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Singhania, R.R.; Dixit, P.; Patel, A.K.; Giri, B.S.; Kuo, C.H.; Chen, C.W.; Di Dong, C. Role and significance of lytic polysaccharide monooxygenases (LPMOs) in lignocellulose deconstruction. Bioresour. Technol. 2021, 335, 125261. [Google Scholar] [CrossRef]
- Manavalan, T.; Stepnov, A.A.; Hegnar, O.A.; Eijsink, V.G. Sugar oxidoreductases and LPMOs–two sides of the same polysaccharide degradation story? Carbohydr. Res. 2021, 505, 108350. [Google Scholar] [CrossRef]
- Walton, P.H.; Davies, G.J. On the catalytic mechanisms of lytic polysaccharide monooxygenases. Curr. Opin. Chem. Biol. 2016, 31, 195–207. [Google Scholar] [CrossRef]
- Sharma, S.; Modi, R.; Kaur, A. Lytic polysaccharide monooxygenases producing microbes: A key indicator for biomass-degrading enzymes. Biocatal. Agric. Biotechnol. 2024, 60, 103337. [Google Scholar] [CrossRef]
- Rieder, L.; Sørlie, M. Recent advances in understanding LPMO catalysis. Biochemistry 2023, 62, 3170–3172. [Google Scholar] [CrossRef]
- Hagemann, M.M.; Hedegård, E.D. Molecular mechanism of substrate oxidation in lytic polysaccharide monooxygenases: Insight from theoretical investigations. Chem. A Eur. J. 2023, 29, e202202379. [Google Scholar] [CrossRef]
- Ipsen, J.Ø.; Hallas-Møller, M.; Brander, S.; Lo Leggio, L.; Johansen, K.S. Lytic polysaccharide monooxygenases and other histidine-brace copper proteins: Structure, oxygen activation and biotechnological applications. Biochem. Soc. Trans. 2021, 49, 531–540. [Google Scholar] [CrossRef]
- Liu, Y.; Harnden, K.A.; Van Stappen, C.; Dikanov, S.A.; Lu, Y. A designed Copper Histidine-brace enzyme for oxidative depolymerization of polysaccharides as a model of lytic polysaccharide monooxygenase. Proc. Natl. Acad. Sci. USA 2023, 120, e2308286120. [Google Scholar] [CrossRef]
- Guo, X.; An, Y.; Jiang, L.; Zhang, J.; Lu, F.; Liu, F. The discovery and enzymatic characterization of a novel AA10 LPMO from Bacillus amyloliquefaciens with dual substrate specificity. Int. J. Biol. Macromol. 2022, 203, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, Y.; Liu, Y.; Li, Y.; Yu, H. Heterologous expression and characterization of a novel lytic polysaccharide monooxygenase from Natrialbaceae archaeon and its application for chitin biodegradation. Bioresour. Technol. 2022, 354, 127174. [Google Scholar] [CrossRef] [PubMed]
- Loose, J.S.M.; Boudes, M.; Bergoin, M.; Coulibaly, F.; Vaaje-Kolstad, G. The Melolontha melolontha entomopoxvirus fusolin protein is a chitin-active lytic polysaccharide monooxygenase that displays extreme stability. FEBS Lett. 2023, 597, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Contato, A.G.; Borelli, T.C.; Buckeridge, M.S.; Rogers, J.; Hartson, S.; Prade, R.A.; Polizeli, M.L.T.M. Secretome analysis of Thermothelomyces thermophilus LMBC 162 cultivated with Tamarindus indica seeds reveals CAZymes for degradation of lignocellulosic biomass. J. Fungi 2024, 10, 121. [Google Scholar] [CrossRef]
- Lange, L.; Pilgaard, B.; Herbst, F.A.; Busk, P.K.; Gleason, F.; Pedersen, A.G. Origin of fungal biomass degrading enzymes: Evolution, diversity and function of enzymes of early lineage fungi. Fungal Biol. Rev. 2019, 33, 82–97. [Google Scholar] [CrossRef]
- Resl, P.; Bujold, A.R.; Tagirdzhanova, G.; Meidl, P.; Freire Rallo, S.; Kono, M.; Fernández-Brime, S.; Guðmundsson, H.; Andrésson, O.S.; Muggia, L.; et al. Large differences in carbohydrate degradation and transport potential among lichen fungal symbionts. Nat. Commun. 2022, 13, 2634. [Google Scholar] [CrossRef]
- Lebreton, A.; Zeng, Q.; Miyauchi, S.; Kohler, A.; Dai, Y.C.; Martin, F.M. Evolution of the mode of nutrition in symbiotic and saprotrophic fungi in forest ecosystems. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 385–404. [Google Scholar] [CrossRef]
- Li, H.; Young, S.E.; Poulsen, M.; Currie, C.R. Symbiont-mediated digestion of plant biomass in fungus-farming insects. Annu. Rev. Entomol. 2021, 66, 297–316. [Google Scholar] [CrossRef]
- Tõlgo, M.; Hegnar, O.A.; Østby, H.; Várnai, A.; Vilaplana, F.; Eijsink, V.G.; Olsson, L. Comparison of six lytic polysaccharide monooxygenases from Thermothielavioides terrestris shows that functional variation underlies the multiplicity of LPMO genes in filamentous fungi. Appl. Environ. Microbiol. 2022, 88, e0009622. [Google Scholar] [CrossRef] [PubMed]
- Contato, A.G.; de Oliveira, T.B.; Aranha, G.M.; de Freitas, E.N.; Vici, A.C.; Nogueira, K.M.V.; de Lucas, R.C.; Scarcella, A.S.A.; Buckeridge, M.S.; Silva, R.N.; et al. Prospection of fungal lignocellulolytic enzymes produced from jatoba (Hymenaea courbaril) and tamarind (Tamarindus indica) seeds: Scaling for bioreactor and saccharification profile of sugarcane bagasse. Microorganisms 2021, 9, 533. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Y.; Yu, J.; Wang, L. Recent advances in the efficient degradation of lignocellulosic metabolic networks by lytic polysaccharide monooxygenase: Advancing lignocellulose degradation with LPMOs. Acta Biochim. Biophys. Sin. 2023, 55, 529. [Google Scholar] [CrossRef]
- Kuusk, S.; Lipp, M.; Mahajan, S.; Väljamäe, P. On the pH dependency of the catalysis by a Lytic Polysaccharide Monooxygenase from the fungus Trichoderma reesei. ACS Catal. 2024, 14, 13408–13419. [Google Scholar] [CrossRef]
- Hegnar, O.A.; Østby, H.; Petrović, D.M.; Olsson, L.; Várnai, A.; Eijsink, V.G. Quantifying oxidation of cellulose-associated glucuronoxylan by two lytic polysaccharide monooxygenases from Neurospora crassa. Appl. Environ. Microbiol. 2021, 87, e0165221. [Google Scholar] [CrossRef]
- Chorozian, K.; Karnaouri, A.; Tryfona, T.; Kondyli, N.G.; Karantonis, A.; Topakas, E. Characterization of a novel AA16 lytic polysaccharide monooxygenase from Thermothelomyces thermophilus and comparison of biochemical properties with an LPMO from AA9 family. Carbohydr. Polym. 2024, 342, 122387. [Google Scholar] [CrossRef]
- Patel, I.; Kracher, D.; Ma, S.; Garajova, S.; Haon, M.; Faulds, C.B.; Berrin, J.G.; Ludwig, R.; Record, E. Salt-responsive lytic polysaccharide monooxygenases from the mangrove fungus Pestalotiopsis sp. NCi6. Biotechnol. Biofuels 2016, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Busk, P.K.; Lange, M.; Pilgaard, B.; Lange, L. Several genes encoding enzymes with the same activity are necessary for aerobic fungal degradation of cellulose in nature. PLoS ONE 2014, 9, e114138. [Google Scholar] [CrossRef]
- Rieder, L.; Petrović, D.; Valjamae, P.; Eijsink, V.G.; Sørlie, M. Kinetic characterization of a putatively chitin-active LPMO reveals a preference for soluble substrates and absence of monooxygenase activity. ACS Catal. 2021, 11, 11685–11695. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, H. Current understanding of substrate specificity and regioselectivity of LPMOs. Bioresour. Bioprocess. 2020, 7, 11. [Google Scholar] [CrossRef]
- Kipping, L.; Jehmlich, N.; Moll, J.; Noll, M.; Gossner, M.M.; Van Den Bossche, T.; Edelmann, P.; Borken, W.; Hofrichter, M.; Kellner, H. Enzymatic machinery of wood-inhabiting fungi that degrade temperate tree species. ISME J. 2024, 18, wrae050. [Google Scholar] [CrossRef]
- Shabaev, A.V.; Moiseenko, K.V.; Glazunova, O.A.; Savinova, O.S.; Fedorova, T.V. Comparative analysis of Peniophora lycii and Trametes hirsuta exoproteomes demonstrates “Shades of Gray” in the concept of white-rotting fungi. Int. J. Mol. Sci. 2022, 23, 10322. [Google Scholar] [CrossRef]
- Zhu, Y.; Plaza, N.; Kojima, Y.; Yoshida, M.; Zhang, J.; Jellison, J.; Pingali, S.V.; O’Neill, H.; Goodell, B. Nanostructural analysis of enzymatic and non-enzymatic brown rot fungal deconstruction of the lignocellulose cell wall. Front. Microbiol. 2020, 11, 1389. [Google Scholar] [CrossRef]
- Rosso, M.N.; Berrin, J.G.; Lomascolo, A. Plant wastes and sustainable refineries: What can we learn from fungi? Curr. Opin. Green Sustain. Chem. 2022, 34, 100602. [Google Scholar] [CrossRef]
- Barbi, F.; Kohler, A.; Barry, K.; Baskaran, P.; Daum, C.; Fauchery, L.; Ihrmark, K.; Kuo, A.; LaButti, K.; Lipzen, A.; et al. Fungal ecological strategies reflected in gene transcription-a case study of two litter decomposers. Environ. Microbiol. 2020, 22, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Scarcella, A.S.D.A.; Pasin, T.M.; de Lucas, R.C.; Ferreira-Nozawa, M.S.; de Oliveira, T.B.; Contato, A.G.; Grandis, A.; Buckeridge, M.S.; Polizeli, M.L.T.M. Holocellulase production by filamentous fungi: Potential in the hydrolysis of energy cane and other sugarcane varieties. Biomass Convers. Biorefinery 2023, 13, 1163–1174. [Google Scholar] [CrossRef]
- Tang, C.; Cavka, A.; Bui, M.; Jönsson, L.J. Comparison of simultaneous saccharification and fermentation with LPMO-supported hybrid hydrolysis and fermentation. Front. Bioeng. Biotechnol. 2024, 12, 1419723. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Altyar, A.E.; Mohamed, S.G.; Mohamed, G.A. Genus Thielavia: Phytochemicals, industrial importance and biological relevance. Nat. Prod. Res. 2022, 36, 5108–5123. [Google Scholar] [CrossRef] [PubMed]
- Barandiaran, L.; Alonso-Lerma, B.; Reifs, A.; Larraza, I.; Olmos-Juste, R.; Fernandez-Calvo, A.; Jabalera, Y.; Eceiza, A.; Perez-Jimenez, R. Enzymatic upgrading of nanochitin using an ancient lytic polysaccharide monooxygenase. Commun. Mater. 2022, 3, 55. [Google Scholar] [CrossRef]
- Angeltveit, C.F.; Várnai, A.; Eijsink, V.G.; Horn, S.J. Enhancing enzymatic saccharification yields of cellulose at high solid loadings by combining different LPMO activities. Biotechnol. Biofuels Bioprod. 2024, 17, 39. [Google Scholar] [CrossRef]
- Long, L.; Hu, Y.; Sun, F.; Gao, W.; Hao, Z.; Yin, H. Advances in lytic polysaccharide monooxygenases with the cellulose-degrading auxiliary activity family 9 to facilitate cellulose degradation for biorefinery. Int. J. Biol. Macromol. 2022, 219, 68–83. [Google Scholar] [CrossRef]
- Koskela, S.; Wang, S.; Xu, D.; Yang, X.; Li, K.; Berglund, L.A.; McKee, L.S.; Bulone, V.; Zhou, Q. Lytic polysaccharide monooxygenase (LPMO) mediated production of ultra-fine cellulose nanofibres from delignified softwood fibres. Green Chem. 2019, 21, 5924–5933. [Google Scholar] [CrossRef]
- Zerva, A.; Tsafantakis, N.; Topakas, E. Evaluation of Basidiomycetes wild strains grown in agro-industrial residues for their anti-tyrosinase and antioxidant potential and for the production of biocatalysts. Fermentation 2021, 7, 19. [Google Scholar] [CrossRef]
- Agrawal, D.; Basotra, N.; Balan, V.; Tsang, A.; Chadha, B.S. Discovery and expression of thermostable LPMOs from thermophilic fungi for producing efficient lignocellulolytic enzyme cocktails. Appl. Biochem. Biotechnol. 2020, 191, 463–481. [Google Scholar] [CrossRef] [PubMed]
- Bunterngsook, B.; Mhuantong, W.; Kanokratana, P.; Iseki, Y.; Watanabe, T.; Champreda, V. Identification and characterization of a novel AA9-type lytic polysaccharide monooxygenase from a bagasse metagenome. Appl. Microbiol. Biotechnol. 2021, 105, 197–210. [Google Scholar] [CrossRef]
- Frandsen, K.E.; Haon, M.; Grisel, S.; Henrissat, B.; Leggio, L.L.; Berrin, J.G. Identification of the molecular determinants driving the substrate specificity of fungal lytic polysaccharide monooxygenases (LPMOs). J. Biol. Chem. 2021, 296, 100086. [Google Scholar] [CrossRef]
- Mazurkewich, S.; Seveso, A.; Hüttner, S.; Brändén, G.; Larsbrink, J. Structure of a C1/C4-oxidizing AA9 lytic polysaccharide monooxygenase from the thermophilic fungus Malbranchea cinnamomea. Biol. Crystallogr. 2021, 77, 1019–1026. [Google Scholar] [CrossRef]
- Calderaro, F.; Keser, M.; Akeroyd, M.; Bevers, L.E.; Eijsink, V.G.H.; Várnai, A.; van Den Berg, M.A. Characterization of an AA9 LPMO from Thielavia australiensis, Taus LPMO9B, under industrially relevant lignocellulose saccharification conditions. Biotechnol. Biofuels 2020, 13, 195. [Google Scholar] [CrossRef]
- Jagadeeswaran, G.; Gainey, L.; Mort, A.J. An AA9-LPMO containing a CBM1 domain in Aspergillus nidulans is active on cellulose and cleaves cello-oligosaccharides. AMB Express 2018, 8, 171. [Google Scholar] [CrossRef]
- Waheed, A.; Chen, Y.; Rizwan, H.M.; Adnan, M.; Ma, X.; Liu, G. Genomic characterization and expression profiling of the lytic polysaccharide monooxygenases AA9 family in thermophilic fungi Thermothelomyces fergusii in response to carbon source media. Int. J. Biol. Macromol. 2024, 265, 130740. [Google Scholar] [CrossRef]
- Garrido, M.M.; Landoni, M.; Sabbadin, F.; Valacco, M.P.; Couto, A.; Bruce, N.C.; Wirth, S.A.; Campos, E. Ps AA9A, a C1-specific AA9 lytic polysaccharide monooxygenase from the white-rot basidiomycete Pycnoporus sanguineus. Appl. Microbiol. Biotechnol. 2020, 104, 9631–9643. [Google Scholar] [CrossRef]
- Leggio, L.L.; Weihe, C.D.; Poulsen, J.C.N.; Sweeney, M.; Rasmussen, F.; Lin, J.; De Maria, L.; Wogulis, M. Structure of a lytic polysaccharide monooxygenase from Aspergillus fumigatus and an engineered thermostable variant. Carbohydr. Res. 2018, 469, 55–59. [Google Scholar] [CrossRef]
- Yao, R.A.; Reyre, J.L.; Tamburrini, K.C.; Haon, M.; Tranquet, O.; Nalubothula, A.; Mukherjee, S.; Le Gall, S.; Grisel, S.; Longhi, S.; et al. The Ustilago maydis AA10 LPMO is active on fungal cell wall chitin. Appl. Environ. Microbiol. 2023, 89, e0057323. [Google Scholar] [CrossRef]
- Støpamo, F.G.; Røhr, Å.K.; Mekasha, S.; Petrović, D.M.; Várnai, A.; Eijsink, V.G. Characterization of a lytic polysaccharide monooxygenase from Aspergillus fumigatus shows functional variation among family AA11 fungal LPMOs. J. Biol. Chem. 2021, 297, 101421. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, Z.; Kong, Z.; Wang, M.; Li, T.; Zhu, H.; Wan, Q.; Liu, D.; Shen, Q. Functional characterization of a novel copper-dependent lytic polysaccharide monooxygenase TgAA11 from Trichoderma guizhouense NJAU 4742 in the oxidative degradation of chitin. Carbohydr. Polym. 2021, 258, 117708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Haider, J.; Yew, M.; Yang, J.; Zhu, L. Discovery and functional characterization of new starch-active lytic polysaccharide monooxygenases. Int. J. Biol. Macromol. 2025, 304, 140833. [Google Scholar] [CrossRef]
- Momeni, M.H.; Leth, M.L.; Sternberg, C.; Schoof, E.; Nielsen, M.W.; Holck, J.; Workman, C.T.; Hoof, J.B.; Hachem, M.A. Loss of AA13 LPMOs impairs degradation of resistant starch and reduces the growth of Aspergillus nidulans. Biotechnol. Biofuels 2020, 13, 135. [Google Scholar] [CrossRef]
- Matsuzawa, T. Plant polysaccharide degradation-related enzymes in Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2024, 88, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Tuveng, T.R.; Østby, H.; Tamburrini, K.C.; Bissaro, B.; Hegnar, O.A.; Stepnov, A.A.; Várnai, A.; Berrin, J.G.; Eijsink, V.G. Revisiting the AA14 family of lytic polysaccharide monooxygenases and their catalytic activity. FEBS Lett. 2023, 597, 2086–2102. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, X.; Zhang, P.; Long, L.; Ding, S. A novel AA14 LPMO from Talaromyces rugulosus with bifunctional cellulolytic/hemicellulolytic activity boosted cellulose hydrolysis. Biotechnol. Biofuels Bioprod. 2024, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Zhao, X.; Zhang, P.; Long, L.; Ding, S. A novel cellulolytic/xylanolytic SbAA14 from Sordaria brevicollis with a branched chain preference and its synergistic effects with glycoside hydrolases on lignocellulose. Int. J. Biol. Macromol. 2024, 260, 129504. [Google Scholar] [CrossRef]
- Mäkinen, M.; Kuuskeri, J.; Laine, P.; Smolander, O.P.; Kovalchuk, A.; Zeng, Z.; Asiegbu, F.O.; Paulin, L.; Auvinen, P.; Lundell, T. Genome description of Phlebia radiata 79 with comparative genomics analysis on lignocellulose decomposition machinery of phlebioid fungi. BMC Genom. 2019, 20, 430. [Google Scholar] [CrossRef]
- Couturier, M.; Ladeveze, S.; Sulzenbacher, G.; Ciano, L.; Fanuel, M.; Moreau, C.; Villares, A.; Cathala, B.; Chaspoul, F.; Frandsen, K.E.; et al. Lytic xylan oxidases from wood-decay fungi unlock biomass degradation. Nat. Chem. Biol. 2018, 14, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Guo, X.; Tian, S.; Yang, Q.; Kim, M.; Mun, S.; Noh, M.Y.; Kramer, K.J.; Muthukrishnan, S.; Arakane, Y. AA15 lytic polysaccharide monooxygenase is required for efficient chitinous cuticle turnover during insect molting. Commun. Biol. 2022, 5, 518. [Google Scholar] [CrossRef] [PubMed]
- Cairo, J.P.L.F.; Almeida, D.V.; Damasio, A.; Garcia, W.; Squina, F.M. The periplasmic expression and purification of AA15 lytic polysaccharide monooxygenases from insect species in Escherichia coli. Protein Expr. Purif. 2022, 190, 105994. [Google Scholar] [CrossRef]
- Sun, P.; Huang, Z.; Banerjee, S.; Kadowaki, M.A.; Veersma, R.J.; Magri, S.; Hilgers, R.; Muderspach, S.J.; Laurent, C.V.F.P.; Ludwig, R.; et al. AA16 oxidoreductases boost cellulose-active AA9 lytic polysaccharide monooxygenases from Myceliophthora thermophila. ACS Catal. 2023, 13, 4454–4467. [Google Scholar] [CrossRef]
- Filiatrault-Chastel, C.; Navarro, D.; Haon, M.; Grisel, S.; Herpoël-Gimbert, I.; Chevret, D.; Fanuel, M.; Henrissat, B.; Heiss-Blanquet, S.; Margeot, M.; et al. AA16, a new lytic polysaccharide monooxygenase family identified in fungal secretomes. Biotechnol. Biofuels 2019, 12, 55. [Google Scholar] [CrossRef]
- Cairo, J.P.L.F.; Almeida, D.V.; Andrade, V.B.; Terrasan, C.R.; Telfer, A.; Gonçalves, T.A.; Diaz, D.E.; Figueiredo, F.L.; Brenelli, L.B.; Walton, P.H.; et al. Biochemical and structural insights of a recombinant AA16 LPMO from the marine and sponge-symbiont Peniophora sp. Int. J. Biol. Macromol. 2024, 280, 135596. [Google Scholar]
- Peña, A.; Babiker, R.; Chaduli, D.; Lipzen, A.; Wang, M.; Chovatia, M.; Rencoret, J.; Marques, G.; Sánchez-Ruiz, M.I.; Kijpornyongpan, T.; et al. A multiomic approach to understand how Pleurotus eryngii transforms non-woody lignocellulosic material. J. Fungi 2021, 7, 426. [Google Scholar] [CrossRef]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef]
- Guo, X.; An, Y.; Liu, F.; Lu, F.; Wang, B. Lytic polysaccharide monooxygenase—A new driving force for lignocellulosic biomass degradation. Bioresour. Technol. 2022, 362, 127803. [Google Scholar] [CrossRef]
- Nekiunaite, L.; Arntzen, M.Ø.; Svensson, B.; Vaaje-Kolstad, G.; Hachem, M.A. Lytic polysaccharide monooxygenases and other oxidative enzymes are abundantly secreted by Aspergillus nidulans grown on different starches. Biotechnol. Biofuels 2016, 9, 187. [Google Scholar] [CrossRef]
- Vu, V.V.; Marletta, M.A. Starch-degrading polysaccharide monooxygenases. Cell. Mol. Life Sci. 2016, 73, 2809–2819. [Google Scholar] [CrossRef]
- Lo Leggio, L.; Simmons, T.J.; Poulsen, J.C.N.; Frandsen, K.E.; Hemsworth, G.R.; Stringer, M.A.; von Freiesleben, P.; Tovborg, C.; Johansen, K.S.; De Maria, L.; et al. Structure and boosting activity of a starch-degrading lytic polysaccharide monooxygenase. Nat. Commun. 2015, 6, 5961. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Sharma, V.K.; Goel, A.; Kumar, A. The upsurge of lytic polysaccharide monooxygenases in biomass deconstruction: Characteristic functions and sustainable applications. FEBS J. 2024, 291, 5081–5101. [Google Scholar] [CrossRef] [PubMed]
- Tandrup, T.; Frandsen, K.E.; Johansen, K.S.; Berrin, J.G.; Lo Leggio, L. Recent insights into lytic polysaccharide monooxygenases (LPMOs). Biochem. Soc. Trans. 2018, 46, 1431–1447. [Google Scholar] [CrossRef]
- Martino, E.; Morin, E.; Grelet, G.A.; Kuo, A.; Kohler, A.; Daghino, S.; Barry, K.W.; Cichocki, N.; Clum, A.; Dockter, R.B.; et al. Comparative genomics and transcriptomics depict ericoid mycorrhizal fungi as versatile saprotrophs and plant mutualists. New Phytol. 2018, 217, 1213–1229. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, S.; Kiss, E.; Kuo, A.; Drula, E.; Kohler, A.; Sánchez-García, M.; Morin, E.; Andrepoulos, B.; Barry, K.W.; Bonito, G.; et al. Large-scale genome sequencing of mycorrhizal fungi provides insights into the early evolution of symbiotic traits. Nat. Commun. 2020, 11, 5125. [Google Scholar] [CrossRef] [PubMed]
- Busk, P.K.; Lange, L. Classification of fungal and bacterial lytic polysaccharide monooxygenases. BMC Genom. 2015, 16, 368. [Google Scholar] [CrossRef]
- Monclaro, A.V.; Ferreira Filho, E.X. Fungal lytic polysaccharide monooxygenases from family AA9: Recent developments and application in lignocelullose breakdown. Int. J. Biol. Macromol. 2017, 102, 771–778. [Google Scholar] [CrossRef]
- Vandhana, T.M.; Reyre, J.L.; Sushmaa, D.; Berrin, J.G.; Bissaro, B.; Madhuprakash, J. On the expansion of biological functions of lytic polysaccharide monooxygenases. New Phytol. 2022, 233, 2380–2396. [Google Scholar] [CrossRef]
- Kommedal, E.G.; Sæther, F.; Hahn, T.; Eijsink, V.G. Natural photoredox catalysts promote light-driven lytic polysaccharide monooxygenase reactions and enzymatic turnover of biomass. Proc. Natl. Acad. Sci. USA 2022, 119, e2204510119. [Google Scholar] [CrossRef]
- Chorozian, K.; Karnaouri, A.; Karantonis, A.; Souli, M.; Topakas, E. Characterization of a dual cellulolytic/xylanolytic AA9 lytic polysaccharide monooxygenase from Thermothelomyces thermophilus and its utilization toward nanocellulose production in a multi-step bioprocess. ACS Sustain. Chem. Eng. 2022, 10, 8919–8929. [Google Scholar] [CrossRef]
- Zarattini, M.; Corso, M.; Kadowaki, M.A.; Monclaro, A.; Magri, S.; Milanese, I.; Jolivet, S.; de Godoy, M.O.; Hermans, C.; Fagard, M.; et al. LPMO-oxidized cellulose oligosaccharides evoke immunity in Arabidopsis conferring resistance towards necrotrophic fungus B. cinerea. Commun. Biol. 2021, 4, 727. [Google Scholar] [CrossRef]
- Chalak, A.; Villares, A.; Moreau, C.; Haon, M.; Grisel, S.; d’Orlando, A.; Herpoël-Gimbert, I.; Labourel, A.; Cathala, B.; Berrin, J.G. Influence of the carbohydrate-binding module on the activity of a fungal AA9 lytic polysaccharide monooxygenase on cellulosic substrates. Biotechnol. Biofuels 2019, 12, 206. [Google Scholar] [CrossRef]
- Monclaro, A.V.; Petrović, D.M.; Alves, G.S.; Costa, M.M.; Midorikawa, G.E.; Miller, R.N.; Ximenes Filho, E.; Eijsink, V.G.H.; Várnai, A. Characterization of two family AA9 LPMOs from Aspergillus tamarii with distinct activities on xyloglucan reveals structural differences linked to cleavage specificity. PLoS ONE 2020, 15, e0235642. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ma, F.; Zhao, H.; Zhang, S.; Wang, L.; Zhang, X.; Yu, H. A lytic polysaccharide monooxygenase from a white-rot fungus drives the degradation of lignin by a versatile peroxidase. Appl. Environ. Microbiol. 2019, 85, e02803–e02818. [Google Scholar] [CrossRef]
- Kojima, Y.; Várnai, A.; Ishida, T.; Sunagawa, N.; Petrovic, D.M.; Igarashi, K.; Jellison, J.; Goodell, B.; Alfredsen, G.; Westereng, B.; et al. A lytic polysaccharide monooxygenase with broad xyloglucan specificity from the brown-rot fungus Gloeophyllum trabeum and its action on cellulose-xyloglucan complexes. Appl. Environ. Microbiol. 2016, 82, 6557–6572. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.L.R.; dos Santos, L.V.; Pereira, G.A.G. AA9 and AA10: From enigmatic to essential enzymes. Appl. Microbiol. Biotechnol. 2016, 100, 9–16. [Google Scholar] [CrossRef]
- Turunen, R.; Tuveng, T.R.; Forsberg, Z.; Schiml, V.C.; Eijsink, V.G.; Arntzen, M.Ø. Functional characterization of two AA10 lytic polysaccharide monooxygenases from Cellulomonas gelida. Protein Sci. 2025, 34, e70060. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, Z.; Røhr, Å.K.; Mekasha, S.; Andersson, K.K.; Eijsink, V.G.; Vaaje-Kolstad, G.; Sørlie, M. Comparative study of two chitin-active and two cellulose-active AA10-type lytic polysaccharide monooxygenases. Biochemistry 2014, 53, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Bissaro, B.; Isaksen, I.; Vaaje-Kolstad, G.; Eijsink, V.G.; Røhr, Å.K. How a lytic polysaccharide monooxygenase binds crystalline chitin. Biochemistry 2018, 57, 1893–1906. [Google Scholar] [CrossRef]
- Pan, D.; Liu, J.; Xiao, P.; Xie, Y.; Zhou, X.; Zhang, Y. Research progress of lytic chitin monooxygenase and its utilization in chitin resource fermentation transformation. Fermentation 2023, 9, 754. [Google Scholar] [CrossRef]
- Hüttner, S.; Várnai, A.; Petrović, D.M.; Bach, C.X.; Kim Anh, D.T.; Thanh, V.N.; Eijsink, V.G.H.; Larsbrink, J.; Olsson, L. Specific xylan activity revealed for AA9 lytic polysaccharide monooxygenases of the thermophilic fungus Malbranchea cinnamomea by functional characterization. Appl. Environ. Microbiol. 2019, 85, e01408–e01419. [Google Scholar] [CrossRef]
- Sabbadin, F.; Hemsworth, G.R.; Ciano, L.; Henrissat, B.; Dupree, P.; Tryfona, T.; Marques, R.D.S.; Sweeney, R.S.; Besser, K.; Elias, L.; et al. An ancient family of lytic polysaccharide monooxygenases with roles in arthropod development and biomass digestion. Nat. Commun. 2018, 9, 756. [Google Scholar] [CrossRef]
- Cairo, J.P.L.F.; Cannella, D.; Oliveira, L.C.; Gonçalves, T.A.; Rubio, M.V.; Terrasan, C.R.; Tramontina, R.; Mofatto, L.S.; Garcia, W.; Felby, C.; et al. On the roles of AA15 lytic polysaccharide monooxygenases derived from the termite Coptotermes gestroi. J. Inorg. Biochem. 2021, 216, 111316. [Google Scholar] [CrossRef]
- Calusinska, M.; Marynowska, M.; Bertucci, M.; Untereiner, B.; Klimek, D.; Goux, X.; Sillam-Dussès, D.; Gawron, P.; Helder, R.; Wilmes, P.; et al. Integrative omics analysis of the termite gut system adaptation to Miscanthus diet identifies lignocellulose degradation enzymes. Commun. Biol. 2020, 3, 275. [Google Scholar] [CrossRef]
- Wu, G.; Miyauchi, S.; Morin, E.; Kuo, A.; Drula, E.; Varga, T.; Kohler, A.; Feng, B.; Cao, Y.; Lipzen, A.; et al. Evolutionary innovations through gain and loss of genes in the ectomycorrhizal Boletales. New Phytol. 2022, 233, 1383–1400. [Google Scholar] [CrossRef] [PubMed]
- Ayuso-Fernandez, I.; Molpeceres, G.; Camarero, S.; Ruiz-Dueñas, F.J.; Martínez, A.T. Ancestral sequence reconstruction as a tool to study the evolution of wood decaying fungi. Front. Fungal Biol. 2022, 3, 1003489. [Google Scholar] [CrossRef]
- Sharma, G.; Kaur, B.; Singh, V.; Raheja, Y.; Falco, M.D.; Tsang, A.; Chadha, B.S. Genome and secretome insights: Unravelling the lignocellulolytic potential of Myceliophthora verrucosa for enhanced hydrolysis of lignocellulosic biomass. Arch. Microbiol. 2024, 206, 236. [Google Scholar] [CrossRef] [PubMed]
- Contato, A.G.; Inácio, F.D.; Bueno, P.S.A.; Nolli, M.M.; Janeiro, V.; Peralta, R.M.; de Souza, C.G.M. Pleurotus pulmonarius: A protease-producing white rot fungus in lignocellulosic residues. Int. Microbiol. 2023, 26, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Zanellati, A.; Spina, F.; Bonaterra, M.; Dinuccio, E.; Varese, G.C.; Scarpeci, T.E. Screening and evaluation of phenols and furans degrading fungi for the biological pretreatment of lignocellulosic biomass. Int. Biodeterior. Biodegrad. 2021, 161, 105246. [Google Scholar] [CrossRef]
- Pasari, N.; Gupta, M.; Sinha, T.; Ogunmolu, F.E.; Yazdani, S.S. Systematic identification of CAZymes and transcription factors in the hypercellulolytic fungus Penicillium funiculosum NCIM1228 involved in lignocellulosic biomass degradation. Biotechnol. Biofuels Bioprod. 2023, 16, 150. [Google Scholar] [CrossRef]
- De Freitas, E.N.; Alnoch, R.C.; Contato, A.G.; Nogueira, K.M.V.; Crevelin, E.J.; Moraes, L.A.B.; Silva, R.N.; Martínez, C.A.; Polizeli, M.L.T.M. Enzymatic pretreatment with laccases from Lentinus sajor-caju induces structural modification in Lignin and enhances the digestibility of Tropical Forage Grass (Panicum maximum) grown under future climate conditions. Int. J. Mol. Sci. 2021, 22, 9445. [Google Scholar] [CrossRef]
- Contato, A.G.; Vici, A.C.; Pinheiro, V.E.; Oliveira, T.B.D.; Freitas, E.N.D.; Aranha, G.M.; Valvassora-Junior, A.L.A.; Vargas-Rechia, C.G.; Buckeridge, M.S.; Polizeli, M.L.T.M. Comparison of Trichoderma longibrachiatum xyloglucanase production using tamarind (Tamarindus indica) and jatoba (Hymenaea courbaril) seeds: Factorial design and immobilization on ionic supports. Fermentation 2022, 8, 510. [Google Scholar] [CrossRef]
- Helal, G.A.; Khalil, R.R.; Galal, Y.G.; Soliman, S.M.; Abd Elkader, R.S. Studies on cellulases of some cellulose-degrading soil fungi. Arch. Microbiol. 2022, 204, 65. [Google Scholar] [CrossRef]
- Moon, M.; Lee, J.P.; Park, G.W.; Lee, J.S.; Park, H.J.; Min, K. Lytic polysaccharide monooxygenase (LPMO)-derived saccharification of lignocellulosic biomass. Bioresour. Technol. 2022, 359, 127501. [Google Scholar] [CrossRef]
- Shabaev, A.V.; Savinova, O.S.; Moiseenko, K.V.; Glazunova, O.A.; Fedorova, T.V. Saprotrophic wood decay ability and plant cell wall degrading enzyme system of the white rot fungus Crucibulum laeve: Secretome, Metabolome and Genome Investigations. J. Fungi 2024, 11, 21. [Google Scholar] [CrossRef]
- Danneels, B.; Tanghe, M.; Desmet, T. Structural features on the substrate-binding surface of fungal lytic polysaccharide monooxygenases determine their oxidative regioselectivity. Biotechnol. J. 2019, 14, 1800211. [Google Scholar] [CrossRef]
- Kojima, Y.; Várnai, A.; Eijsink, V.G.; Yoshida, M. The role of lytic polysaccharide monooxygenases in wood rotting basidiomycetes. Trends Glycosci. Glycotechnol. 2020, 32, E135–E143. [Google Scholar] [CrossRef]
- Kommedal, E.G.; Angeltveit, C.F.; Klau, L.J.; Ayuso-Fernández, I.; Arstad, B.; Antonsen, S.G.; Stenstrøm, Y.; Ekeberg, D.; Gírio, F.; Carvalheiro, F.; et al. Visible light-exposed lignin facilitates cellulose solubilization by lytic polysaccharide monooxygenases. Nat. Commun. 2023, 14, 1063. [Google Scholar] [CrossRef]
- Ma, L.; Li, G.; Xu, H.; Liu, Z.; Wan, Q.; Liu, D.; Shen, Q. Structural and functional study of a novel lytic polysaccharide monooxygenase cPMO2 from compost sample in the oxidative degradation of cellulose. Chem. Eng. J. 2022, 433, 134509. [Google Scholar] [CrossRef]
- Labourel, A.; Frandsen, K.E.; Zhang, F.; Brouilly, N.; Grisel, S.; Haon, M.; Ciano, L.; Ropartz, D.; Fanuel, M.; Martin, F.; et al. A fungal family of lytic polysaccharide monooxygenase-like copper proteins. Nat. Chem. Biol. 2020, 16, 345–350. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Davies, G.J.; Walton, P.H.; Rovira, C. Activation of O2 and H2O2 by lytic polysaccharide monooxygenases. ACS Catal. 2020, 10, 12760–12769. [Google Scholar] [CrossRef]
- Liu, B.; Olson, Å.; Wu, M.; Broberg, A.; Sandgren, M. Biochemical studies of two lytic polysaccharide monooxygenases from the white-rot fungus Heterobasidion irregulare and their roles in lignocellulose degradation. PLoS ONE 2017, 12, e0189479. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.S. Lytic polysaccharide monooxygenases: The microbial power tool for lignocellulose degradation. Trends Plant Sci. 2016, 21, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Jagadeeswaran, G.; Veale, L.; Mort, A.J. Do lytic polysaccharide monooxygenases aid in plant pathogenesis and herbivory? Trends Plant Sci. 2021, 26, 142–155. [Google Scholar] [CrossRef]
- Contato, A.G.; Nogueira, K.M.V.; Buckeridge, M.S.; Silva, R.N.; Polizeli, M.L.T.M. Trichoderma longibrachiatum and Thermothelomyces thermophilus co-culture: Improvement the saccharification profile of different sugarcane bagasse varieties. Biotechnol. Lett. 2023, 45, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.; Borelli, C.; Korting, H.C.; Hube, B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses 2005, 48, 365–377. [Google Scholar] [CrossRef]
- Bissaro, B.; Várnai, A.; Røhr, Å.K.; Eijsink, V.G. Oxidoreductases and reactive oxygen species in conversion of lignocellulosic biomass. Microbiol. Mol. Biol. Rev. 2018, 82, 10–1128. [Google Scholar] [CrossRef]
- Blanco-Ulate, B.; Morales-Cruz, A.; Amrine, K.C.; Labavitch, J.M.; Powell, A.L.; Cantu, D. Genome-wide transcriptional profiling of Botrytis cinerea genes targeting plant cell walls during infections of different hosts. Front. Plant Sci. 2014, 5, 435. [Google Scholar] [CrossRef]
- Nekiunaite, L.; Petrović, D.M.; Westereng, B.; Vaaje-Kolstad, G.; Hachem, M.A.; Várnai, A.; Eijsink, V.G. Fg LPMO 9A from Fusarium graminearum cleaves xyloglucan independently of the backbone substitution pattern. FEBS Lett. 2016, 590, 3346–3356. [Google Scholar] [CrossRef]
- Nekiunaite, L.; Isaksen, T.; Vaaje-Kolstad, G.; Abou Hachem, M. Fungal lytic polysaccharide monooxygenases bind starch and β-cyclodextrin similarly to amylolytic hydrolases. FEBS Lett. 2016, 590, 2737–2747. [Google Scholar] [CrossRef]
- Polonio, Á.; Fernández-Ortuño, D.; de Vicente, A.; Pérez-García, A. A haustorial-expressed lytic polysaccharide monooxygenase from the cucurbit powdery mildew pathogen Podosphaera xanthii contributes to the suppression of chitin-triggered immunity. Mol. Plant Pathol. 2021, 22, 580–601. [Google Scholar] [CrossRef]
- Molina, A.; Sánchez-Vallet, A.; Jordá, L.; Carrasco-López, C.; Rodríguez-Herva, J.J.; López-Solanilla, E. Plant cell walls: Source of carbohydrate-based signals in plant-pathogen interactions. Curr. Opin. Plant Biol. 2024, 82, 102630. [Google Scholar] [CrossRef]
- Mitsuhashi, W.; Shimura, S.; Miyamoto, K.; Sugimoto, T.N. Spatial distribution of orally administered viral fusolin protein in the insect midgut and possible synergism between fusolin and digestive proteases to disrupt the midgut peritrophic matrix. Arch. Virol. 2019, 164, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Berrin, J.G.; Rosso, M.N.; Hachem, M.A. Fungal secretomics to probe the biological functions of lytic polysaccharide monooxygenases. Carbohydr. Res. 2017, 448, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Levy-Booth, D.J.; Giesbrecht, I.J.; Kellogg, C.T.; Heger, T.J.; D’Amore, D.V.; Keeling, P.J.; Hallam, S.J.; Mohn, W.W. Seasonal and ecohydrological regulation of active microbial populations involved in DOC, CO2, and CH4 fluxes in temperate rainforest soil. ISME J. 2019, 13, 950–963. [Google Scholar] [CrossRef] [PubMed]
- Sabbadin, F.; Henrissat, B.; Bruce, N.C.; McQueen-Mason, S.J. Lytic polysaccharide monooxygenases as chitin-specific virulence factors in crayfish plague. Biomolecules 2021, 11, 1180. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Xia, Y.; Zhang, H.; Cui, J.L. The microscopic mechanism between endophytic fungi and host plants: From recognition to building stable mutually beneficial relationships. Microbiol. Res. 2022, 261, 127056. [Google Scholar] [CrossRef]
- Lu, H.; Wei, T.; Lou, H.; Shu, X.; Chen, Q. A critical review on communication mechanism within plant-endophytic fungi interactions to cope with biotic and abiotic stresses. J. Fungi 2021, 7, 719. [Google Scholar] [CrossRef] [PubMed]
- Redkar, A.; Sabale, M.; Zuccaro, A.; Di Pietro, A. Determinants of endophytic and pathogenic lifestyle in root colonizing fungi. Curr. Opin. Plant Biol. 2022, 67, 102226. [Google Scholar] [CrossRef]
- Marcianò, D.; Kappel, L.; Ullah, S.F.; Srivastava, V. From glycans to green biotechnology: Exploring cell wall dynamics and phytobiota impact in plant glycopathology. Crit. Rev. Biotechnol. 2025, 45, 314–332. [Google Scholar] [CrossRef]
- Osono, T. Ecology of ligninolytic fungi associated with leaf litter decomposition. Ecol. Res. 2007, 22, 955–974. [Google Scholar] [CrossRef]
- Martin, F.; Kohler, A.; Murat, C.; Veneault-Fourrey, C.; Hibbett, D.S. Unearthing the roots of ectomycorrhizal symbioses. Nat. Rev. Microbiol. 2016, 14, 760–773. [Google Scholar] [CrossRef]
- Maillard, F.; Kohler, A.; Morin, E.; Hossann, C.; Miyauchi, S.; Ziegler-Devin, I.; Gérant, D.; Angeli, N.; Lipzen, A.; Keymanesh, K.; et al. Functional genomics gives new insights into the ectomycorrhizal degradation of chitin. New Phytol. 2023, 238, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Czjzek, M.; Ficko-Blean, E.; Berrin, J.G. A special issue of Essays in Biochemistry on current advances about CAZymes and their impact and key role in human health and environment. Essays Biochem. 2023, 67, 325–329. [Google Scholar] [CrossRef]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef]
- Gong, Y.; Lebreton, A.; Zhang, F.; Martin, F. Role of carbohydrate-active enzymes in mycorrhizal symbioses. Essays Biochem. 2023, 67, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Bissaro, B.; Kommedal, E.; Røhr, Å.K.; Eijsink, V.G. Controlled depolymerization of cellulose by light-driven lytic polysaccharide oxygenases. Nat. Commun. 2020, 11, 890. [Google Scholar] [CrossRef]
- Wang, B.; Walton, P.H.; Rovira, C. Molecular mechanisms of oxygen activation and hydrogen peroxide formation in lytic polysaccharide monooxygenases. ACS Catal. 2019, 9, 4958–4969. [Google Scholar] [CrossRef]
- Bissaro, B.; Eijsink, V.G. Lytic polysaccharide monooxygenases: Enzymes for controlled and site-specific Fenton-like chemistry. Essays Biochem. 2023, 67, 575–584. [Google Scholar]
- Gudmundsson, M.; Kim, S.; Wu, M.; Ishida, T.; Momeni, M.H.; Vaaje-Kolstad, G.; Lundberg, D.; Royant, A.; Ståhlberg, J.; Eijsink, V.G.H.; et al. Structural and electronic snapshots during the transition from a Cu (II) to Cu (I) metal center of a lytic polysaccharide monooxygenase by X-ray photoreduction. J. Biol. Chem. 2014, 289, 18782–18792. [Google Scholar] [CrossRef]
- Zhao, J.; Zhuo, Y.; Diaz, D.E.; Shanmugam, M.; Telfer, A.J.; Lindley, P.J.; Kracher, D.; Hayashi, T.; Seibt, L.S.; Hardy, F.J.; et al. Mapping the initial stages of a protective pathway that enhances catalytic turnover by a lytic polysaccharide monooxygenase. J. Am. Chem. Soc. 2023, 145, 20672–20682. [Google Scholar] [CrossRef]
- Bissaro, B.; Røhr, Å.K.; Müller, G.; Chylenski, P.; Skaugen, M.; Forsberg, Z.; Horn, S.J.; Vaaje-Kolstad, G.; Eijsink, V.G. Oxidative cleavage of polysaccharides by monocopper enzymes depends on H2O2. Nat. Chem. Biol. 2017, 13, 1123–1128. [Google Scholar] [CrossRef]
- Kuusk, S.; Kont, R.; Kuusk, P.; Heering, A.; Sørlie, M.; Bissaro, B.; Eijsink, V.G.H.; Väljamäe, P. Kinetic insights into the role of the reductant in H2O2-driven degradation of chitin by a bacterial lytic polysaccharide monooxygenase. J. Biol. Chem. 2019, 294, 1516–1528. [Google Scholar] [CrossRef]
- Schwaiger, L.; Csarman, F.; Chang, H.; Golten, O.; Eijsink, V.G.; Ludwig, R. Electrochemical monitoring of heterogeneous peroxygenase reactions unravels LPMO kinetics. ACS Catal. 2024, 14, 1205–1219. [Google Scholar] [CrossRef] [PubMed]
- Kadić, A.; Várnai, A.; Eijsink, V.G.; Horn, S.J.; Lidén, G. In situ measurements of oxidation–reduction potential and hydrogen peroxide concentration as tools for revealing LPMO inactivation during enzymatic saccharification of cellulose. Biotechnol. Biofuels 2021, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ma, X.; Waheed, A.; Adnan, M.; Xie, N.; Liu, G. Characterization of a thermostable cellobiose dehydrogenase from Myceliophthora thermophila and its function of electron-donating for lytic polysaccharide monooxygenase. Biofuels Bioprod. Biorefining 2024, 18, 171–183. [Google Scholar] [CrossRef]
- Laurent, C.V.; Breslmayr, E.; Tunega, D.; Ludwig, R.; Oostenbrink, C. Interaction between cellobiose dehydrogenase and lytic polysaccharide monooxygenase. Biochemistry 2019, 58, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Várnai, A.; Umezawa, K.; Yoshida, M.; Eijsink, V.G. The pyrroloquinoline-quinone-dependent pyranose dehydrogenase from Coprinopsis cinerea drives lytic polysaccharide monooxygenase action. Appl. Environ. Microbiol. 2018, 84, e00156-18. [Google Scholar] [CrossRef]
- Ji, T.; Liaqat, F.; Khazi, M.I.; Liaqat, N.; Nawaz, M.Z.; Zhu, D. Lignin biotransformation: Advances in enzymatic valorization and bioproduction strategies. Ind. Crops Prod. 2024, 216, 118759. [Google Scholar] [CrossRef]
- Singh, A.K.; Bilal, M.; Iqbal, H.M.; Meyer, A.S.; Raj, A. Bioremediation of lignin derivatives and phenolics in wastewater with lignin modifying enzymes: Status, opportunities and challenges. Sci. Total Environ. 2021, 777, 145988. [Google Scholar] [CrossRef]
- Mattila, H.; Österman-Udd, J.; Mali, T.; Lundell, T. Basidiomycota fungi and ROS: Genomic perspective on key enzymes involved in generation and mitigation of reactive oxygen species. Front. Fungal Biol. 2022, 3, 837605. [Google Scholar] [CrossRef]
- Raheja, Y.; Gaur, P.; Islam, T.; Chaurasia, A.K.; Gaur, V.K.; Chadha, B.S. Advancement in lignocellulolytic enzyme production: Tailored strategies to overcome challenges in biomass hydrolysis. Syst. Microbiol. Biomanufacturing 2025, 5, 948–968. [Google Scholar] [CrossRef]
- Long, L.; Wang, H.; Wang, C.; Feng, S.; Yang, H.; Meng, X.; Huang, C.; Ragauskas, A.J. Glyceryl lignin enhances lytic polysaccharide monooxygenase (LPMO) oxidative activity via electron transfer and H2O2 generation: Mechanistic insights from glycerol pretreatment. Chem. Eng. J. 2025, 516, 163851. [Google Scholar] [CrossRef]
- Stepnov, A.A.; Forsberg, Z.; Sørlie, M.; Nguyen, G.S.; Wentzel, A.; Røhr, Å.K.; Eijsink, V.G. Unraveling the roles of the reductant and free copper ions in LPMO kinetics. Biotechnol. Biofuels 2021, 14, 28. [Google Scholar] [CrossRef]
- Angeltveit, C.F.; Kommedal, E.G.; Stepnov, A.A.; Eijsink, V.G.; Horn, S.J. Light exposure of lignin affects the saccharification efficiency of lpmo-containing cellulolytic enzyme cocktails. ACS Sustain. Chem. Eng. 2024, 12, 9777–9786. [Google Scholar] [CrossRef]
- Magri, S.; Cannella, D. Producing Value-Added Products from Organic Bioresources via Photo-BioCatalytic Processes. In Production of Biofuels and Chemicals from Sustainable Recycling of Organic Solid Waste; Springer Nature: Singapore, 2022; pp. 245–282. [Google Scholar]
- Chen, K.; Zhang, X.; Long, L.; Ding, S. Comparison of C4-oxidizing and C1/C4-oxidizing AA9 LPMOs in substrate adsorption, H2O2-driven activity and synergy with cellulase on celluloses of different crystallinity. Carbohydr. Polym. 2021, 269, 118305. [Google Scholar] [CrossRef]
- Tandrup, T.; Tryfona, T.; Frandsen, K.E.H.; Johansen, K.S.; Dupree, P.; Lo Leggio, L. Oligosaccharide binding and thermostability of two related AA9 lytic polysaccharide monooxygenases. Biochemistry 2020, 59, 3347–3358. [Google Scholar] [CrossRef]
- Zhou, X.; Qi, X.; Huang, H.; Zhu, H. Sequence and structural analysis of AA9 and AA10 LPMOs: An insight into the basis of substrate specificity and regioselectivity. Int. J. Mol. Sci. 2019, 20, 4594. [Google Scholar] [CrossRef]
- Sabbadin, F.; Pesante, G.; Elias, L.; Besser, K.; Li, Y.; Steele-King, C.; Stark, M.; Rathbone, D.A.; Dowle, A.A.; Bates, R.; et al. Uncovering the molecular mechanisms of lignocellulose digestion in shipworms. Biotechnol. Biofuels 2018, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, J.; Liu, X.; Pan, X.; Hou, J.; Ran, C.; Zhou, Z. Improving extracellular production of Serratia marcescens lytic polysaccharide monooxygenase CBP21 and Aeromonas veronii B565 chitinase Chi92 in Escherichia coli and their synergism. AMB Express 2017, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Muderspach, S.J.; Tandrup, T.; Frandsen, K.E.; Santoni, G.; Poulsen, J.C.N.; Leggio, L.L. Further structural studies of the lytic polysaccharide monooxygenase Ao AA13 belonging to the starch-active AA13 family. Amylase 2019, 3, 41–54. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, J.; Li, Z.; Haider, J.; Zhou, Y.; Ji, Y.; Schwaneberg, U.; Zhu, L. Influences of the carbohydrate-binding module on a fungal starch-active lytic polysaccharide monooxygenase. J. Agric. Food Chem. 2023, 71, 18405–18413. [Google Scholar] [CrossRef]
- Frommhagen, M.; Koetsier, M.J.; Westphal, A.H.; Visser, J.; Hinz, S.W.; Vincken, J.P.; van Berkel, W.J.L.; Kabel, M.A.; Gruppen, H. Lytic polysaccharide monooxygenases from Myceliophthora thermophila C1 differ in substrate preference and reducing agent specificity. Biotechnol. Biofuels 2016, 9, 186. [Google Scholar] [CrossRef]
- Liu, B.; Krishnaswamyreddy, S.; Muraleedharan, M.N.; Olson, Å.; Broberg, A.; Ståhlberg, J.; Sandgren, M. Side-by-side biochemical comparison of two lytic polysaccharide monooxygenases from the white-rot fungus Heterobasidion irregulare on their activity against crystalline cellulose and glucomannan. PLoS ONE 2018, 13, e0203430. [Google Scholar] [CrossRef]
- Beeson, W.T.; Vu, V.V.; Span, E.A.; Phillips, C.M.; Marletta, M.A. Cellulose degradation by polysaccharide monooxygenases. Annu. Rev. Biochem. 2015, 84, 923–946. [Google Scholar] [CrossRef]
- Laurent, C.V.; Sun, P.; Scheiblbrandner, S.; Csarman, F.; Cannazza, P.; Frommhagen, M.; van Berkel, W.J.H.; Oostenbrink, C.; Kabel, M.A.; Ludwig, R. Influence of lytic polysaccharide monooxygenase active site segments on activity and affinity. Int. J. Mol. Sci. 2019, 20, 6219. [Google Scholar] [CrossRef]
- Forsberg, Z.; Courtade, G. On the impact of carbohydrate-binding modules (CBMs) in lytic polysaccharide monooxygenases (LPMOs). Essays Biochem. 2023, 67, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Várnai, A.; Siika-Aho, M.; Viikari, L. Carbohydrate-binding modules (CBMs) revisited: Reduced amount of water counterbalances the need for CBMs. Biotechnol. Biofuels 2013, 6, 30. [Google Scholar] [CrossRef]
- Christiansen, C.; Hachem, M.A.; Janeček, Š.; Viksø-Nielsen, A.; Blennow, A.; Svensson, B. The carbohydrate-binding module family 20–diversity, structure, and function. FEBS J. 2009, 276, 5006–5029. [Google Scholar] [CrossRef]
- Zhao, X.; Xie, F.; Chen, K.; Long, L.; Ding, S. The Effect of CBM1 and linker on the oxidase, peroxidase and monooxygenase activities of AA9 LPMOs: Insight into their correlation with the nature of reductants and crystallinity of celluloses. Int. J. Mol. Sci. 2024, 25, 12616. [Google Scholar] [CrossRef]
- Méndez-Líter, J.A.; Ayuso-Fernández, I.; Csarman, F.; de Eugenio, L.I.; Míguez, N.; Plou, F.J.; Prieto, A.; Ludwig, R.; Martínez, M.J. Lytic polysaccharide monooxygenase from Talaromyces amestolkiae with an enigmatic linker-like region: The role of this enzyme on cellulose saccharification. Int. J. Mol. Sci. 2021, 22, 13611. [Google Scholar] [CrossRef]
- Meier, K.K.; Jones, S.M.; Kaper, T.; Hansson, H.; Koetsier, M.J.; Karkehabadi, S.; Solomon, E.I.; Sandgren, M.; Kelemen, B. Oxygen activation by Cu LPMOs in recalcitrant carbohydrate polysaccharide conversion to monomer sugars. Chem. Rev. 2017, 118, 2593–2635. [Google Scholar] [CrossRef] [PubMed]
- Ayuso-Fernández, I.; Emrich-Mills, T.Z.; Haak, J.; Golten, O.; Hall, K.R.; Schwaiger, L.; Moe, T.S.; Stepnov, A.A.; Ludwig, R.; Cutsai III, G.E.; et al. Mutational dissection of a hole hopping route in a lytic polysaccharide monooxygenase (LPMO). Nat. Commun. 2024, 15, 3975. [Google Scholar] [CrossRef] [PubMed]
- Dan, M.; Zheng, Y.; Zhao, G.; Hsieh, Y.S.; Wang, D. Current insights of factors interfering the stability of lytic polysaccharide monooxygenases. Biotechnol. Adv. 2023, 67, 108216. [Google Scholar] [CrossRef]
- Loose, J.S.; Arntzen, M.Ø.; Bissaro, B.; Ludwig, R.; Eijsink, V.G.; Vaaje-Kolstad, G. Multipoint precision binding of substrate protects lytic polysaccharide monooxygenases from self-destructive off-pathway processes. Biochemistry 2018, 57, 4114–4124. [Google Scholar] [CrossRef]
- Karnaouri, A.; Chorozian, K.; Zouraris, D.; Karantonis, A.; Topakas, E.; Rova, U.; Christakopoulos, P. Lytic polysaccharide monooxygenases as powerful tools in enzymatically assisted preparation of nano-scaled cellulose from lignocellulose: A review. Bioresour. Technol. 2022, 345, 126491. [Google Scholar] [CrossRef]
- Støpamo, F.G.; Sulaeva, I.; Budischowsky, D.; Rahikainen, J.; Marjamaa, K.; Potthast, A.; Kruus, K.; Eijsink, V.G.H.; Várnai, A. Oxidation of cellulose fibers using LPMOs with varying allomorphic substrate preferences, oxidative regioselectivities, and domain structures. Carbohydr. Polym. 2024, 330, 121816. [Google Scholar] [CrossRef]
- Muraleedharan, M.N.; Zouraris, D.; Karantonis, A.; Topakas, E.; Sandgren, M.; Rova, U.; Christakopoulos, P.; Karnaouri, A. Effect of lignin fractions isolated from different biomass sources on cellulose oxidation by fungal lytic polysaccharide monooxygenases. Biotechnol. Biofuels 2018, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, R.A.; Tuveng, T.R.; Stenstrøm, Y.H.; Eijsink, V.G.; Sørlie, M. The characterization of the glucono-δ-lactone-carboxylic acid equilibrium in the products of chitin-active lytic polysaccharide monooxygenases. J. Chem. Thermodyn. 2017, 106, 10–15. [Google Scholar] [CrossRef]
- Westereng, B.; Arntzen, M.Ø.; Aachmann, F.L.; Várnai, A.; Eijsink, V.G.; Agger, J.W. Simultaneous analysis of C1 and C4 oxidized oligosaccharides, the products of lytic polysaccharide monooxygenases acting on cellulose. J. Chromatogr. A 2016, 1445, 46–54. [Google Scholar] [CrossRef]
- Tokin, R.; Ipsen, J.Ø.; Westh, P.; Johansen, K.S. The synergy between LPMOs and cellulases in enzymatic saccharification of cellulose is both enzyme-and substrate-dependent. Biotechnol. Lett. 2020, 42, 1975–1984. [Google Scholar] [CrossRef]
- Sun, P.; Laurent, C.V.; Boerkamp, V.J.; van Erven, G.; Ludwig, R.; van Berkel, W.J.; Kabel, M.A. Regioselective C4 and C6 double oxidation of cellulose by lytic polysaccharide monooxygenases. ChemSusChem 2022, 15, e202102203. [Google Scholar] [CrossRef] [PubMed]
- Muthuramalingam, S.; Maheshwaran, D.; Velusamy, M.; Mayilmurugan, R. Regioselective oxidative carbon-oxygen bond cleavage catalysed by copper (II) complexes: A relevant model study for lytic polysaccharides monooxygenases activity. J. Catal. 2019, 372, 352–361. [Google Scholar] [CrossRef]
- Frommhagen, M.; Westphal, A.H.; van Berkel, W.J.; Kabel, M.A. Distinct substrate specificities and electron-donating systems of fungal lytic polysaccharide monooxygenases. Front. Microbiol. 2018, 9, 1080. [Google Scholar] [CrossRef]
- Calderaro, F.; Bevers, L.E.; van den Berg, M.A. Oxidative power: Tools for assessing LPMO activity on cellulose. Biomolecules 2021, 11, 1098. [Google Scholar] [CrossRef]
- Agger, J.W.; Isaksen, T.; Várnai, A.; Vidal-Melgosa, S.; Willats, W.G.; Ludwig, R.; Horn, S.V.; Eijsink, V.G.H.; Westereng, B. Discovery of LPMO activity on hemicelluloses shows the importance of oxidative processes in plant cell wall degradation. Proc. Natl. Acad. Sci. USA 2014, 111, 6287–6292. [Google Scholar] [CrossRef] [PubMed]
- Mafa, M.S.; Pletschke, B.I.; Malgas, S. Defining the frontiers of synergism between cellulolytic enzymes for improved hydrolysis of lignocellulosic feedstocks. Catalysts 2021, 11, 1343. [Google Scholar] [CrossRef]
- Khamassi, A.; Dumon, C. Enzyme synergy for plant cell wall polysaccharide degradation. Essays Biochem. 2023, 67, 521–531. [Google Scholar] [CrossRef]
- Hu, J.; Tian, D.; Renneckar, S.; Saddler, J.N. Enzyme mediated nanofibrillation of cellulose by the synergistic actions of an endoglucanase, lytic polysaccharide monooxygenase (LPMO) and xylanase. Sci. Rep. 2018, 8, 3195. [Google Scholar] [CrossRef]
- Sharma, R.; Putera, K.; Banaszak Holl, M.M.; Garnier, G.; Haritos, V.S. Laccase and LPMO-driven biocatalysis produces surface carboxylic acids on lignocellulose and promotes nanofiber production. ACS Sustain. Chem. Eng. 2023, 11, 12541–12551. [Google Scholar] [CrossRef]
- Chorozian, K.; Karnaouri, A.; Georgaki-Kondyli, N.; Karantonis, A.; Topakas, E. Assessing the role of redox partners in Tth LPMO9G and its mutants: Focus on H2O2 production and interaction with cellulose. Biotechnol. Biofuels Bioprod. 2024, 17, 19. [Google Scholar] [CrossRef]
- Stepnov, A.A.; Eijsink, V.G.; Forsberg, Z. Enhanced in situ H2O2 production explains synergy between an LPMO with a cellulose-binding domain and a single-domain LPMO. Sci. Rep. 2022, 12, 6129. [Google Scholar] [CrossRef]
- Sun, S.; Li, F.; Li, M.; Zhang, W.; Jiang, Z.; Zhao, H.; Pu, Y.; Ragauskas, A.J.; Dai, S.Y.; Zhang, X.; et al. Lytic polysaccharide monooxygenase synergized with lignin-degrading enzymes for efficient lignin degradation. IScience 2023, 26, 870. [Google Scholar] [CrossRef] [PubMed]
- Reyre, J.L.; Grisel, S.; Lechar, A.; Margeot, A.; Arragain, S.; Berrin, J.G.; Bissaro, B. Implementation of CDH in LPMO-containing minimal cellulase cocktails to tune bioproduct production from lignocellulosic biomass. ACS Sustain. Chem. Eng. 2024, 12, 8128–8138. [Google Scholar] [CrossRef]
- Müller, G.; Várnai, A.; Johansen, K.S.; Eijsink, V.G.; Horn, S.J. Harnessing the potential of LPMO-containing cellulase cocktails poses new demands on processing conditions. Biotechnol. Biofuels 2015, 8, 187. [Google Scholar] [CrossRef]
- Courtade, G.; Le, S.B.; Sætrom, G.I.; Brautaset, T.; Aachmann, F.L. A novel expression system for lytic polysaccharide monooxygenases. Carbohydr. Res. 2017, 448, 212–219. [Google Scholar] [CrossRef]
- Raheja, Y.; Singh, V.; Kumar, N.; Agrawal, D.; Sharma, G.; Di Falco, M.; Tsang, A.; Chadha, B.S. Transcriptional and secretome analysis of Rasamsonia emersonii lytic polysaccharide mono-oxygenases. Appl. Microbiol. Biotechnol. 2024, 108, 444. [Google Scholar] [CrossRef]
- Möllers, K.B.; Mikkelsen, H.; Simonsen, T.I.; Cannella, D.; Johansen, K.S.; Bjerrum, M.J.; Felby, C. On the formation and role of reactive oxygen species in light-driven LPMO oxidation of phosphoric acid swollen cellulose. Carbohydr. Res. 2017, 448, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Xin, D.; Xing, M.; Ran, G.; Blossom, B.M. The influence of photosynthetic pigment chlorophyllin in light-driven LPMO system on the hydrolytic action of cellulases. Int. J. Biol. Macromol. 2024, 281, 136714. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, N.; Pearce, R.; Yi, S.; Zhao, X. Comparative secretomics analysis reveals the major components of Penicillium oxalicum 16 and Trichoderma reesei RUT-C30. Microorganisms 2021, 9, 2042. [Google Scholar] [CrossRef] [PubMed]
- Arntzen, M.Ø.; Bengtsson, O.; Várnai, A.; Delogu, F.; Mathiesen, G.; Eijsink, V.G. Quantitative comparison of the biomass-degrading enzyme repertoires of five filamentous fungi. Sci. Rep. 2020, 10, 20267. [Google Scholar] [CrossRef]
- Samal, A.; Craig, J.P.; Coradetti, S.T.; Benz, J.P.; Eddy, J.A.; Price, N.D.; Glass, N.L. Network reconstruction and systems analysis of plant cell wall deconstruction by Neurospora crassa. Biotechnol. Biofuels 2017, 10, 225. [Google Scholar] [CrossRef]
- Dixit, P.; Basu, B.; Puri, M.; Tuli, D.K.; Mathur, A.S.; Barrow, C.J. A screening approach for assessing lytic polysaccharide monooxygenase activity in fungal strains. Biotechnol. Biofuels 2019, 12, 185. [Google Scholar] [CrossRef]
- Singh, G.; Sahu, S.; Bharti, S.; Arya, S.K. Significance of enzymes for the recycling of wasted non-food biomass to value added products: A sustainable stewardship towards the cleaner environment. Process Saf. Environ. Prot. 2024, 190, 395–412. [Google Scholar] [CrossRef]
- Subramanian, K.; Sarkar, M.K.; Wang, H.; Qin, Z.H.; Chopra, S.S.; Jin, M.; Kumar, V.; Chen, C.; Tsang, C.W.; Lin, C.S.K. An overview of cotton and polyester, and their blended waste textile valorisation to value-added products: A circular economy approach–research trends, opportunities and challenges. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3921–3942. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, X.; Gu, R.; Zhao, B.; Zhao, X.; Song, H.; Zheng, H.; Xu, J.; Bai, W. A novel starch-active lytic polysaccharide monooxygenase discovered with bioinformatics screening and its application in textile desizing. BMC Biotechnol. 2024, 24, 2. [Google Scholar] [CrossRef]
- Müller, G.; Kalyani, D.C.; Horn, S.J. LPMOs in cellulase mixtures affect fermentation strategies for lactic acid production from lignocellulosic biomass. Biotechnol. Bioeng. 2017, 114, 552–559. [Google Scholar] [CrossRef]
- Othman, A.M.; Mechichi, T.; Chowdhary, P.; Suleiman, W.B. Ligninolytic enzymes and their potential applications. Front. Microbiol. 2023, 14, 1235206. [Google Scholar] [CrossRef] [PubMed]
- Arfi, Y.; Shamshoum, M.; Rogachev, I.; Peleg, Y.; Bayer, E.A. Integration of bacterial lytic polysaccharide monooxygenases into designer cellulosomes promotes enhanced cellulose degradation. Proc. Natl. Acad. Sci. USA 2014, 111, 9109–9114. [Google Scholar] [CrossRef]
- Amicucci, M.J.; Nandita, E.; Lebrilla, C.B. Function without structures: The need for in-depth analysis of dietary carbohydrates. J. Agric. Food Chem. 2019, 67, 4418–4424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R. Functional characterization of cellulose-degrading AA9 lytic polysaccharide monooxygenases and their potential exploitation. Appl. Microbiol. Biotechnol. 2020, 104, 3229–3243. [Google Scholar] [CrossRef] [PubMed]
- Berhe, M.H.; Song, X.; Yao, L. Improving the enzymatic activity and stability of a lytic polysaccharide monooxygenase. Int. J. Mol. Sci. 2023, 24, 8963. [Google Scholar] [CrossRef]
- Rieder, L.; Ebner, K.; Glieder, A.; Sørlie, M. Novel molecular biological tools for the efficient expression of fungal lytic polysaccharide monooxygenases in Pichia pastoris. Biotechnol. Biofuels 2021, 14, 122. [Google Scholar] [CrossRef]
- Afreen, S.; Mishra, S. Production of high-value oxidative enzymes by Cyathus bulleri on agricultural and agri-food wastes for application in the textile sector. World J. Microbiol. Biotechnol. 2023, 39, 329. [Google Scholar] [CrossRef]
- Brück, S.A.; Contato, A.G.; Gamboa-Trujillo, P.; de Oliveira, T.B.; Cereia, M.; de Moraes Polizeli, M.L.T.M. Prospection of psychrotrophic filamentous fungi isolated from the High Andean Paramo Region of Northern Ecuador: Enzymatic activity and molecular identification. Microorganisms 2022, 10, 282. [Google Scholar] [CrossRef]
- Hasegawa, N.; Sugiyama, M.; Igarashi, K. Acetylxylan esterase is the key to the host specialization of wood-decay fungi predicted by random forest machine-learning algorithm. J. Wood Sci. 2024, 70, 44. [Google Scholar] [CrossRef]
- Karnaouri, A.; Muraleedharan, M.N.; Dimarogona, M.; Topakas, E.; Rova, U.; Sandgren, M.; Christakopoulos, P. Recombinant expression of thermostable processive Mt EG5 endoglucanase and its synergism with Mt LPMO from Myceliophthora thermophila during the hydrolysis of lignocellulosic substrates. Biotechnol. Biofuels 2017, 10, 126. [Google Scholar] [CrossRef]
- Hernández-Rollán, C.; Falkenberg, K.B.; Rennig, M.; Bertelsen, A.B.; Ipsen, J.Ø.; Brander, S.; Daley, D.O.; Johansen, K.S.; Nørholm, M.H. LyGo: A platform for rapid screening of lytic polysaccharide monooxygenase production. ACS Synth. Biol. 2021, 10, 897–906. [Google Scholar] [CrossRef]
- Du, L.; Ma, L.; Ma, Q.; Guo, G.; Han, X.; Xiao, D. Hydrolytic boosting of lignocellulosic biomass by a fungal lytic polysaccharide monooxygenase, AnLPMO15g from Aspergillus niger. Ind. Crops Prod. 2018, 126, 309–315. [Google Scholar] [CrossRef]
- Liang, Y.; Si, T.; Ang, E.L.; Zhao, H. Engineered pentafunctional minicellulosome for simultaneous saccharification and ethanol fermentation in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2014, 80, 6677–6684. [Google Scholar] [CrossRef]
- Forsberg, Z.; Stepnov, A.A.; Nærdal, G.K.; Klinkenberg, G.; Eijsink, V.G. Engineering lytic polysaccharide monooxygenases (LPMOs). In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2020; Volume 644, pp. 1–34. [Google Scholar]
- Zhou, X.; Xu, Z.; Li, Y.; He, J.; Zhu, H. Improvement of the stability and activity of an LPMO through rational disulfide bonds design. Front. Bioeng. Biotechnol. 2022, 9, 815990. [Google Scholar] [CrossRef]
- Wahart, A.J.; Staniland, J.; Miller, G.J.; Cosgrove, S.C. Oxidase enzymes as sustainable oxidation catalysts. R. Soc. Open Sci. 2022, 9, 211572. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Purohit, A.; Yadav, S.K. Kinetic characterization of a lytic polysaccharide monooxygenase reveals a unique specificity for depolymerization at β-O-4 of lignin compounds. ACS Sustain. Chem. Eng. 2023, 11, 4398–4408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).