1. Introduction
Polymers are widely used materials found in various forms, colors, and everyday objects, with the annual production of petroleum-derived plastics projected to reach approximately 300 million tons by 2025 [
1]. The large-scale production of these petroleum-based polymers has led to significant environmental impacts, primarily due to carbon dioxide (CO
2) emissions and the accumulation of materials that are highly resistant to environmental degradation [
2,
3]. This situation underscores the urgent need for sustainable alternatives derived from renewable resources. Current applications increasingly demand materials with properties that traditional polymers do not offer, such as degradability, mechanical strength, electrical conductivity, and more. In recent years, research and development efforts have focused on the creation of natural-based polymers for specialized applications, including food packaging, metal coatings, tissue engineering, filtration, and general-purpose colloids [
2].
Among these new types of polymers are bio-based biopolymers synthesized from agricultural waste and other renewable resources, such as polylactic acid (PLA), polycaprolactone (PCL), chitosan, and cellulose acetate (AC), which have been developed to replace 100% petroleum-based synthetic polymers [
4]. For instance, starch has been used to produce biopolymers that generate nearly 60% lower greenhouse gas emissions compared to polystyrene packaging.
Although biopolymers are considered environmentally friendly materials, they present certain limitations, including high production costs, low resistance to mechanical loads, low elastic modulus, poor UV resistance, and inadequate electronic properties, among others [
5,
6]. However, these limitations can be mitigated by using polymers derived from agricultural waste and by adopting alternative processing methods to produce composite materials. Such materials can combine different degradable substances to form membranes, films, fibers, and/or particles.
One of the key materials in biopolymer development is starch, obtained from sources such as potatoes, cassava, corn, and barley. Starch is well known for its excellent film-forming capacity and its effectiveness as an oxygen barrier, making it a promising substitute for synthetic polymers in applications like food packaging [
7,
8]. It is composed mainly of two components: linear amylose and branched amylopectin [
9]. However, starch-based films tend to be brittle and weak, resulting in poor mechanical performance.
Another natural material that enhances the mechanical properties of biopolymers is cellulose. This polymer offers high rigidity and chemical stability, making it an ideal candidate for composite reinforcement. It is often used to reinforce thermoplastics in the form of nano- or microfibers [
10]. Cellulose can be derived from various plant sources, particularly from the cell walls of plant stems. However, isolating pure cellulose is challenging due to the need to separate it from lignin and hemicellulose [
11].
Cellulose can be chemically modified by replacing hydroxyl groups with appropriate functional groups (such as carboxyl, alkyl, acetate, and nitrate) onto the hydroxyl groups along its carbon chain, although these reactions are not typically stoichiometric. Various chemical modifications of cellulose exist, including esters (e.g., cellulose acetate), ethers (e.g., carboxymethyl cellulose), and derivatives such as rayon and cellophane (from cellulose xanthate). Among commercially available cellulose types, carboxymethyl cellulose (CMC) is one of the most widely used, with applications in the detergent, food, paper, textile, pharmaceutical, and paint industries [
12,
13]. CMC is especially valued for its high viscosity, non-toxicity, non-allergenic nature, and low cost. Its numerous hydroxyl and carboxyl groups make it highly absorbent, enabling it to retain significant water content, biodegrade rapidly, and serve various functions [
14]. In fiber form, it has been shown that the Young’s modulus of CMC increases as fiber diameter decreases.
Research has been conducted using a thermoplastic matrix of plasticized cassava starch reinforced with cellulose nanofibers derived from cassava bagasse. The structural integrity of this material was evaluated using Dynamic Mechanical Analysis (DMA) [
2]. The results indicated that reinforcement enhanced both mechanical strength and Young’s modulus, likely due to additional plasticization from sugars produced during the acid hydrolysis of starch. Another study examining the effect of CMC concentration on the physical properties of biodegradable cassava starch-based films concluded that the incorporation of CMC fibers increased tensile strength and decreased elongation at break, possibly due to the strong interaction between cassava starch and CMC [
8].
One of the techniques for producing micro- and nanofibers from cellulose derivatives is electrospinning, in which a polymer solution is subjected to a voltage above 5 kV, causing the solution to solidify in the air as it travels from the injector tip to the collector [
15,
16]. This technique has been applied in areas such as tissue engineering, filtration, membranes, and energy cells [
17]. Micro- and nanofibers from starch, cellulose, and other agricultural waste materials have been successfully produced using this method [
18,
19].
In recent years, electrospinning has been used to prepare nanofibers from CMC/PEO (polyethylene oxide) for use in drug delivery systems [
20]. These systems have produced core–shell nanofibers, composed of a CMC and PEO mixture, loaded with drugs. This approach can be extended to encapsulate other drugs for various biomedical applications, including tissue engineering.
In the Boyacá region of Colombia, as in other parts of the world, efforts are underway to utilize waste from potato plantations (e.g., stems and leaves) as part of circular economy strategies aimed at reducing polymer use [
5,
10]. However, this research area is still in its early stages, particularly in the field of nanotechnology. One area of interest is the development of potato starch membranes reinforced with cellulose nanofibers, though this is complicated by the difficulty of solubilizing cellulose for electrospinning, which may require alternative processes such as cellulose acetylation.
Based on this context, the objective of the present study was to prepare and characterize CMC/PEO nanofibers using electrospinning in order to reinforce potato starch membranes. The aim was to enhance adhesion and, consequently, increase the tensile strength of the membranes while assessing their potential applications as biopolymers. The development of new materials from agro-industrial waste not only offers a promising route to replace conventional polymers but may also provide economic benefits to the agricultural sector.
2. Materials and Methods
2.1. Materials
Tubers (
Solanum tuberosum) and potato stems were obtained from the municipality of Sora, Boyacá, Colombia. Potato starch was extracted from the tubers using the methodology described by Cárdenas and Gómez [
17]. Nitric acid (CAS No. 7697-37-2, 70%), acetic acid (CAS No. 64-19-7, 99%), polyethylene oxide (PEO) (CAS No. 75-21-8), sodium hydroxide (CAS No. 1310-73-2, 88%), monochloroacetic acid (CAS No. 79-11-8, 99.8%), ethanol (CAS No. 64-17-5, 99%), glycerol (CAS No. 56-81-5, 99%), and citric acid (CAS No. 77-92-9, 99%) were all purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Preparation of Films Using the Thermal Gelatinization Method
The films were prepared using a potato starch concentration of 4%
w/
w and glycerol at 2%
w/
w. Starch and glycerol were mixed directly with distilled water to form batches weighing 100 g, following the methodology described by Vasanthan and Bergthaller [
12]. The film-forming solutions were heated from 30 °C to 95 °C and maintained at 95 °C for 10 min. The films were produced via casting: the gelatinized suspensions were poured into sterile polystyrene Petri dishes (110 × 20 mm) and dried in an oven at 65 °C for 5 h. After drying, the films were removed and stored in a desiccator with a desiccant.
2.3. Cellulose Extraction from Potato Stems
For cellulose extraction, potato stems were cut into small pieces of approximately 1 cm3. The dried stems were ground into a powder and passed through a 20-mesh sieve. The cellulose powder was treated with an 8% NaOH solution at a ratio of 1:20 (w/v) and heated at 100 °C for 3.5 h. The resulting suspension was filtered; the solid residue was washed with distilled water to remove residual solvents and then dried at 60 °C.
2.4. Synthesis of Carboxymethyl Cellulose (CMC)
CMC was synthesized according to the methodology proposed by Cruz et al. [
21]. In total, 15 g of cellulose was mixed with 200 mL of isopropanol under constant agitation at 150 rpm for 15 min, during which, an increase in the solution’s viscosity was observed. A 26.6%
w/
w NaOH solution was prepared separately and added to the cellulose mixture. Subsequently, a solution of monochloroacetic acid in 87%
v/
v isopropanol was added, and the mixture was stirred for 3 h at 60 °C. The resulting product was filtered and neutralized with 2–3 mL of acetic acid.
After neutralization, the CMC was washed three times with ethanol (70–80% v/v), and the ethanol residues were recovered for reuse. Following the third wash, the CMC was agitated to disaggregate it and then dried at 60 °C for 3 h.
2.5. Production of CMC and PEO Nanofibers
CMC/PEO nanofibers were produced following the procedure described by Basu et al. [
22]. A 4%
w/
w CMC/PEO solution was prepared and stirred vigorously for 48 h at room temperature. The solution was loaded into a 10 mL syringe fitted with a needle of 0.8 mm diameter.
Electrospinning was performed using equipment consisting of an injection pump (New Era Pump Systems, Inc. NE4000, Farmingdale, NY, USA), a high-voltage power supply (Glassman High Voltage, Inc., Whitehouse Station, NJ, USA), and an aluminum-coated copper collector plate. This equipment was developed at the Materials Research Institute (IIM) of the National Autonomous University of Mexico (UNAM) (Mexico City, Mexico) [
19].
Three concentrations of CMC/PEO (3%, 4%, and 5%) were prepared 48 h before electrospinning. Parameters such as needle–collector distance, applied voltage, and flow rate were determined through preliminary optimization tests, resulting in a needle–collector distance of 20 cm, a flow rate of 0.5 mL/h, and an applied voltage of 20 kV.
2.6. Mechanical Properties in Uniaxial Tension
Mechanical properties under uniaxial tension, including Young’s modulus (elastic modulus), maximum stress, and stress at break, were determined according to ASTM D882-9a using a 25 kg load cell at 24 ± 1 °C and 45% relative humidity. Dog-bone-shaped specimens were cut with dimensions of 5 cm in length and 5 mm in width. The film specimens were stored in a desiccator for 3 days to control moisture content. The thickness of each specimen was measured at three random points using a manual micrometer, and the average of these measurements was used to calculate the cross-sectional area. A strain rate of 1.5 mm/s was used for testing.
2.7. Chemical Analysis
Samples of carboxymethylcellulose (CMC1) and commercial carboxymethylcellulose were analyzed using a Fourier Transform Infrared (FTIR) spectrometer (NICOLET IS20, Thermo Scientific Inc., UNAM, Mexico City, Mexico). Each sample was scanned 10 times with a resolution of 4 cm−1 in a range of 400–4000 cm−1. Samples were applied directly to the ATR crystal surface, and spectra were collected at room temperature and processed using the OMNIC Professional V8.1 software.
2.8. Morphological Determination
The diameter, porosity, and morphology of the starch films, CMC/PEO nanofibers, and composite material (starch films with nanofibers) were examined using scanning electron microscopy (SEM) (JEOL JSM-7600 F) Tokyo, Japan. Samples were cut into 0.5 × 0.5 cm pieces, mounted onto aluminum holders using adhesive tape, and coated with gold by plasma-assisted sputtering to prevent image loss due to surface electron interaction. Sputtering was performed at a current of 30 mA for 1 min.
2.9. Analysis of Crystallinity
The arrangement and crystallinity of the CMC, powdered potato starch, starch films, and starch films reinforced with CMC/PEO nanofibers were analyzed using X-ray diffraction (XRD) with a Siemens D500 diffractometer, Dallas, TX, USA. The scanning angle (2θ) ranged from 4° to 40°, with a step size of 0.02°/s.
2.10. Thermal Analysis
To detect decomposition, sublimation, and/or absorption in the films and fibers, Thermogravimetric Analysis (TGA) was conducted using a TGA Q5000 IR (TA Instruments, New Castle, DE, USA). Samples of approximately 5 mg were heated from 30 °C to 500 °C at a rate of 5 °C/min under a nitrogen atmosphere. Differential Scanning Calorimetry (DSC) was performed using a DSC Q2000 (TA Instruments) to identify thermal transitions such as glass transition temperature (Tg), crystallization temperature (Tc), and melting temperature (Tf), also under a nitrogen atmosphere. Data were processed using the TA Instruments Universal Analysis 2000 software.
3. Results
3.1. Mechanical Testing
Table 1 presents the tensile strength and elongation properties of potato starch films, starch–CMC films, and starch films reinforced with CMC/PEO nanofibers.
The results in
Table 1 indicate that CMC/PEO nanofibers had a significant effect among the materials tested. A moderate increase in tensile strength—approximately 7 MPa—was observed compared to the starch–CMC blend.
The average maximum tensile strength was 13.6 MPa for the pure starch film and approximately 7.17 MPa for the starch–CMC films. In contrast, the starch film reinforced with nanofibers exhibited a tensile strength of 14.1 MPa.
The starch films exhibited a linear trend up to approximately 20% deformation, followed by a nonlinear response, reaching an average elongation maximum of 56.35% at a maximum stress of 13.6 MPa. Four repetitions were conducted for each film to ensure replicability and to obtain an average value.
The behavior of the starch films with CMC nanofibers is similar to that of the pure starch films. They exhibit a linear trend up to a stress of about 8 MPa, after which, the behavior becomes nonlinear, reaching rupture at approximately 14.1 MPa and 60% deformation. The tensile strength increased with the addition of CMC/PEO nanofibers, possibly because the very thin fibers (on the nanometer scale) are less likely to introduce defects into the material, resulting in greater tensile strength than pure native CMC [
22]. These fibers act as reinforcing elements for the native potato starch film.
Figure 1 illustrates the mechanical properties of the starch films, starch–CMC films, and starch–CMC/PEO nanofiber films.
Figure 1 shows the tensile strength of the tested materials. Starch exhibited a tensile strength of 13.6 MPa and a strain of 56.35%. In comparison, starch–CMC exhibited a tensile strength of 7.1 MPa and a strain above 100%, demonstrating a significant decrease in tensile strength of 6.5 MPa. Starch with CMC/PEO nanofibers had a tensile strength of 14.1 MPa, representing an increase in the strength of starch.
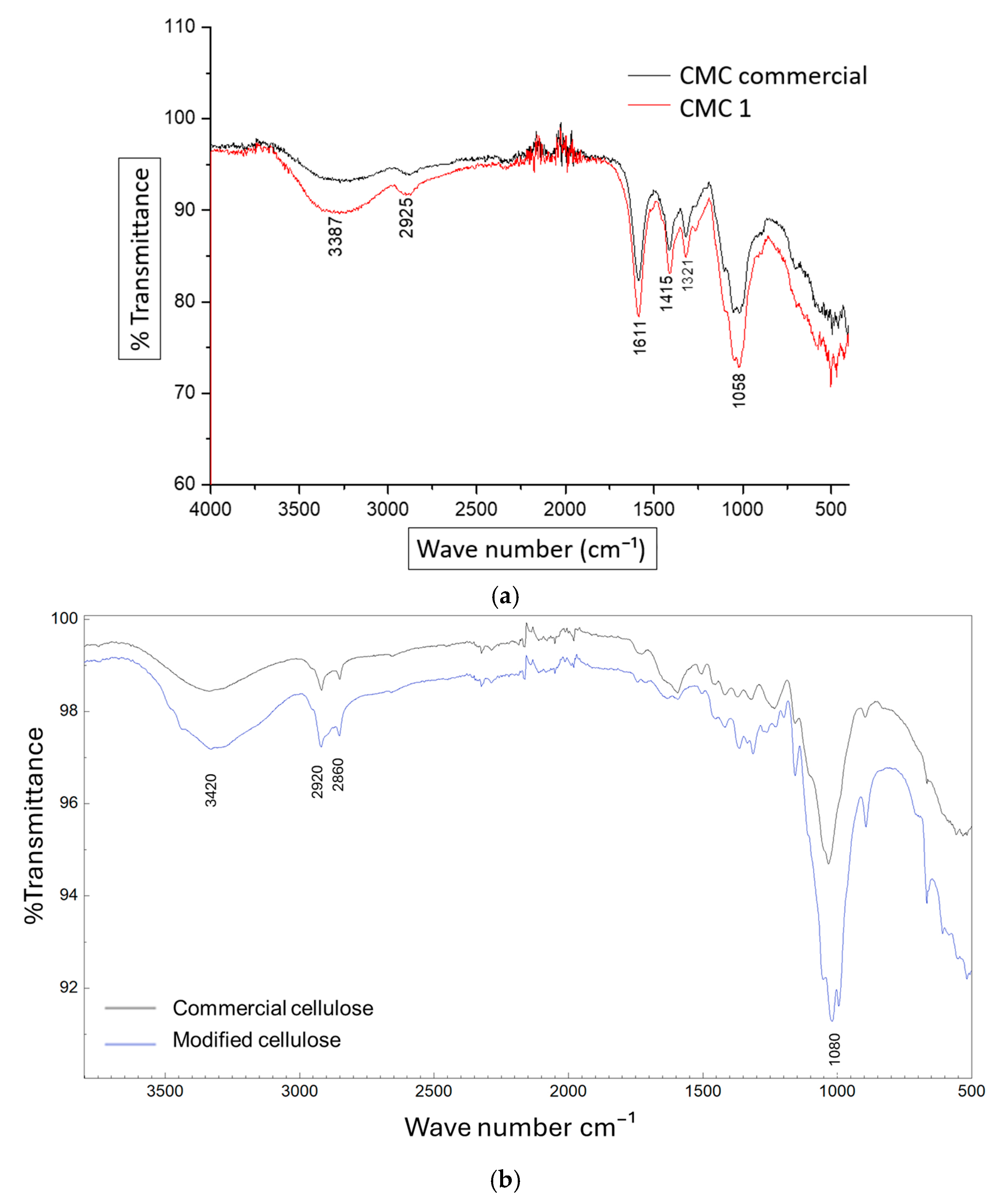
3.2. Functional Groups by FTIR
Figure 2a shows the infrared spectrum (FTIR) of CMC obtained from potato stems (red line) and commercial CMC from Sigma-Aldrich (black line). The two spectra show strong similarity to the infrared analysis reported by Cruz et al. [
21], which presents the representative peaks of the functional groups of carboxymethyl cellulose. The peak at 3387 cm
−1 corresponds to the stretching of –OH groups and intermolecular and intramolecular hydrogen bonding. The signal at 2925 cm
−1 corresponds to the stretching of the C–H group, while the signal at 1415 cm
−1 is possibly due to the scissoring motion of the –CH
2 group. The signals at 1321 cm
−1 and 1058 cm
−1 are due to the bending of the –OH groups and the stretching of the CH–O–CH
2 group, respectively. The presence of an intense absorption band at 1611 cm
−1 confirms the presence of the COO⁻ group. Based on the similarity of the two IR spectra for the synthesized CMC (a) and the commercial CMC, it can be concluded that the cellulose extraction process from potato stems and the synthesis of CMC were successfully carried out.
In
Figure 2b, the comparative FTIR spectra of commercial cellulose versus modified cellulose are shown, revealing significant differences that indicate structural alterations in the treated material. In the region between 3600 and 3000 cm
−1, associated with the stretching of hydroxyl groups (–OH), the modified cellulose shows a decrease in the intensity of the characteristic broadband, suggesting alterations in hydrogen bonding, possibly due to reduced crystallinity or the incorporation of new functional groups. In the 3000 to 2800 cm
−1 region, corresponding to aliphatic C–H stretching, a slight reduction in intensity can also be observed in the modified sample.
A notable difference appears around 1720–1740 cm−1, where an additional band is present in the spectrum of the modified cellulose but absent in the commercial cellulose. This may indicate the presence of carbonyl (C=O) groups introduced during the chemical modification process. In the region between 1500 and 1200 cm−1, variations in band shape and intensity can be observed, likely due to structural changes in glucose rings or the incorporation of new functional groups. Likewise, in the 1200 to 900 cm−1 region, corresponding to vibrations from C–O–C and C–O bonds, changes can be observed that reflect modifications in glycosidic link structures.
Finally, in the region from 900 to 500 cm−1, more subtle differences can be identified, which may be related to a decrease in structural order, indicating a reduction in the crystallinity of the modified cellulose. Taken together, these spectral differences confirm that the cellulose has undergone chemical modifications affecting both its internal structure and functional groups, resulting in chemical similarity to commercial cellulose.
3.3. Morphology by SEM
Electrospinning with a CMC/PEO solution concentration of 3% did not produce fibers on the collector; instead, a liquid residue from the solution adhered to the collector. This suggests that under these conditions, evaporation did not occur—possibly due to the high viscosity of the polymer solution, which prevented sufficient solvent evaporation during its travel from the injector to the collector. At a 5% CMC/PEO concentration, solvent discs adhered to each other on the collector, and similar to the 3% concentration, evaporation was insufficient. Therefore, an intermediate concentration of 4% was selected.
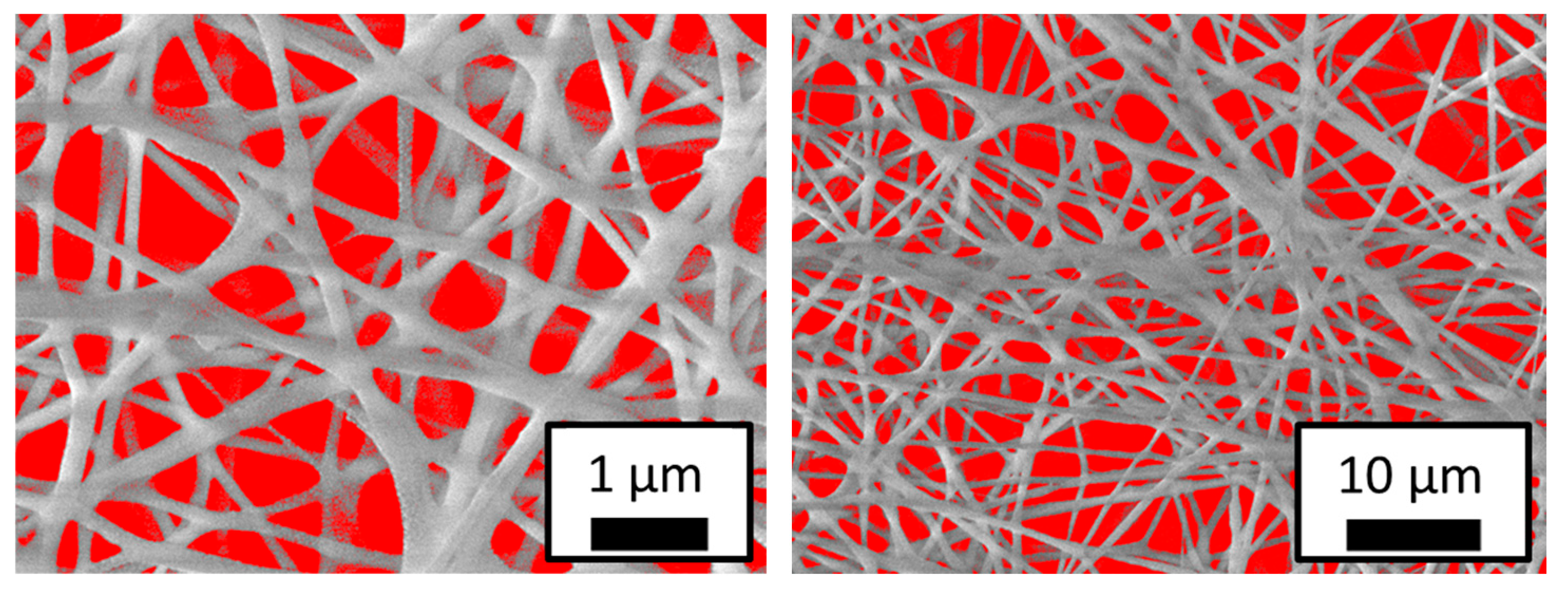
Figure 3 shows SEM micrographs of nanofibers obtained from CMC/PEO at a concentration of 4%, with an injection speed of 0.5 mL/h, an applied voltage of 20 kV, and a needle-to-collector distance of 20 cm. At voltages below 20 kV, no fibers were formed, likely because the electric field generated by the lower potential was insufficient for the solution to reach the collector. At the 4% CMC/PEO concentration,
Figure 4 shows randomly distributed fibers with uniform diameters and high porosity. No defects or droplets can be observed, indicating that these electrospinning parameters allow for a continuous process that produces membranes with high porosity and interconnected fibers.
The ImageJ V13.0.6 software was used to measure the diameters of the fibers in the SEM micrographs (
Figure 3), allowing for the calculation of fiber distribution, average diameter, and standard deviation. At a 4% CMC/PEO concentration, the average nanofiber diameter was 63.3 nm, with a standard deviation of 0.018 nm.
Figure 4 shows the estimated porosity of CMC/PEO membranes at 4%, obtained using an ImageJ function that calculates pore areas based on the color contrast between the solid fibers and the empty spaces (pores). The resulting average porosity was 32.78%.
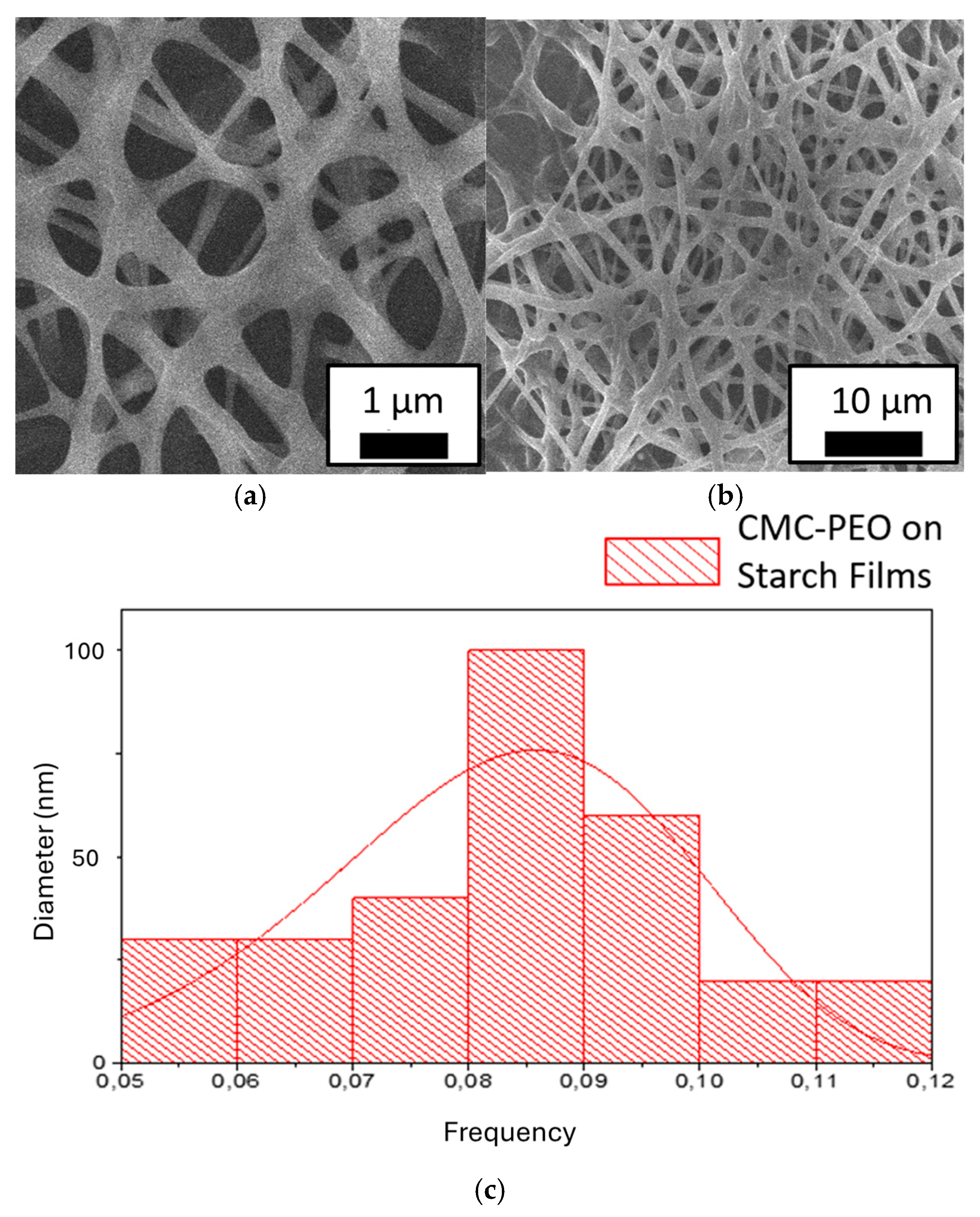
Figure 5 presents SEM micrographs of electrospun CMC/PEO composite fibers deposited on the surface of starch films. The same electrospinning parameters were used as before: 4% CMC/PEO concentration, injection rate of 0.5 mL/h, applied voltage of 20 kV, a 20 cm needle-to-collector distance, ambient temperature of 25 °C, and relative humidity of 28%. In this case, however, the collector was coated with a starch film. It can be observed that the fibers maintained their structure, despite the potato starch film on the collector potentially reducing the applied electric field.
Figure 5c shows that the fiber diameter distribution on the starch film is very similar to that of fibers deposited directly onto metallic collectors, although some degree of flattening is evident. This may be due to the starch film attenuating the electric field, as well as interactions between the polymeric fibers during deposition.
As shown in the histogram in
Figure 5c, the average diameter of the CMC/PEO nanofibers deposited on the surface of the starch films was 82 nm, with a standard deviation of 0.015 nm. This represents an increase in diameter compared to CMC/PEO fibers deposited on a copper collector (63 nm), which can be attributed to the flattening of fibers during deposition onto the starch film.
3.4. X-Ray Diffraction Analysis
The X-ray diffraction (XRD) patterns of the CMC/PEO fibers, potato starch films, and films with CMC/PEO fibers are shown in
Figure 6 The XRD spectrum of the starch film in
Figure 6 displays a semicrystalline structure, characterized by three diffraction peaks at 16.94°, 19.24°, and 21.42°, along with a broad amorphous region. The presence of these peaks indicates the plasticization of starch with glycerol, which limits molecular mobility. Additionally, the heat applied during starch processing may have disrupted the arrangement of amylopectin chains during gelatinization, thereby reducing crystallinity.
In
Figure 6, the XRD spectrum of the starch-CMC films does not show crystalline peaks, indicating a loss of crystallinity in contrast to the typical crystalline peaks observed in the initial CMC and starch. Apparently, the film processing of starch and the addition of PEO during CMC electrospinning inhibit crystallization. However, some degree of lateral chain ordering may still develop during the processing of the starch and CMC/PEO.
The XRD spectrum of CMC shows several peaks, indicating a crystalline structure, with peaks at 2θ angles of 31° and 45°. This could be associated with the degree of substitution of the glucose molecule, and the limited crystallinity may also be related to the substitution of carboxyl groups in the cellulose molecule.
The diffractogram of potato starch in
Figure 6 shows signals at 2θ angles of 15.3° and 17.18°, the latter being the most intense. Peaks at 22.35° and 14.41° are also visible, suggesting a semicrystalline structure. Starch contains crystalline regions within the branched amylopectin, while the linear amylose is largely amorphous.
3.5. Thermal Characterization
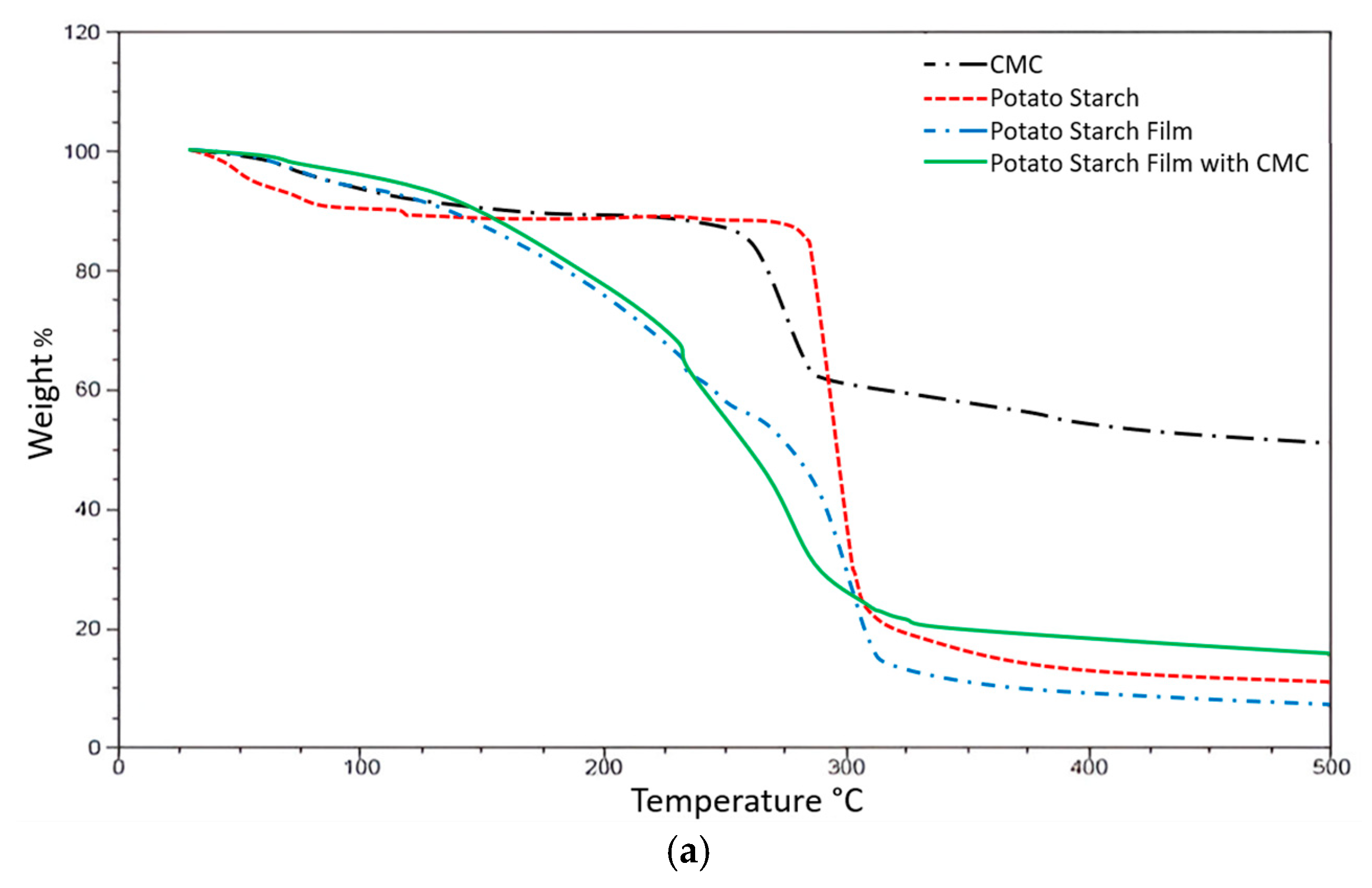
In
Figure 7a, in the starch thermogram (red dotted lines), the starch shows a degradation possibly due to the loss of water or volatile compounds at a temperature of 85.6 °C with a weight loss of 7.393%. It has a degradation transition temperature of 292.26 °C that varies according to the percentage of amylose and amylopectin in the different starches.
In
Figure 7a, the starch thermogram (red dotted lines) shows degradation likely due to the loss of water or volatile compounds at 85.6 °C, corresponding to a weight loss of 7.393%. It has a degradation transition temperature of 292.26 °C that varies according to the percentage of amylose and amylopectin in the different starches.
For CMC (black dotted lines), a weight loss can be observed at 100 °C, indicating moisture loss from the polymer. After this point, thermal stability is maintained until 260 °C, where material degradation begins and continues up to 280 °C.
In starch films, stepwise degradation can be observed, where intermediate products vaporize at temperatures lower than that of pure starch (290 °C). This behavior is typical in films containing plasticizers such as glycerol and is associated with a thermal transition at 223 °C. An initial weight loss of 11.21% can be observed, corresponding to the evaporation of volatile components such as water, followed by a second weight loss of 15.95% recorded at 223.80 °C.
Overall, the degradation profiles of the starch and CMC films are similar. However, a slight difference can be observed in films that exhibit only the lower-temperature transition. For both polymers, the transition temperature decreases with increased water content. In the case of starch films, the presence of glycerol also affects both the location of the thermal transition and the temperature at which degradation begins.
As shown in
Figure 7b, the thermogram for native starch exhibits a melting point of 93.04 °C, a lower temperature than that recorded for cassava starch at 134 °C [
9]. This endothermic peak is associated with the fusion of reorganized crystalline starch domains during retrogradation.
Carboxymethyl cellulose (red line) did not exhibit a clear melting peak; its thermal degradation occurs primarily through mass loss. During volatile pyrolysis, compounds are generated, consumed, and released. Therefore, the first step can already be associated with a phase change, observed at 62 °C.
The melting temperature (Tm) of films made from native starch mixed with CMC was 102.81 °C, which is lower than the Tm of films made from native starch alone (112.9 °C). This shift in Tm can be attributed to interactions between the two biopolymers [
10]. The area under the endothermic peak, representing the total heat of melting, decreased with the addition of CMC to the starch films. This reduction in the heat of melting is likely due to a lower degree of crystallization, possibly caused by molecular interactions between starch and CMC disrupting the rearrangement of polymer chains [
11].
These results suggest that during the gelatinization process, starch loses its crystalline structure as the molecules swell at temperatures above 80 °C.
4. Discussion
The incorporation of carboxymethyl cellulose/polyethylene oxide (CMC/PEO) nanofibers into the potato starch matrix demonstrated effective nanoscale reinforcement, with the effect manifested as a moderate improvement in tensile strength without compromising the deformability of the system. This behavior may be attributed to more efficient stress transfer between the continuous phase (starch) and the dispersed phase (nanofibers), mediated by non-covalent physicochemical interactions between the –OH and –COO functional groups of both biopolymers. The average nanofiber diameter (63.3 nm) and its uniform distribution, as observed via scanning electron microscopy (SEM), promote a high surface-to-volume ratio, which increases the effective interfacial area and, consequently, mechanical compatibility between phases. Previous studies, such as that by Ghanbarzadeh et al. [
16], confirm that the addition of cellulosic fibers can enhance both the strength and cohesion of biocomposites, consistent with the findings of this study.
From a structural perspective, X-ray diffraction (XRD) analysis revealed a reduction in crystallinity in the composite films compared to starch and CMC controls, suggesting that nanofiber inclusion induces greater molecular disorder. This phenomenon may be due to disruption in the organization of amylose and amylopectin chains during thermal gelatinization, further influenced by the presence of PEO and interactions with CMC. This crystallinity suppression effect has been previously reported in similar biocomposites and is associated with improved processability and more homogeneous thermal behavior [
21].
Thermal analysis (TGA/DSC) showed that the incorporation of nanofibers did not compromise the overall thermal stability of the material, with degradation profiles remaining within the characteristic range for biopolymers. The slight decrease in glass transition temperature (Tg) observed may be attributed to a reduction in molecular packing density, an effect linked to the amorphous nature of the nanofibers and the potential plasticizing action of PEO at the interface. Nevertheless, the observed multi-step degradation profile suggests the presence of distinct thermally active phases, which could be exploited in the design of functional materials for controlled release or specific thermal sensitivity.
Regarding mechanical performance, although a quantifiable increase in tensile strength was achieved, Young’s modulus remained largely unchanged. This observation can be explained by the viscoelastic nature of plasticized starch and the random orientation of electrospun nanofibers, which limits their contribution to longitudinal reinforcement. The directed fiber orientation or surface functionalization of the fibers could enhance this property in future studies. Nonetheless, the optimized interface, structural orientation, and homogeneity of fiber dispersion remain critical factors in research, especially for scaling this material for real-world applications in sustainable packaging or other emerging technologies.
5. Conclusions
The results obtained from the tensile tests confirmed that the pure starch films reached 13.6 MPa, and the CMC/PEO-reinforced films showed a slightly higher average tensile strength of 14.1 MPa; therefore, the addition of nanofibers did not have a negative impact on mechanical performance.
FTIR spectroscopy also demonstrated that carboxymethyl cellulose (CMC) can be successfully extracted from the modified stems of Pastusa potato plants, as evidenced by the strong correlation between the FTIR spectra of commercial CMC and those extracted from potato stems. Additionally, scanning electron microscopy (SEM) confirmed the formation of membranes composed of CMC fibers derived from potato stems and PEO, with nanometric diameters of 63.3 ± 1 nm. These membranes achieved a porosity of 32.78% using the electrospinning technique—an effective and widely used method for producing nanofibers with a high surface-to-volume ratio, which enhances both mechanical and functional properties.
An experimental study on electrospinning parameter variations revealed that injection speed, applied voltage, needle-to-collector distance, and solution concentration significantly affect nanofiber morphology. Nanofibers with a diameter of 63.3 nm were obtained under optimal conditions: an applied voltage of 20 kV, a needle-to-collector distance of 20 cm, and an injection rate of 0.5 mL/h.
It can be concluded that a composite material based on native potato starch from Boyacá, reinforced with electrospun CMC nanofibers extracted from potato stems and PEO, can be successfully prepared. This demonstrates the potential for generating materials from natural inputs, such as starch and potato stems. Furthermore, improved mechanical properties were observed in the composite material containing CMC/PEO nanofibers compared to the CMC–potato starch blend. These findings suggest the potential of this material for packaging applications, combining the biodegradable nature of natural sources with the desirable chemical and mechanical characteristics required for such uses.
This work presents a promising alternative for the utilization of agricultural waste, contributing to the generation of biopolymers from renewable resources like starch and cellulose. This approach supports environmental sustainability by reducing plastic waste and offering an alternative to non-renewable, petroleum-based plastics.