Hydrochemical Characteristics and Genesis Analysis of Closed Coal Mining Areas in Southwestern Shandong Province, China
Abstract
1. Introduction
2. Geologic Background
3. Materials and Methods
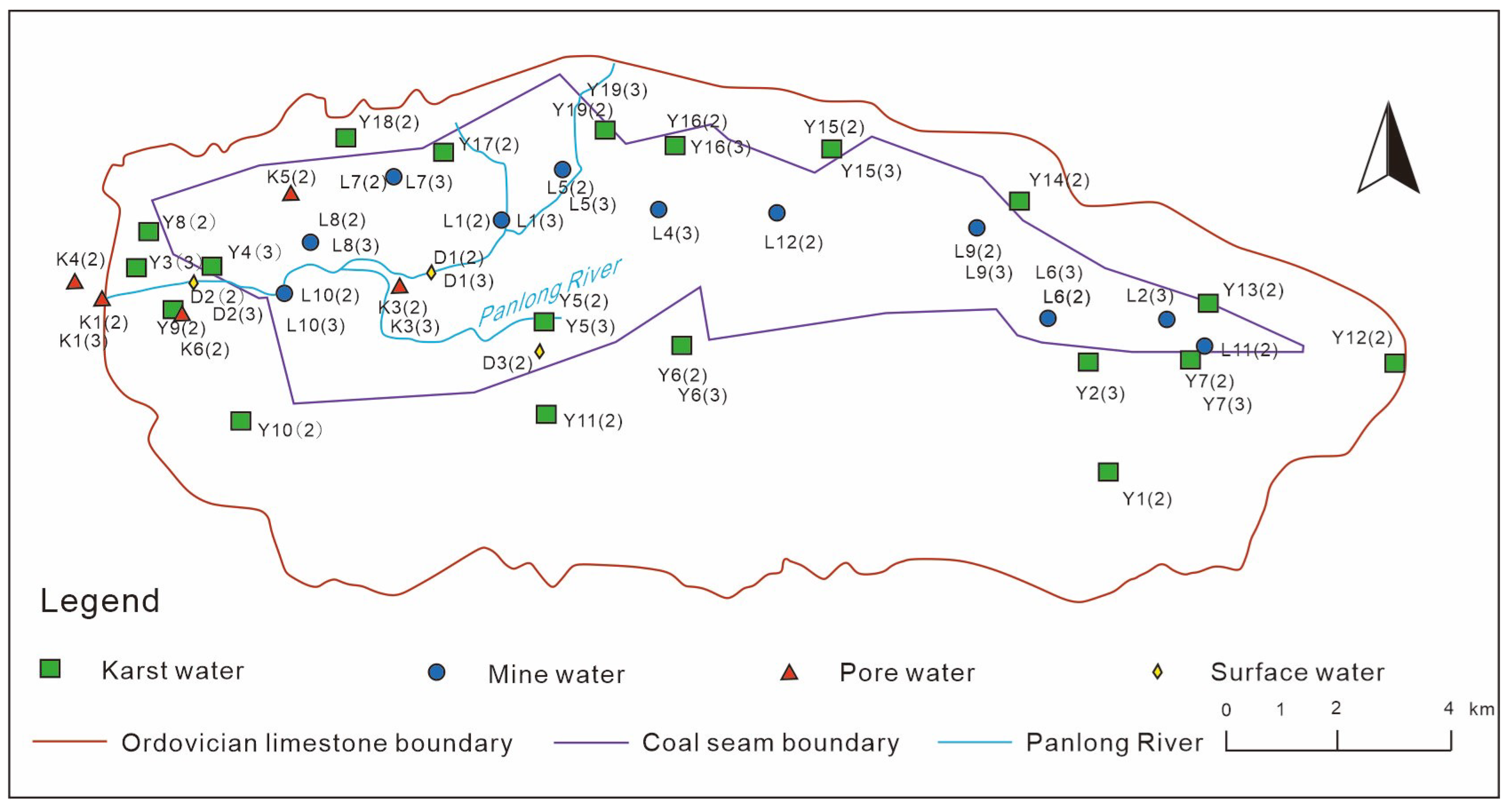
3.1. Sample Collection
3.2. Laboratory Analysis
3.3. Method of Source Analysis
3.3.1. Data Analysis
3.3.2. Mineral Saturation Index Analysis
3.3.3. Ion Activity Ratio Analysis
3.3.4. Positive Matrix Factorization Model
4. Results
4.1. Hydrochemical Characteristics
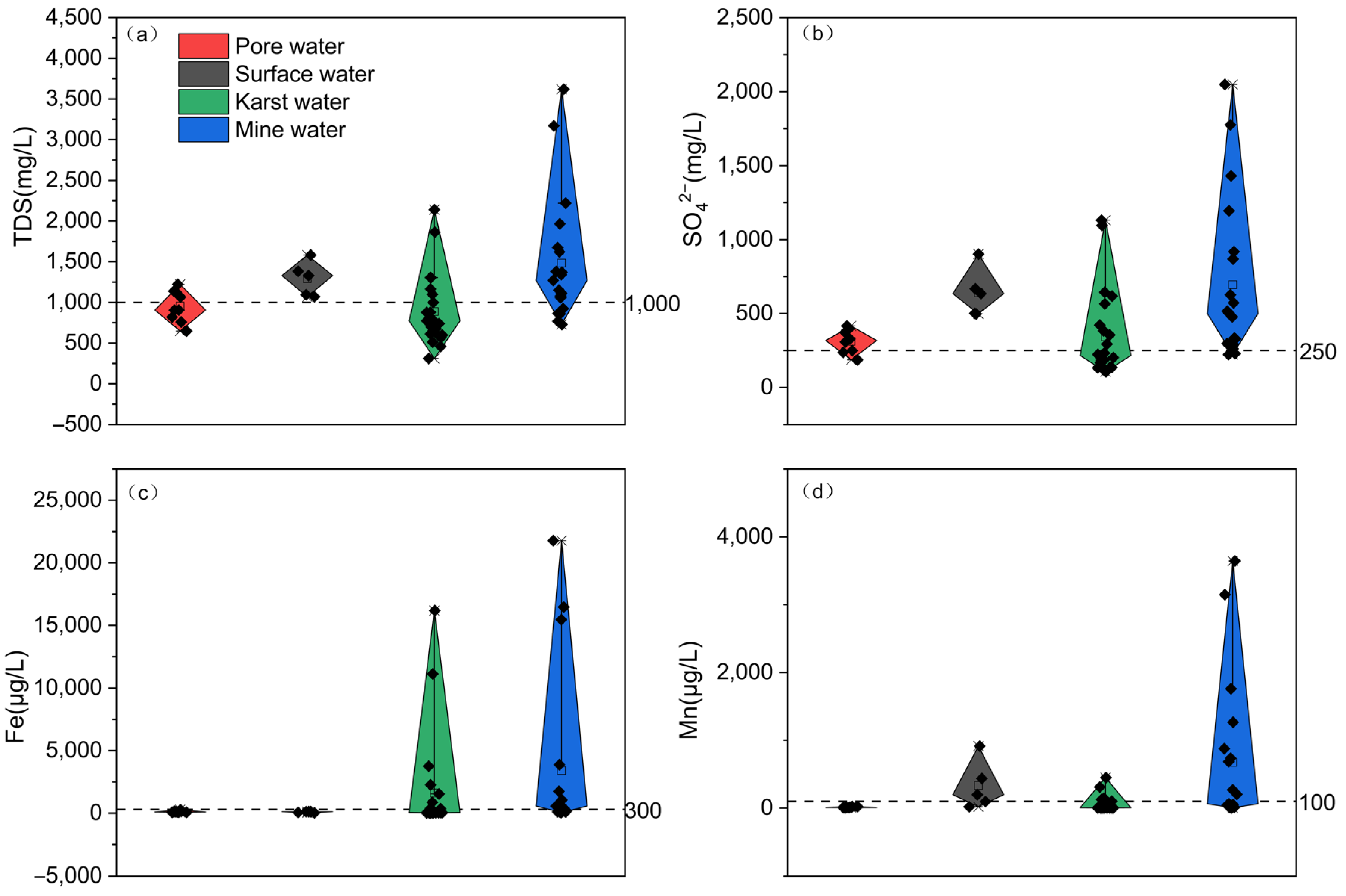
4.1.1. Water Quality Analysis
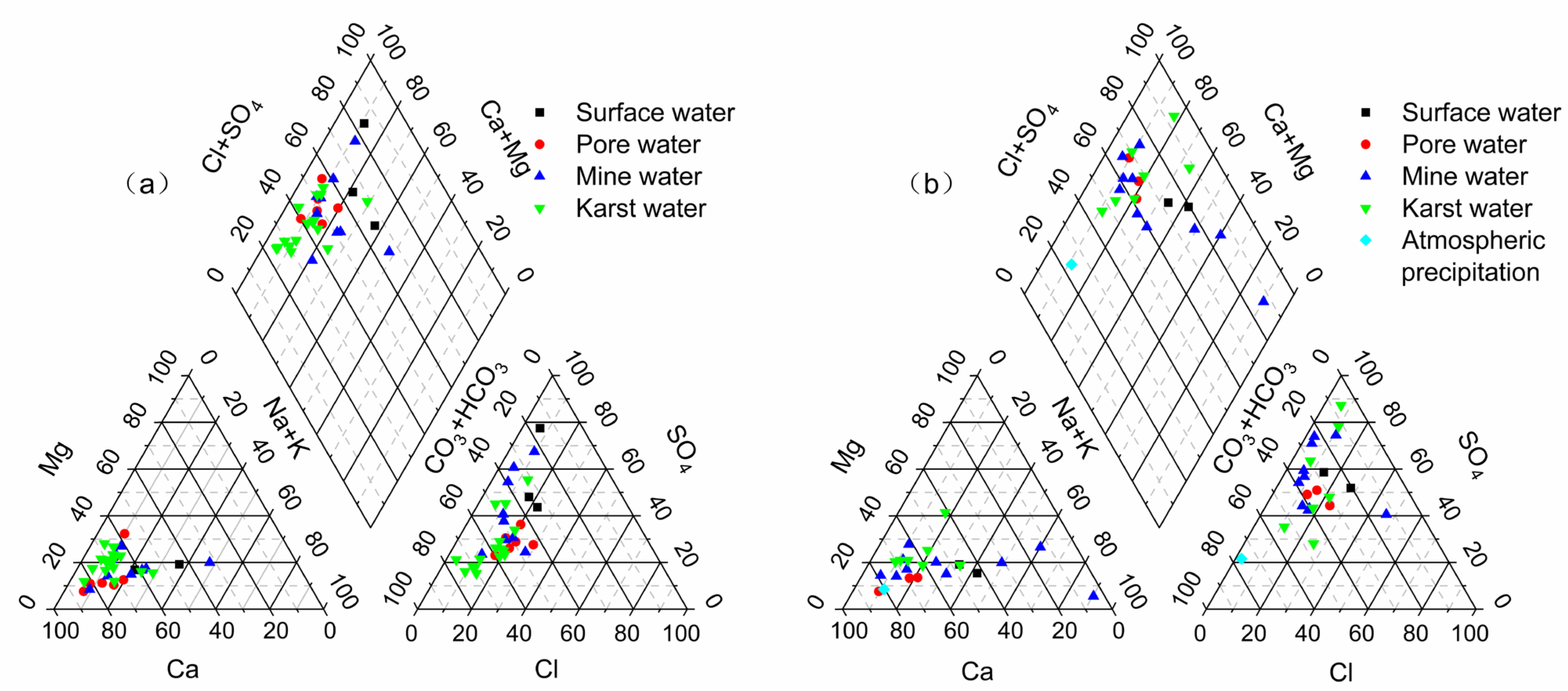
4.1.2. Hydrochemical Types
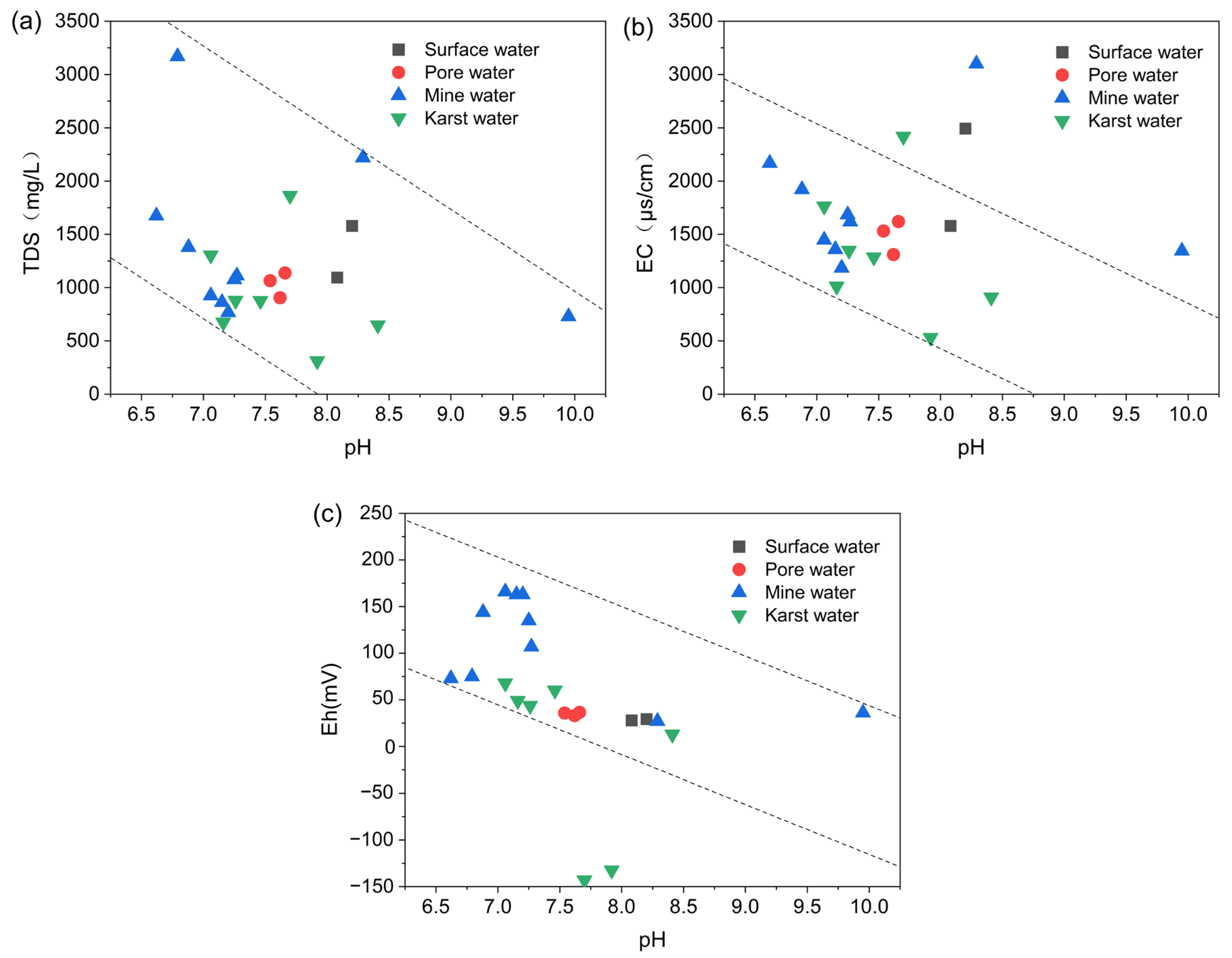
4.1.3. Physicochemical Characteristics
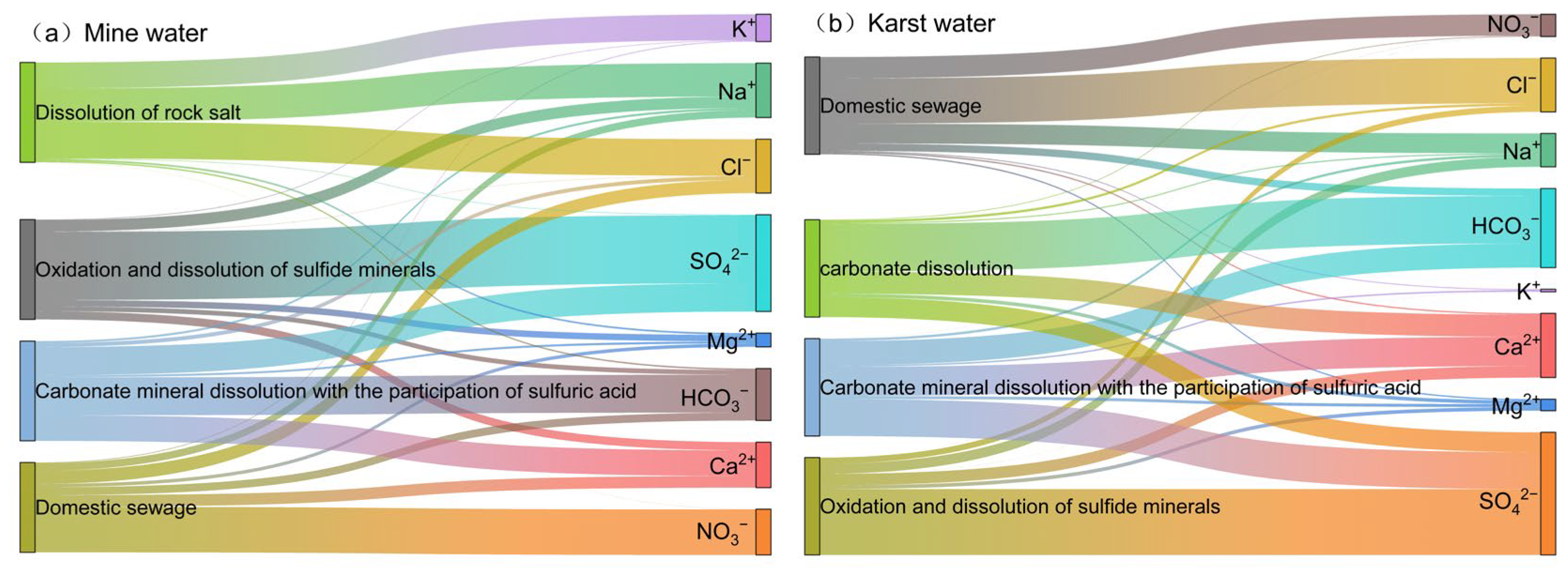
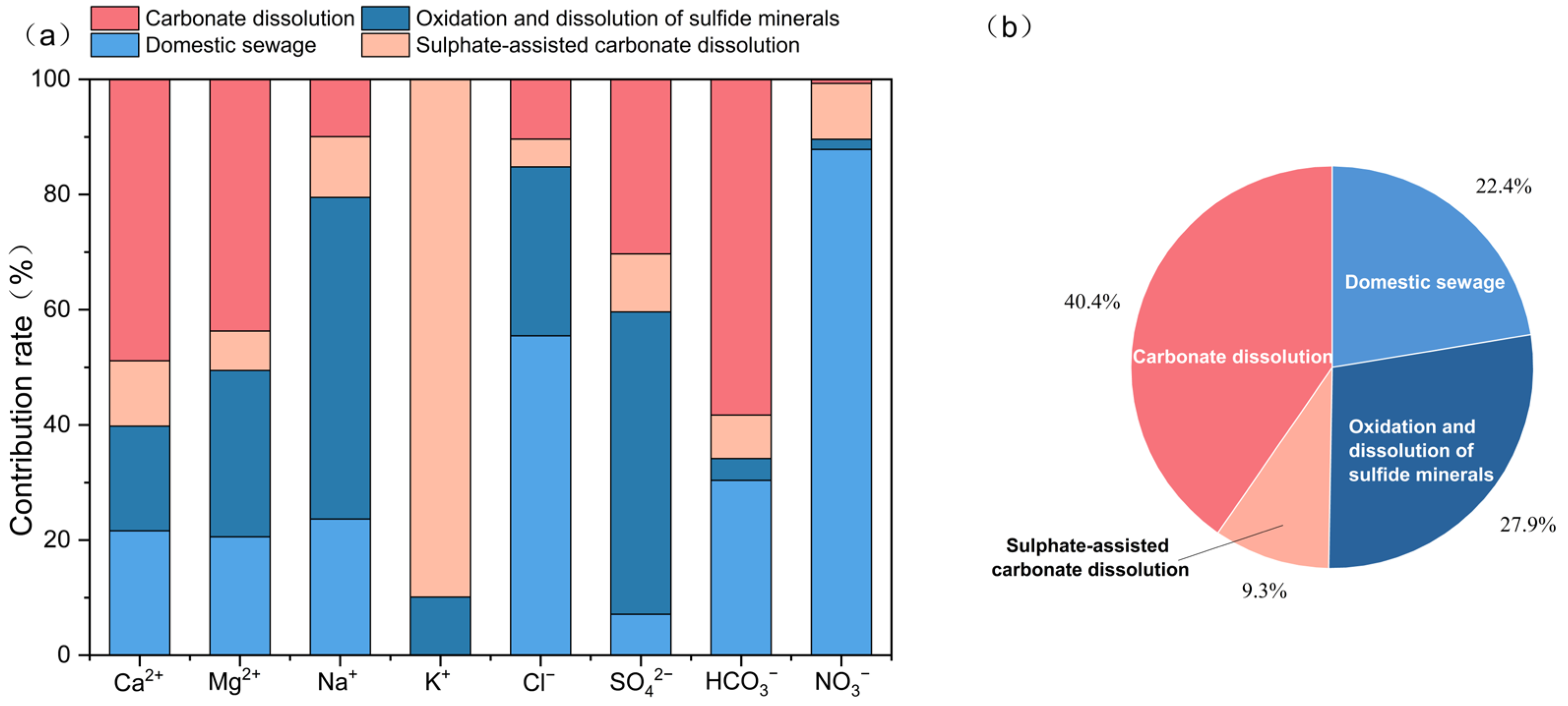
4.2. Analysis of Hydrochemical Genesis
4.2.1. Correlation Analysis
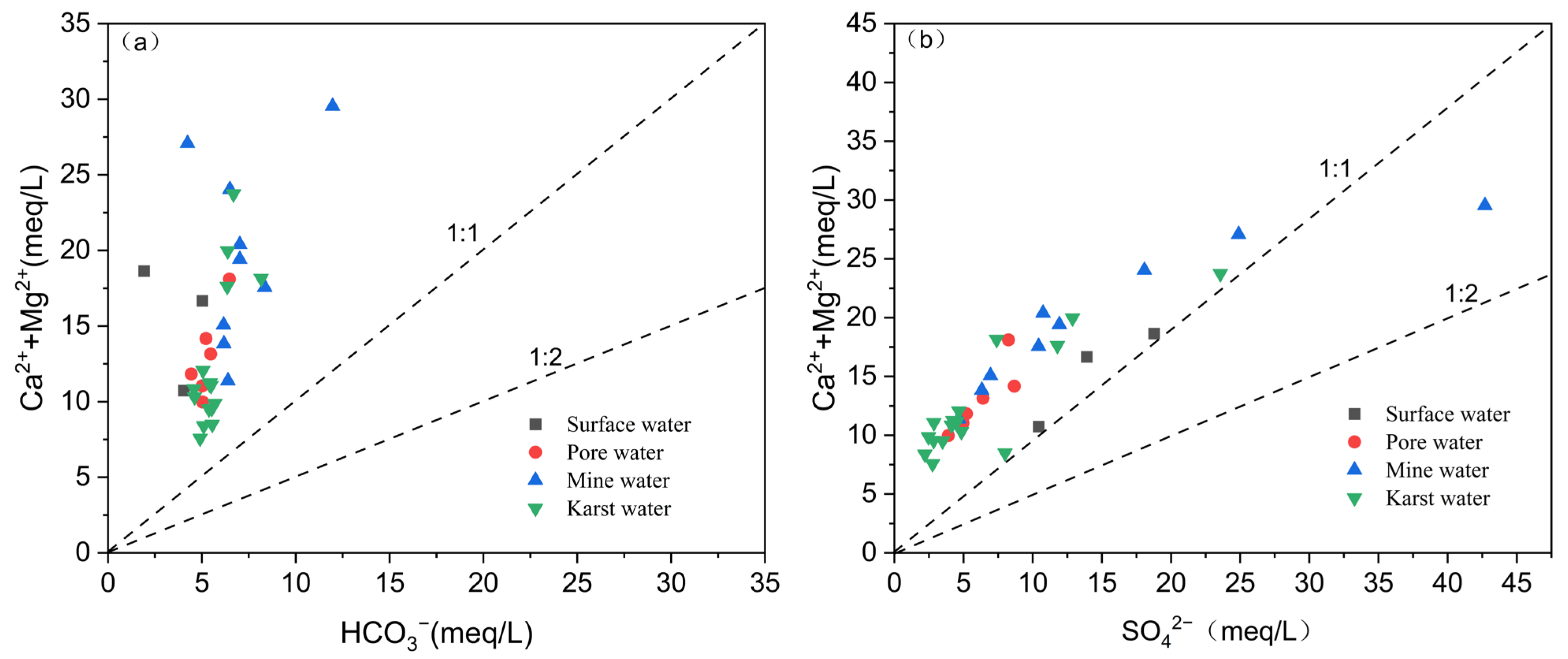
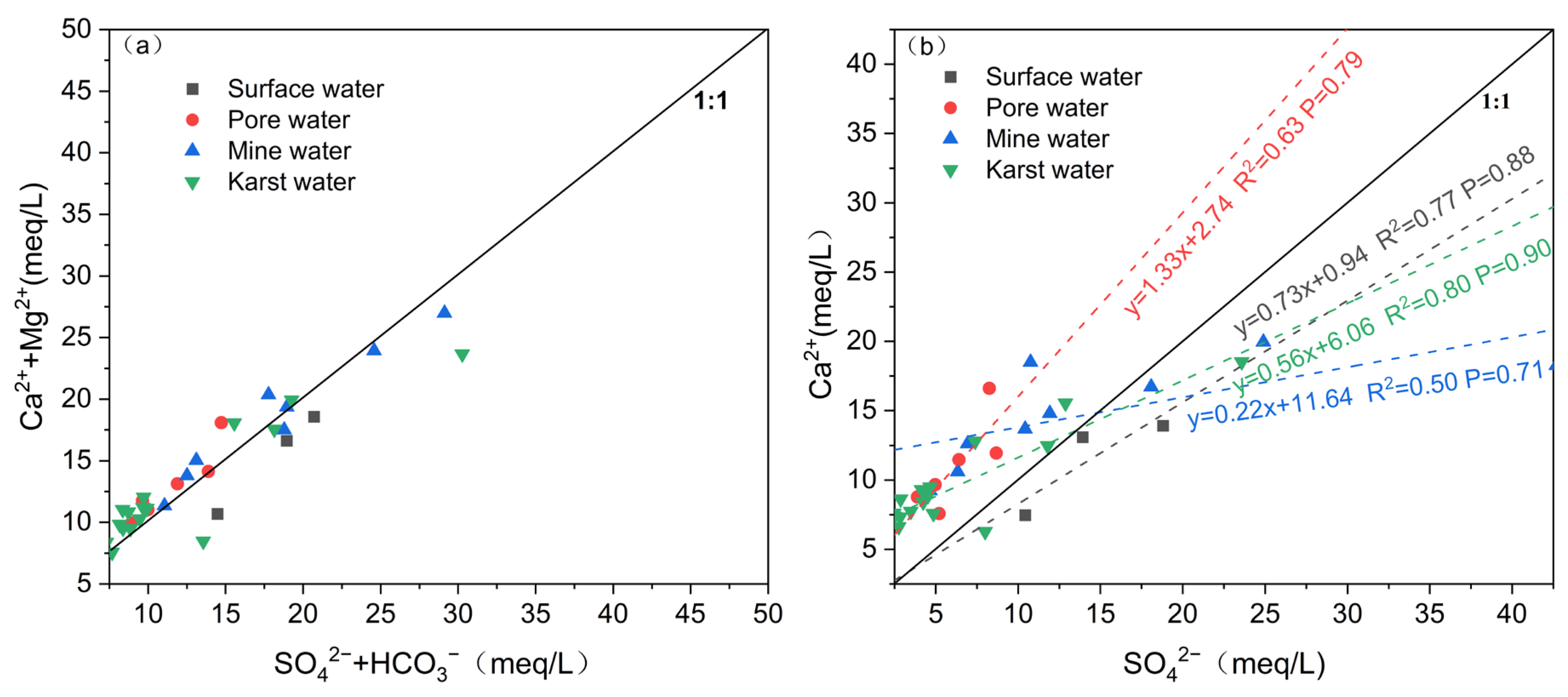
4.2.2. Analysis of Mineral Saturation Indices
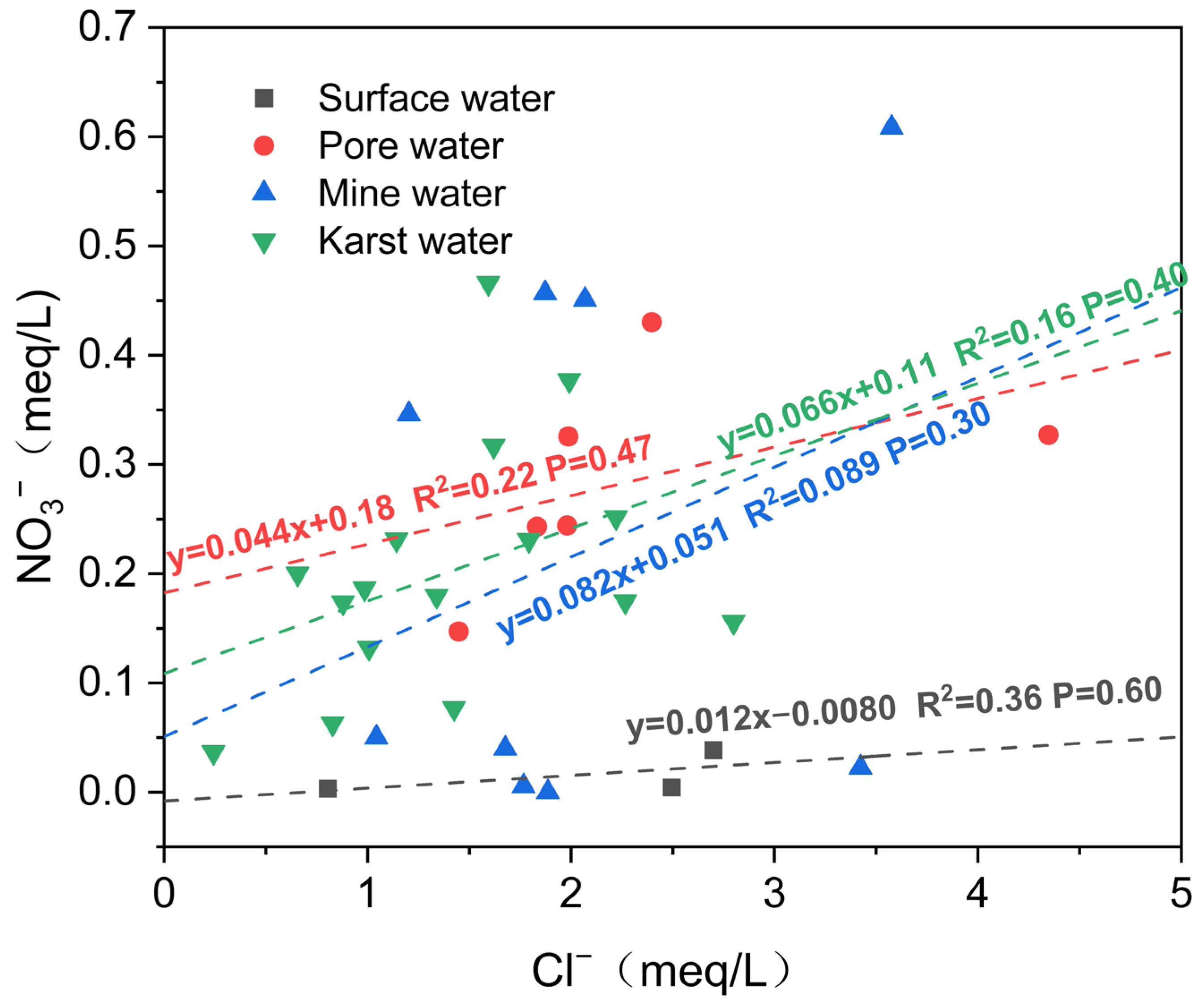
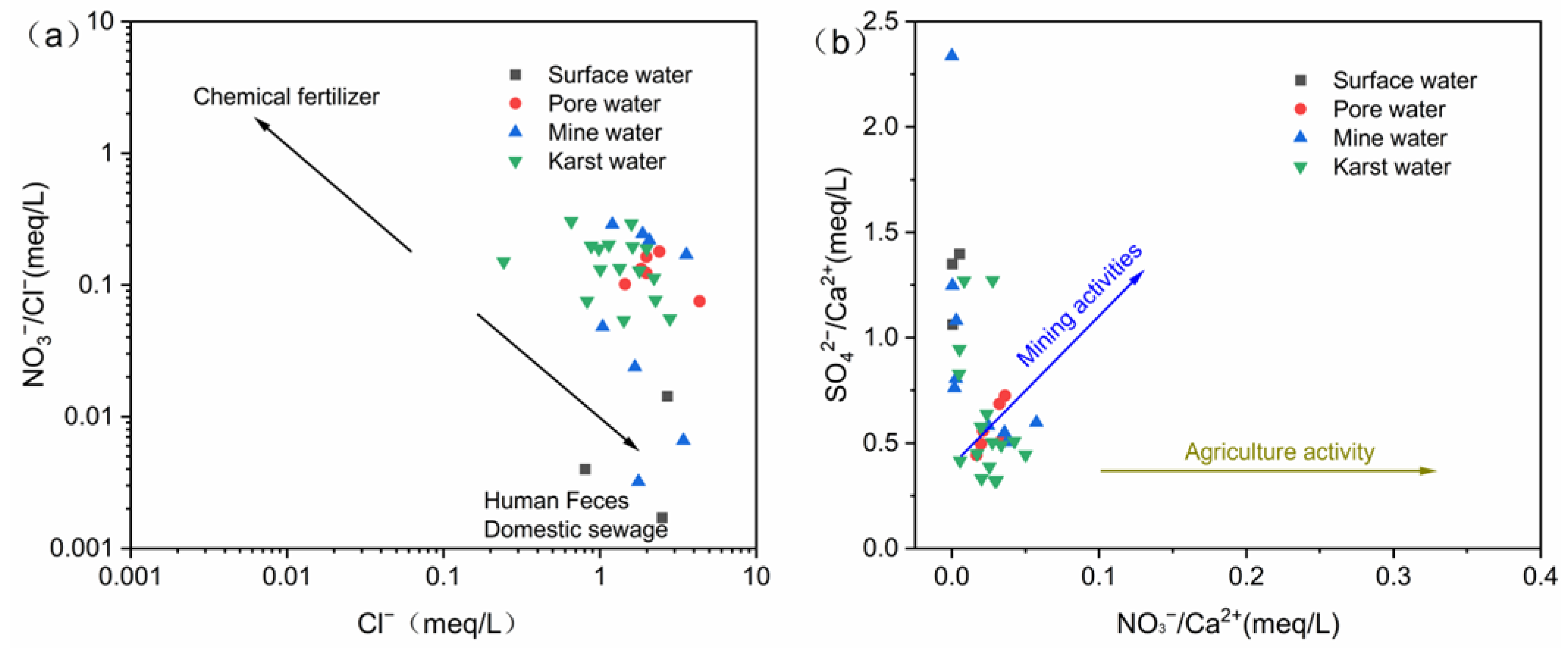
4.2.3. Analysis of Ion Ratios
4.2.4. Quantitative Source Analysis Using PMF
5. Discussion
5.1. Dissolution and Weathering of Minerals
5.2. Cation Exchange Action
5.3. Human Activities
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, L.; Jiang, Y.; Wang, K.; Zhao, Y.; Hao, X.; Xu, C. Precision exploitation and utilization of closed/abandoned mine resources in China. J. China Coal Soc. 2018, 43, 14–20. [Google Scholar]
- Sun, Y.; Xu, Z.; Li, X.; Zhang, L.; Chen, G.; Zhao, X.; Gao, Y.; Liu, Q.; Zhang, S.; Wang, W.; et al. Mine water drainage pollution in China’s coal mining areas and the construction of prevention and control technical system. Coal Geol. Explor. 2021, 49, 1–16. [Google Scholar]
- He, X.; Wu, G.; Zhu, S.; Qiao, J.; Wang, X. The key directions research and consideration of abandoned coal mines geological work in China. China Min. Mag. 2019, 28, 80–84. [Google Scholar]
- Tarasenko, I.; Kholodov, A.; Zin’kov, A.; Chekryzhov, I. Chemical composition of groundwater in abandoned coal mines: Evidence of hydrogeochemical evolution. Appl. Geochem. 2022, 137, 105210. [Google Scholar] [CrossRef]
- Lu, M.; Wu, E.; Li, M. Study on the hydrogeochemistry genesis of high fluorine shallow-bed groundwater in southwestern Shandong Province. Coal Geol. Explor. 2001, 29, 39–42. [Google Scholar]
- Liu, C.; Wang, W.; Yang, L.; Zhu, H.; Guo, Y.; Ma, Y.; Guo, J.; Liu, B. Driving mechanisms of fluorine enrichment characteristics in groundwater, Shandong Province. Acta Geol. Sin. 2021, 95, 1962–1972. [Google Scholar]
- Bian, Y.; Zhao, D.; Han, Y. Hydrochemical characteristics and formation mechanism of ordovician limestone groundwater in the Yanzhou Coalfield. Acta Geosci. Sin. 2017, 38, 236–242. [Google Scholar]
- Abanyie, S.K.; Apea, O.B.; Abagale, S.A.; Amuah, E.E.Y.; Sunkari, E.D. Sources and factors influencing groundwater quality and associated health implications: A review. Emerg. Contam. 2023, 9, 100207. [Google Scholar] [CrossRef]
- Ojo, O.I.; Otieno, F.; Ochieng, G. Groundwater: Characteristics, qualities, pollutions and treatments: An overview. Int. J. Water Res. Environ. Eng. 2012, 4, 162–170. [Google Scholar]
- Deng, J.; Yang, G.; Yan, X.; Du, J.; Tang, Q.; Yu, C.; Pu, S. Quality evaluation and health risk assessment of karst groundwater in Southwest China. Sci. Total Environ. 2024, 946, 174371. [Google Scholar] [CrossRef] [PubMed]
- Krzemień, A.; Sánchez, A.S.; Fernández, P.R.; Zimmermann, K.; Coto, F.G. Towards sustainability in underground coal mine closure contexts: A methodology proposal for environmental risk management. J. Clean. Prod. 2016, 139, 1044–1056. [Google Scholar] [CrossRef]
- Zhang, G.; Mi, M. Sedimentary facies of clastic-platform carbonate sediment strata of epicontinental sea in the Daniudi Gasfield, Ordos Basin. Nat. Gas Ind. B 2021, 8, 239–251. [Google Scholar] [CrossRef]
- HJ 164-2020; Technical Specifications for Environmental Monitoring of Groundwater. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2020.
- DZ/T 0064.49-2021; Geological and Mineral Industry Standards of the People’s Republic of China; Groundwater Quality Analysis Method part 49: Determination of Carbonate, Bicarbonate, and Hydroxide Ions Titration Method. Ministry of Natural Resources of the People’s Republic of China: Beijing, China, 2021.
- Chen, J.; Yan, B.; Xu, T.; Fan, X. Hydrochemical evolution characteristics and mechanism of groundwater funnel areas under artificial governance in Hengshui City, North China. Ecol. Indic. 2023, 148, 110059. [Google Scholar] [CrossRef]
- Han, X.; Pan, B.; Liu, Z.; Hou, B.; Li, D.; Li, M. Relationship among water quality and hydrochemical indices reveals nutrient dynamics and sources in the most sediment-laden river across the continent. J. Environ. Chem. Eng. 2022, 10, 107110. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, W.; Zhu, Y.; Liu, Y.; Li, Y.; Cao, S.; Hao, Q.; Liu, S.; Kong, X.; Han, Z.; et al. Evolution of groundwater hydrochemical characteristics and formation mechanism during groundwater recharge: A case study in the Hutuo River alluvial–pluvial fan, North China Plain. Sci. Total Environ. 2024, 915, 170159. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, D.; Li, Y.; Yang, J.; Zou, S.; Wang, Y.; Huang, X.; Zhang, Z.; Yang, W.; Jia, B. Water sources and factors controlling hydro-chemical compositions in the Yiluo river basin. Environ. Sci. 2020, 41, 1184–1196. [Google Scholar]
- Gaillardet, J.; Dupre, B.; Louvat, P.; Allegre, C. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, Z.; Luo, W.; Ma, T.; Wang, Y. Multiple isotope geochemistry and hydrochemical monitoring of karst water in a rapidly urbanized region. J. Contam. Hydrol. 2018, 218, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, Y.; Luo, W.; Chen, W.; Liu, H. Hydrogeochemical Investigations of Groundwater in the Lingbei Area, Leizhou Peninsula. Environ. Sci. 2020, 41, 4924–4935. [Google Scholar]
- Qu, S.; Shi, Z.; Liang, X.; Wang, G.; Han, J. Multiple factors control groundwater chemistry and quality of multi-layer groundwater system in Northwest China coalfield—Using self-organizing maps (SOM). J. Geochem. Explor. 2021, 227, 106795. [Google Scholar] [CrossRef]
- Shi, X.; Kang, X.; Xu, M.; Deng, H. Hydrochemical characteristics and evolution laws of karst groundwater in the slope zone of the canyon area, Sichuan-Yunnan Plateau. Acta Geol. Sin. 2019, 93, 2975–2984. [Google Scholar]
- Li, G.; Han, Z.; Shen, C.; Zeng, Z. Distribution characteristics and causes of nitrate in waters of typical small karst catchment: A case of the Houzhai river catchment. Earth Sci. 2019, 44, 2899–2908. [Google Scholar]
- Liu, C.; Li, S.; Lang, Y.; Xiao, H. Using δ15N-and δ18O-values to identify nitrate sources in karst ground water, Guiyang, southwest China. Environ. Sci. Technol. 2006, 40, 6928–6933. [Google Scholar] [CrossRef] [PubMed]
- Puig, R.; Soler, A.; Widory, D.; Mas-Pla, J.; Domenech, C.; Otero, N. Characterizing sources and natural attenuation of nitrate contamination in the Baix Ter aquifer system (NE Spain) using a multi-isotope approach. Sci. Total Environ. 2017, 580, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, H.; Wang, Q.; Kou, X.; Cao, X.; Xu, Z.; Wen, L.; Zhuo, Y.; Wang, L. Hydrochemical and stable isotope characteristics of surface water and groundwater in Xiliugou and Wulagai River basin, North China. Ecohydrol. Hydrobiol. 2024, 24, 62–72. [Google Scholar] [CrossRef]
- Fan, L.; Ma, W.; Chang, B.; Sun, K.; Miao, Y.; Lu, B.; Tian, S.; Yang, L. Hydrochemical characteristics and formation mechanism of groundwater in Yushenfu Mining Area. Coal Sci. Technol. 2023, 51, 383–394. [Google Scholar]
- Zheng, T.; Jiao, T.; Hu, B.; Gong, J.; Hou, X.; Wang, H. Hydrochemical characteristics and origin of groundwater in the central Guohe river basin. Environ. Sci. 2021, 42, 766–775. [Google Scholar]
- Jin, D.; Wang, T.; Zhao, B.; Li, D.; Zhou, Z.; Shang, H. Distribution characteristics and formation mechanism of high salinity groundwater in northeast Ningdong Coalfield. Coal Geol. Explor. 2022, 50, 118–127. [Google Scholar]
- Chen, R.; Chen, H.; Song, L.; Yao, Z.; Meng, F.; Teng, Y. Characterization and source apportionment of heavy metals in the sediments of Lake Tai (China) and its surrounding soils. Sci. Total Environ. 2019, 694, 133819. [Google Scholar] [CrossRef] [PubMed]
- GB/T 14848-2017; Standard for Groundwater Quality. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China Standardization Administration of China: Beijing, China, 2017.
- GB 3838-2002; Environmental Quality Standards for Surface Water. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2020.
- Ai, Y.; Chen, H.; Chen, M.; Huang, Y.; Han, Z.; Liu, G.; Gao, X.; Yang, L.; Zhang, W.; Jia, Y.; et al. Characteristics and treatment technologies for acid mine drainage from abandoned coal mines in major coal-producing countries. J. China Coal Soc. 2023, 48, 4521–4535. [Google Scholar]
- Li, X.; Li, C.; Parriaux, A.; Wu, W.; Li, H.; Sun, L.; Liu, C. Multiple resources and their sustainable development in Urban Underground Space. Tunn. Undergr. Space Technol. 2016, 55, 59–66. [Google Scholar] [CrossRef]
- Wang, F.; Liu, C.; Liang, X.; Wei, Z.; Li, J. Acid mining drainage impacts on the separation between iron and manganese in sediments of the Aha Lake, Guizhou Province. Environ. Sci. 2005, 26, 135–140. [Google Scholar]
- Wang, Y.; Jiao, J.; Cherry, J.A.; Lee, C.M. Contribution of the aquitard to the regional groundwater hydrochemistry of the underlying confined aquifer in the Pearl River Delta, China. Sci. Total Environ. 2013, 461–462, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liao, F.; Wang, G.; Qu, S.; Mao, H.; Bai, Y. Hydrogeochemical evolution induced by long-term mining activities in a multi-aquifer system in the mining area. Sci. Total Environ. 2023, 854, 158806. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Routh, J.; Dario, M.; Sarkar, S.; Wei, L.; Luo, D.; Liu, Y. Distribution and mobilization of heavy metals at an acid mine drainage affected region in South China, a postremediation study. Sci. Total Environ. 2020, 724, 138122. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, P.; Zha, X.; Li, X.; Wu, L.; Gu, S. Effects of mining activities on evolution of water chemistry in coal-bearing aquifers in karst region of Midwestern Guizhou, China: Evidences from δ13C of dissolved inorganic carbon and δ34S of sulfate. Environ. Sci. Pollut. Res. 2018, 25, 18038–18048. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Huang, W.; Fu, X.; Zheng, L.; Cheng, S.; Shan, C. Characteristics and genetic mechanism of low sulfate in high-salt groundwater of Permian sandstone in Fudong mining area, Huainan. Coal Geol. Explor. 2023, 51, 74–82. [Google Scholar]
- Yu, L.; Zheng, T.; Zheng, X. Review of nitrate source apportionment and nitrogen isotope fractionation in groundwater. Geosciences 2022, 36, 563–573. [Google Scholar]
- He, W.; Li, F.; Yu, J.; Chen, M.; Deng, Y.; Li, J.; Tang, X.; Chen, Z.; Yan, Z. Risk assessment and source apportionment of trace elements in multiple compartments in the lower reach of the Jinsha River, China. Sci. Rep. 2021, 11, 20041. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, X.; Sun, B.; Sui, H.; Geng, F.; Qi, H.; Ma, X. Hydrochemical characteristics and formation mechanism of geothermal water in Northern Ji’nan. Earth Sci. 2018, 43 (Suppl. 1), 313–325. [Google Scholar]
- Li, S.; Calmels, D.; Han, G.; Gaillardet, J.; Liu, C. Sulfuric acid as an agent of carbonate weathering constrained by δ13CDIC: Examples from Southwest China. Earth Planet. Sci. Lett. 2008, 270, 189–199. [Google Scholar] [CrossRef]
- Jiang, W.; Sheng, Y.; Shi, Z.; Guo, H.; Chen, X.; Mao, H.; Liu, F.; Ning, H.; Liu, N.; Wang, G. Hydrogeochemical characteristics and evolution of formation water in the continental sedimentary basin: A case study in the Qaidam Basin, China. Sci. Total Environ. 2024, 957, 177672. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, Q.; Liu, Y.; Peng, W.; Wang, Z.; Zhao, X. Hydrochemical characteristics and source analysis of deep groundwater in Qianyingzi Coal Mine. Coal Geol. Explor. 2022, 50, 99–106. [Google Scholar]
- Linghu, C.; Cun, D.; Yin, L.; Liu, Z.; Tu, C.; Pang, L. Hydrochemical characteristics and controlling Factors of surface water and groundwater in typical coal mining areas in Eastern Yunnan. Sci. Technol. Eng. 2023, 23, 13728–13740. [Google Scholar]
- Xia, Q.; He, J.; Li, B.; He, B.; Huang, J.; Guo, M.; Luo, D. Hydrochemical evolution characteristics and genesis of groundwater under long-term infiltration (2007–2018) of reclaimed water in Chaobai River, Beijing. Water Res. 2022, 226, 119222. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, H.; Zhang, F.; Han, Z.; Shi, H.; Meng, J.; Feng, Q.; Chen, D. Study on the impact of closed coal mines on groundwater in the Panlong River Basin (Shangdong Province, China) based on sulfur and oxygen isotopes. Water 2024, 16, 1634. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, S.; Zhao, Z.; Li, H.; Yang, J.; Duan, H.; Guo, W.; Liu, Y. The Water-Sediment Regulation Scheme at Xiaolangdi Reservoir and Its Impact on Sulfur Cycling in the Yellow River Basin. Earth Sci. 2022, 47, 589–606. [Google Scholar]
- Jiang, C.; Cheng, L.; Li, C.; Zheng, L. A hydrochemical and multi-isotopic study of groundwater sulfate origin and contribution in the coal mining area. Ecotoxicol. Environ. Saf. 2022, 248, 114286. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tian, R.; Liu, R. Solute geochemistry and multivariate analysis of water quality in the Guohua Phosphorite Mine, Guizhou Province, China. Expos. Health 2019, 11, 81–94. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Zhou, W.; Jiang, Y.; Ding, H.; Dong, F. Evolution of karst geothermal hydrochemical characteristics and genesis mechanism in northern Jinan, Shandong, Eastern China. ACS Omega 2024, 9, 36299–36313. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, X.; Wang, W.; Li, C. Human activity and hydrogeochemical processes relating to groundwater quality degradation in the Yuncheng Basin, northern China. Int. J. Environ. Res. Public Health 2020, 17, 867. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Yin, L.; He, C.; Cun, D.; Ma, Y.; Linghu, C. Hydrochemical composition characteristics and control factors of Xiaohuangni river basin in the upper pearl river. Environ. Sci. 2022, 43, 1885–1897. [Google Scholar]
- Jin, Y.; Long, J.; Ren, J.; Ni, X. Water source analysis of mine filling foundation based on hydrochemical characteristics. Coal Sci. Techno. 2022, 50, 173–180. [Google Scholar]
- Wang, Y.; Wang, H.; Wang, T.; Xue, J.; Shang, H.; Zhou, Z. Hydrochemical characteristics and source analysis of mine water in shallow coal seams in Shaanxi and Inner Mongolia contiguous area. Coal Geol. Explor. 2023, 51, 85–94. [Google Scholar]
- Zhang, F.; Wang, G.; Zhang, M.; Sun, P.; Han, X. Identification of produced water and characteristics of hydrochemistry and stable hydrogen−oxygen isotopes of contaminated groundwater. Northwest. Geol. 2023, 56, 98–108. [Google Scholar]
- Wang, Z.; Pei, J.; Ruan, C.; Adimalla, N.; Liu, H.; Guo, H. Hydrochemical characteristics and salinity formation mechanism of different water bodies in the southern Tibet, China. Environ. Geochem. Health 2024, 47, 7. [Google Scholar] [CrossRef] [PubMed]

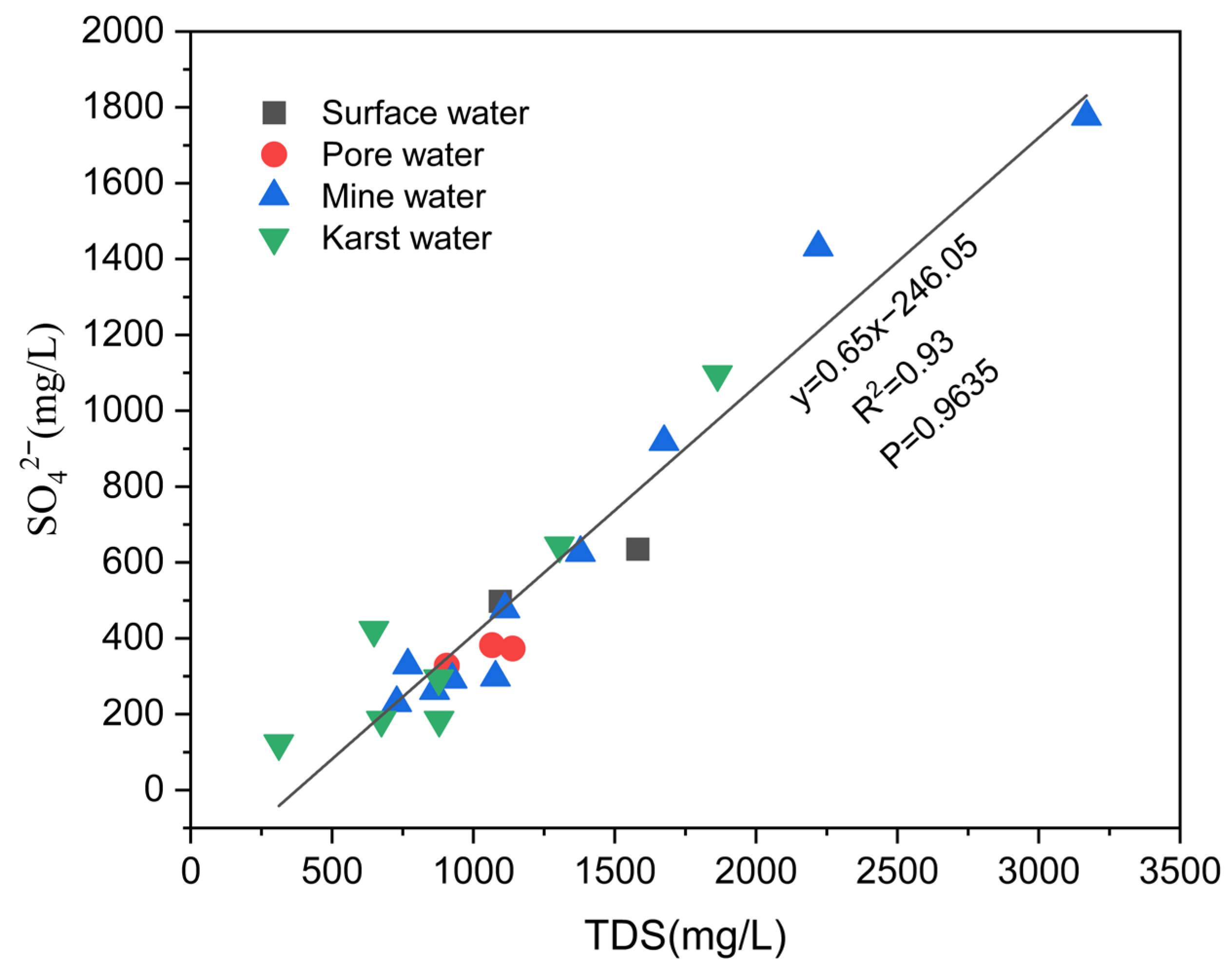
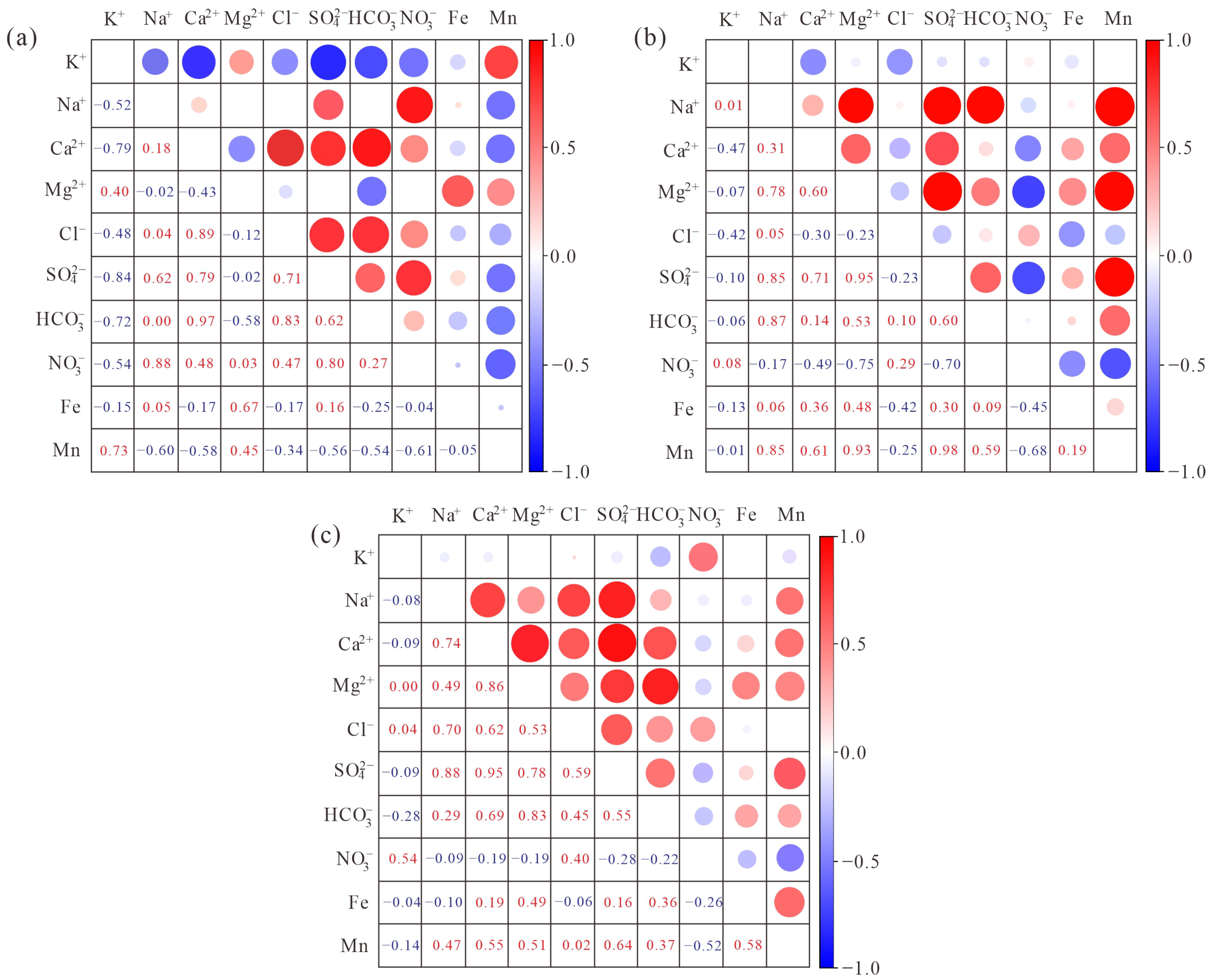

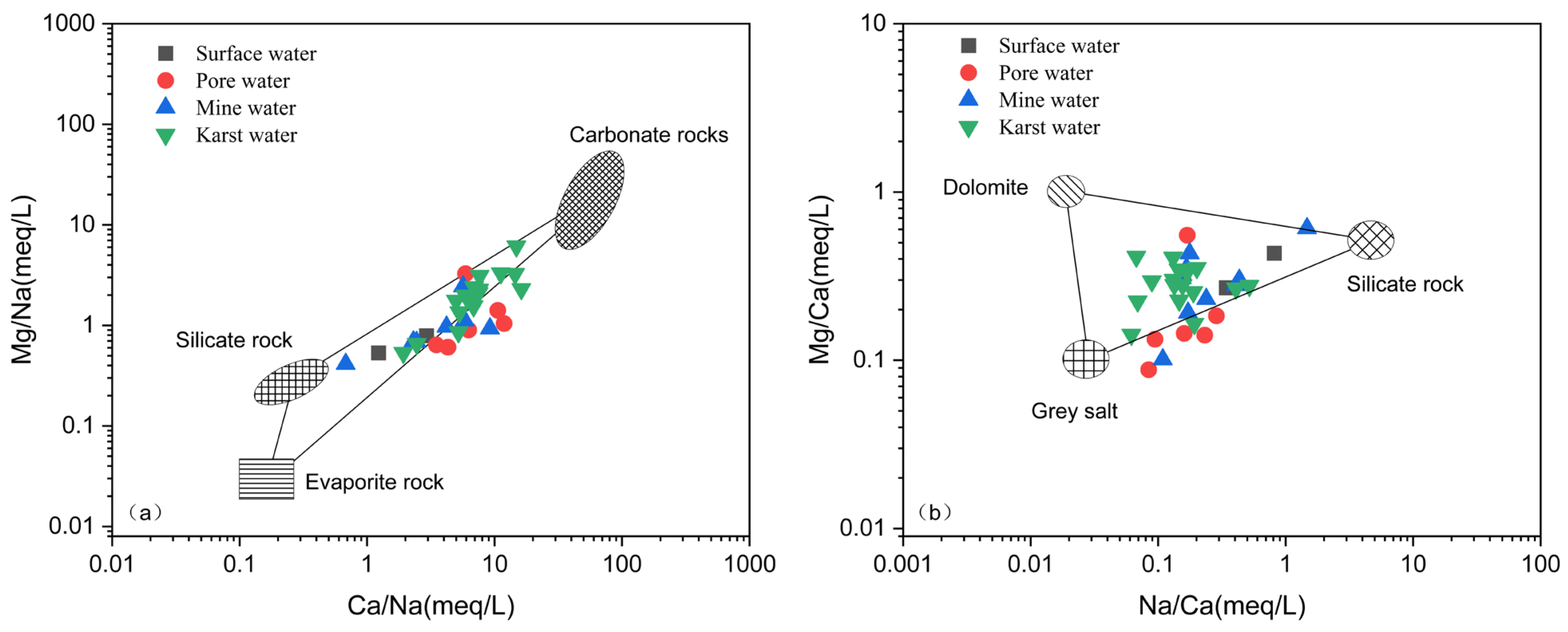
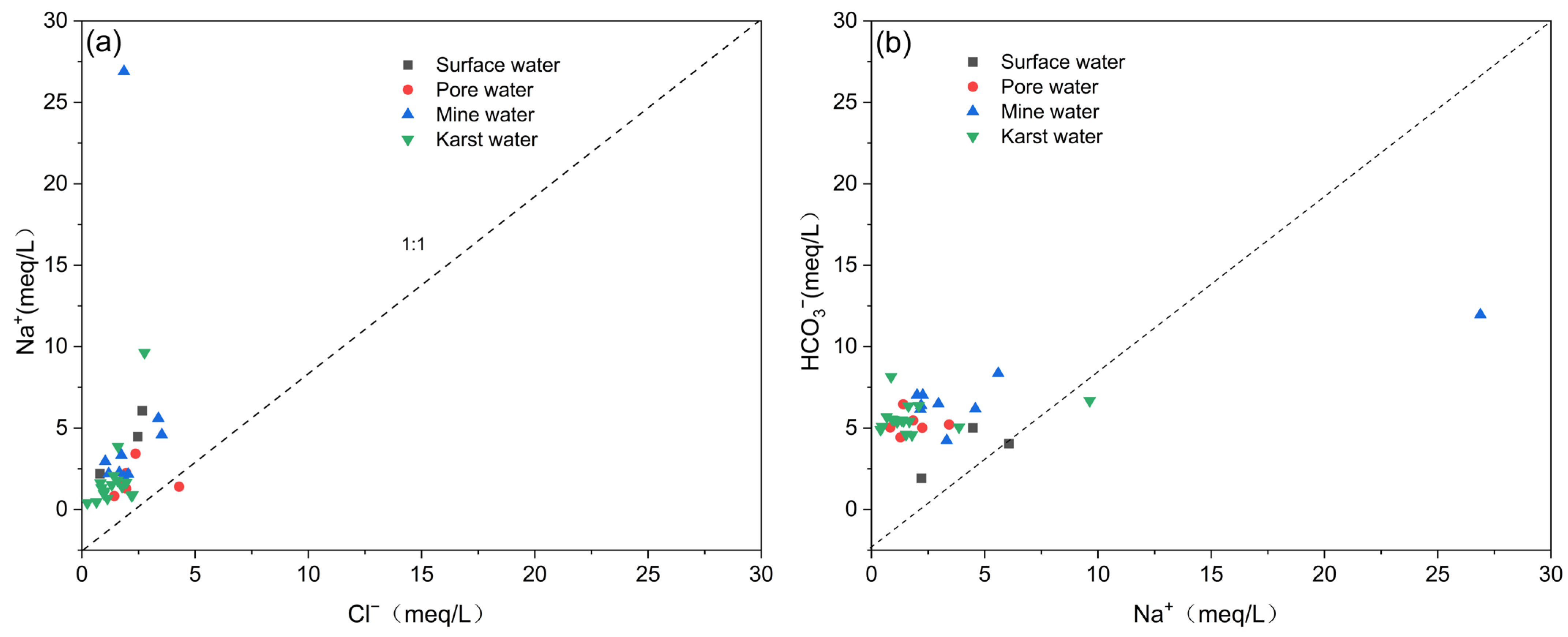


| Type of Water Body | Mineral Phase | SI | Proportion of Different SI Values/% | ||||
|---|---|---|---|---|---|---|---|
| Mineral Name | Chemical Formula | Min | Max | Avg | SI > 0 | SI < 0 | |
| Surface water | Gypsum | CaSO4 | −0.76 | −0.63 | −0.7 | 0 | 100 |
| Rock salt | NaCl, KCl | −6.53 | −5.86 | −6.2 | 0 | 100 | |
| Dolomite | CaMg(CO3)2 | 1.33 | 1.77 | 1.55 | 100 | 0 | |
| Calcite | CaCO3 | 0.9 | 1.13 | 1.02 | 100 | 0 | |
| Pore water | Gypsum | CaSO4 | −0.84 | −0.65 | −0.73 | 0 | 100 |
| Rock salt | NaCl, KCl | −6.95 | −6.81 | −6.89 | 0 | 100 | |
| Dolomite | CaMg(CO3)2 | 0.47 | 0.69 | 0.61 | 100 | 0 | |
| Calcite | CaCO3 | 0.61 | 0.88 | 0.72 | 100 | 0 | |
| Mine water | Gypsum | CaSO4 | −2.15 | −0.27 | −0.826 | 0 | 100 |
| Rock salt | NaCl, KCl | −7.42 | −5.93 | −6.826 | 0 | 100 | |
| Dolomite | CaMg(CO3)2 | −5.03 | 1.11 | −0.452 | 30 | 70 | |
| Calcite | CaCO3 | −2.57 | 0.52 | −0.045 | 80 | 20 | |
| Karst water | Gypsum | CaSO4 | −1.57 | −0.38 | −0.94286 | 0 | 100 |
| Rock salt | NaCl, KCl | −7.66 | −6.28 | −7.13857 | 0 | 100 | |
| Dolomite | CaMg(CO3)2 | −0.23 | 0.69 | 0.204286 | 71 | 29 | |
| Calcite | CaCO3 | 0.05 | 0.55 | 0.304286 | 100 | 0 | |
| Chemical Indicators | Mine Water | Karst Water | ||||||
|---|---|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 1 | Factor 2 | Factor 3 | Factor 4 | |
| Ca2+ | 8.22 | 11.63 | 25.7 | 0.00 | 1.32 | 11.87 | 29.98 | 22.76 |
| Mg2+ | 6.47 | 3.42 | 2.09 | 1.82 | 1.39 | 3.76 | 3.04 | 3.66 |
| Na+ | 11.49 | 7.48 | 2.12 | 33.43 | 20.31 | 10.4 | 2.48 | 1.12 |
| K+ | 0.57 | 0.79 | 0.00 | 25.97 | 0.86 | 0.11 | 1.63 | 0.00 |
| Cl− | 0.23 | 13.15 | 3.94 | 36.69 | 46.56 | 6.19 | 0.00 | 2.49 |
| SO42− | 68.26 | 0.00 | 28.19 | 0.49 | 0.00 | 67.63 | 37.96 | 20.38 |
| HCO3− | 4.76 | 7.91 | 37.96 | 1.47 | 7.62 | 0.00 | 24.79 | 48.88 |
| NO3− | 0.00 | 45.63 | 0.00 | 0.13 | 21.93 | 0.04 | 0.12 | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; He, J.; Zhang, G.; He, J.; Zhao, H.; Wu, M.; Song, X.; Liu, D. Hydrochemical Characteristics and Genesis Analysis of Closed Coal Mining Areas in Southwestern Shandong Province, China. Eng 2025, 6, 164. https://doi.org/10.3390/eng6070164
Wang X, He J, Zhang G, He J, Zhao H, Wu M, Song X, Liu D. Hydrochemical Characteristics and Genesis Analysis of Closed Coal Mining Areas in Southwestern Shandong Province, China. Eng. 2025; 6(7):164. https://doi.org/10.3390/eng6070164
Chicago/Turabian StyleWang, Xiaoqing, Jinxian He, Guchun Zhang, Jianguo He, Heng Zhao, Meng Wu, Xuejuan Song, and Dongfang Liu. 2025. "Hydrochemical Characteristics and Genesis Analysis of Closed Coal Mining Areas in Southwestern Shandong Province, China" Eng 6, no. 7: 164. https://doi.org/10.3390/eng6070164
APA StyleWang, X., He, J., Zhang, G., He, J., Zhao, H., Wu, M., Song, X., & Liu, D. (2025). Hydrochemical Characteristics and Genesis Analysis of Closed Coal Mining Areas in Southwestern Shandong Province, China. Eng, 6(7), 164. https://doi.org/10.3390/eng6070164







