Investigation of the Polyphenol Recovery of Overripe Banana Peel Extract Utilizing Cloud Point Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Chemicals and Reagents
2.2. CPE Procedure
2.3. Polyphenol Recovery
2.4. Quantification of Total Polyphenol Content
2.5. Evaluation of Total Flavonoid Content (TFC)
2.6. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.7. Evaluation of Antiradical Activity (DPPH• Assay)
2.8. HPLC–DAD Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Optimization of the CPE Procedure
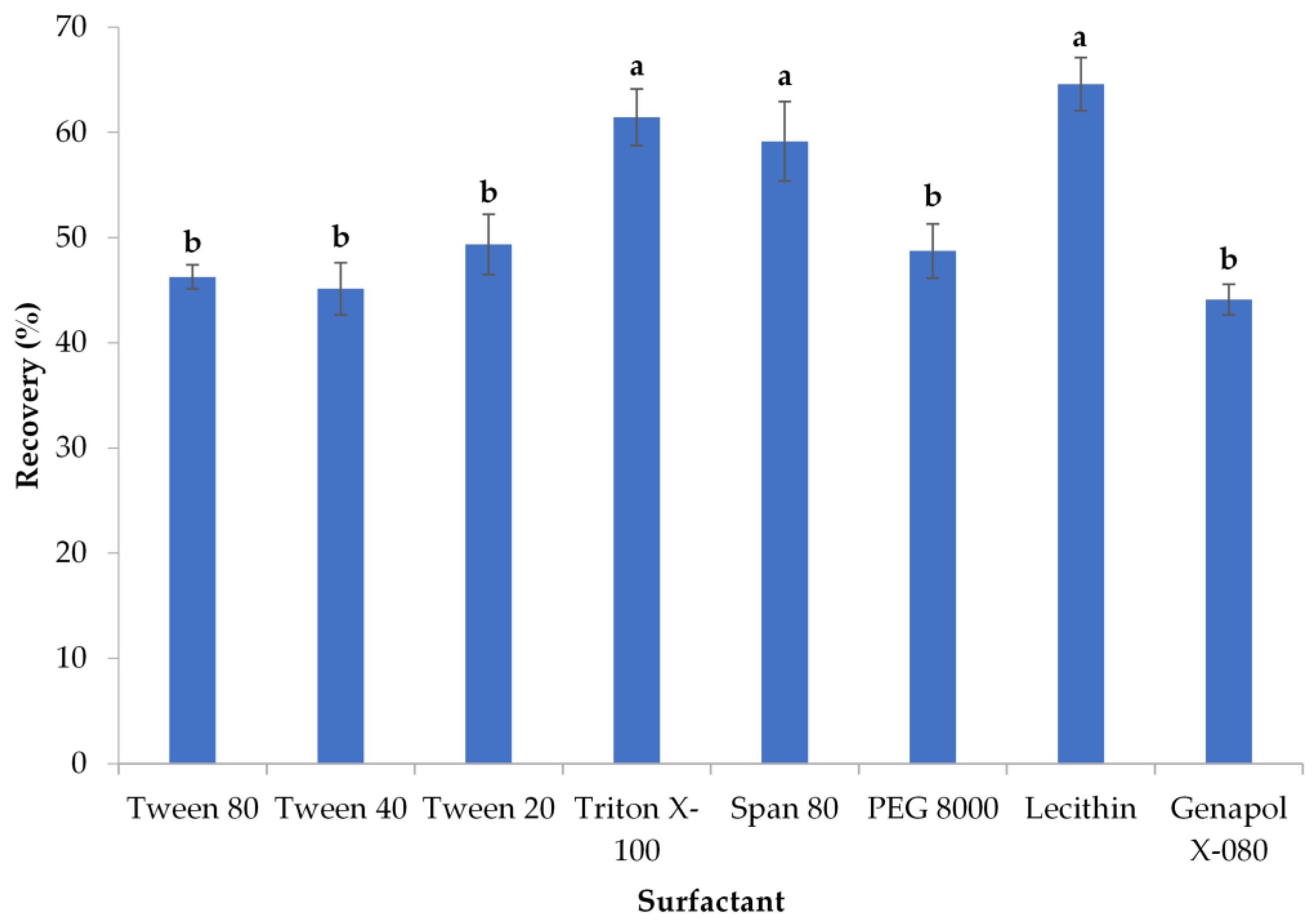
3.1.1. Surfactant Selection
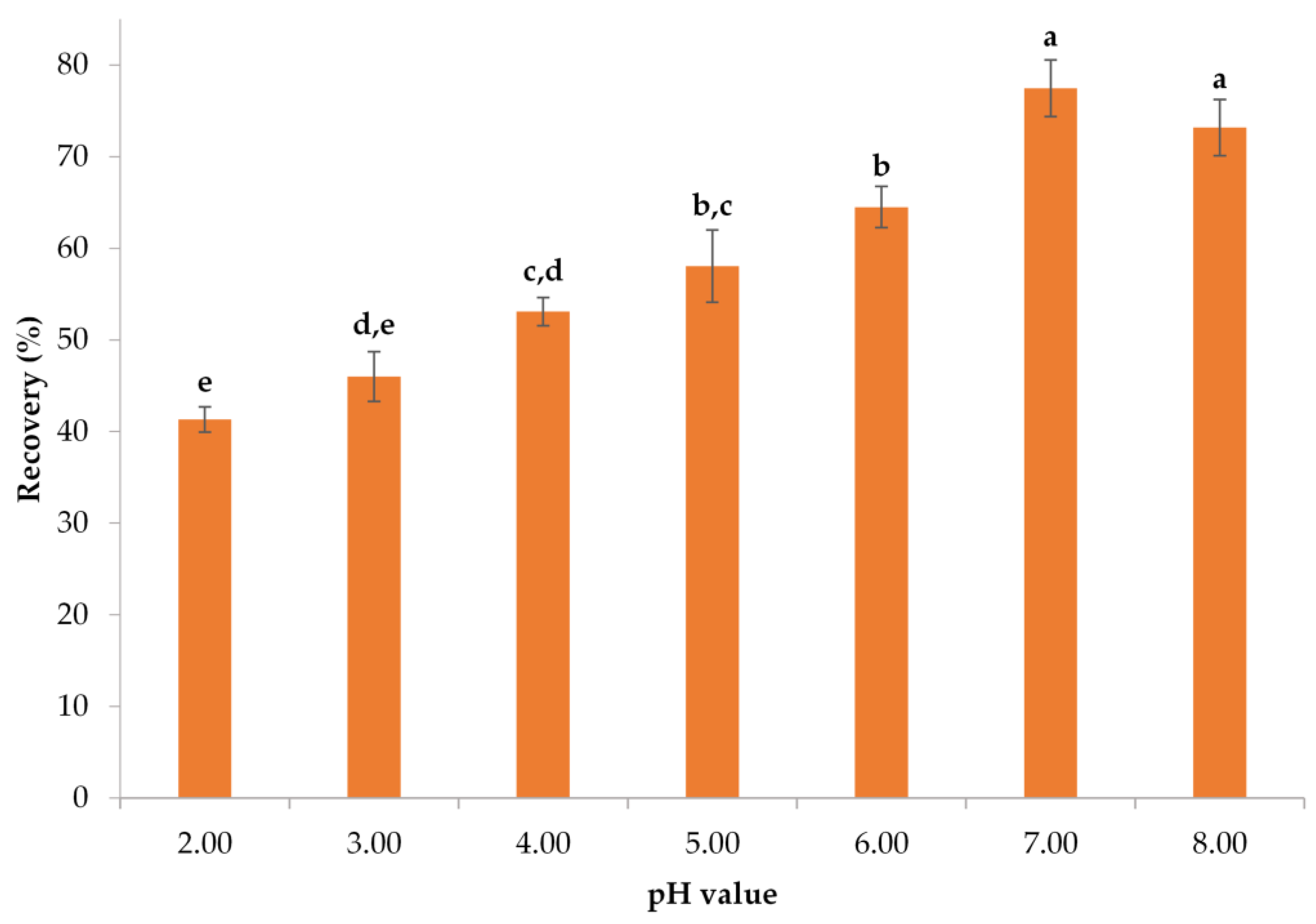
3.1.2. Determination of the Optimal pH
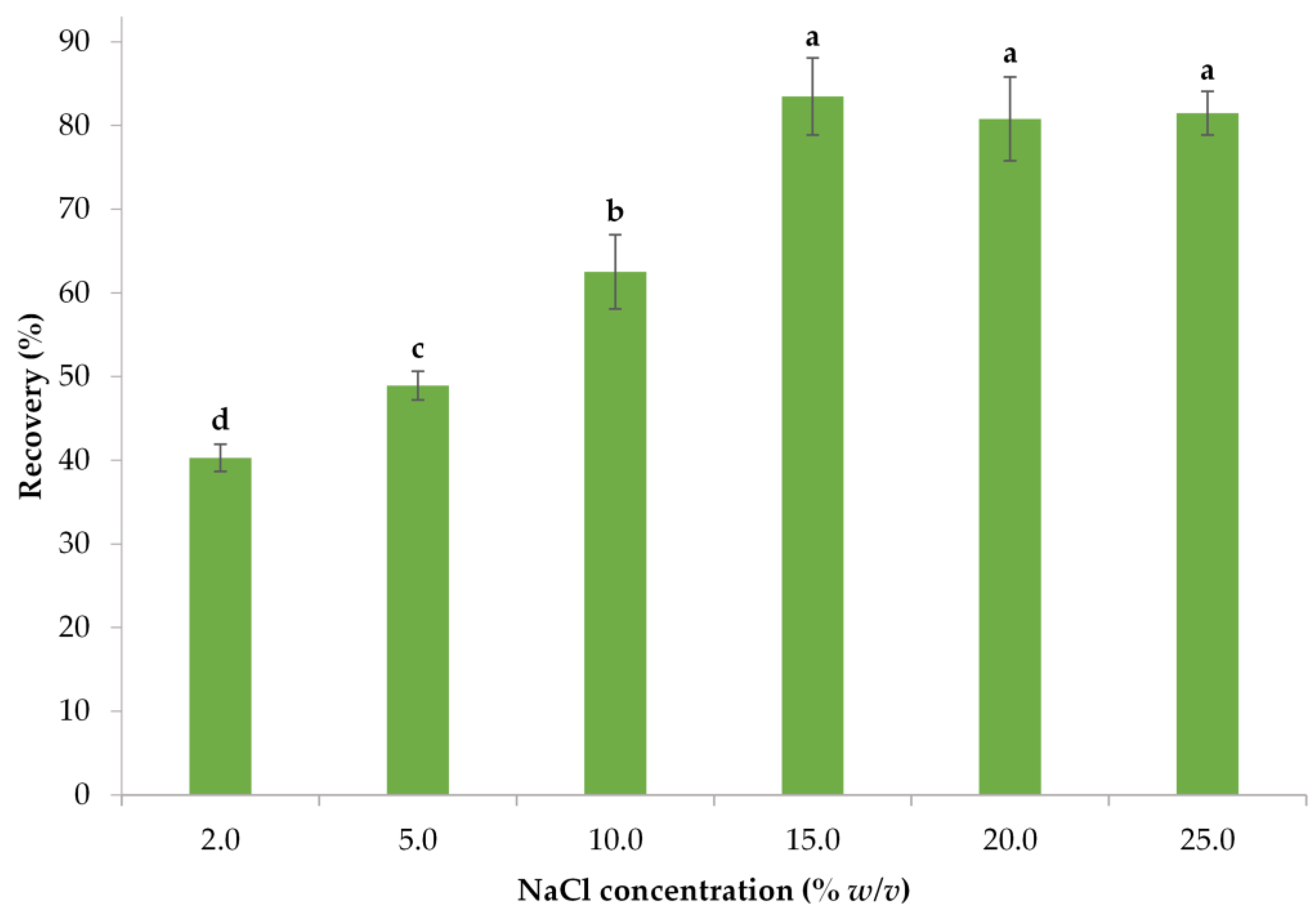
3.1.3. Determination of the Optimal Salt Concentration
3.1.4. Determination of the Optimal Surfactant Concentration
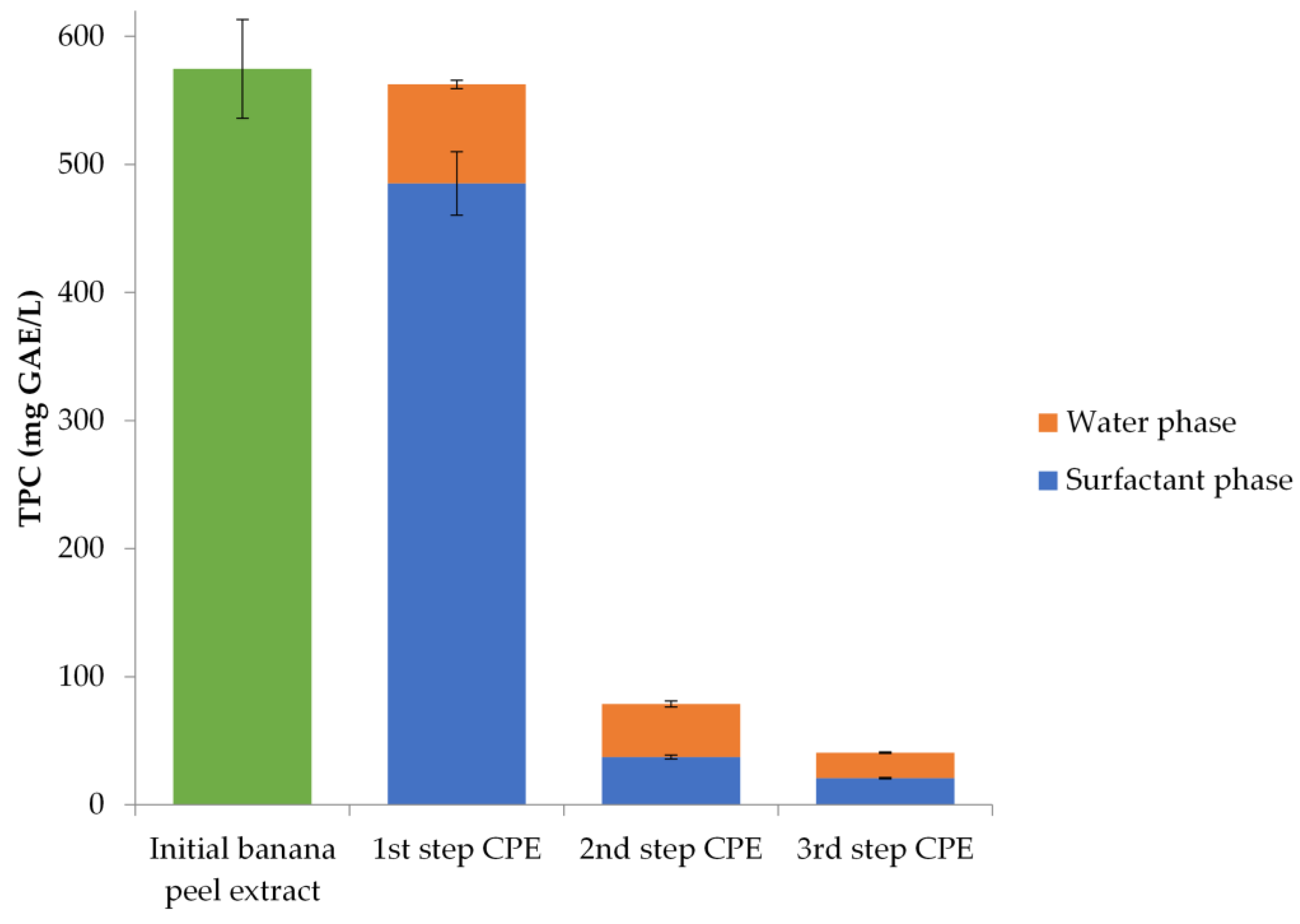
3.2. Analysis of the Optimal Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathew, N.S.; Negi, P.S. Traditional Uses, Phytochemistry and Pharmacology of Wild Banana (Musa acuminata Colla): A Review. J. Ethnopharmacol. 2017, 196, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zaini, H.; Roslan, J.; Saallah, S.; Munsu, E.; Sulaiman, N.S.; Pindi, W. Banana Peels as a Bioactive Ingredient and Its Potential Application in the Food Industry. J. Funct. Foods 2022, 92, 105054. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Phenolic Compounds within Banana Peel and Their Potential Uses: A Review. J. Funct. Foods 2018, 40, 238–248. [Google Scholar] [CrossRef]
- Khamsucharit, P.; Laohaphatanalert, K.; Gavinlertvatana, P.; Sriroth, K.; Sangseethong, K. Characterization of Pectin Extracted from Banana Peels of Different Varieties. Food Sci. Biotechnol. 2018, 27, 623–629. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Changes of Phytochemicals and Antioxidant Capacity of Banana Peel during the Ripening Process; with and without Ethylene Treatment. Sci. Hortic. 2019, 253, 255–262. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Jiang, W. Development of Antioxidant Chitosan Film with Banana Peels Extract and Its Application as Coating in Maintaining the Storage Quality of Apple. Int. J. Biol. Macromol. 2020, 154, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.N.A.; Pasquali, M.A.d.B.; Schnorr, C.E.; Martins, J.J.A.; de Araújo, G.T.; Rocha, A.P.T. Development and Characterization of Blends Formulated with Banana Peel and Banana Pulp for the Production of Blends Powders Rich in Antioxidant Properties. J. Food Sci. Technol. 2019, 56, 5289–5297. [Google Scholar] [CrossRef]
- Al-Sahlany, S.T.G.; Al-musafer, A.M.S. Effect of Substitution Percentage of Banana Peels Flour in Chemical Composition, Rheological Characteristics of Wheat Flour and the Viability of Yeast during Dough Time. J. Saudi Soc. Agric. Sci. 2020, 19, 87–91. [Google Scholar] [CrossRef]
- Serna-Jiménez, J.A.; Luna-Lama, F.; Caballero, Á.; Martín, M.D.L.Á.; Chica, A.F.; Siles, J.Á. Valorisation of Banana Peel Waste as a Precursor Material for Different Renewable Energy Systems. Biomass Bioenergy 2021, 155, 106279. [Google Scholar] [CrossRef]
- Shafi, A.; Ahmad, F.; Mohammad, Z.H. Effect of the Addition of Banana Peel Flour on the Shelf Life and Antioxidant Properties of Cookies. ACS Food Sci. Technol. 2022, 2, 1355–1363. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Schmidt, E.M.; Bonafe, E.G.; Eberlin, M.N.; Sawaya, A.C.H.F.; Visentainer, J.V. Antioxidant Activity, Phenolics and UPLC–ESI(–)–MS of Extracts from Different Tropical Fruits Parts and Processed Peels. Food Res. Int. 2015, 77, 392–399. [Google Scholar] [CrossRef]
- Bashmil, Y.M.; Ali, A.; Bk, A.; Dunshea, F.R.; Suleria, H.A.R. Screening and Characterization of Phenolic Compounds from Australian Grown Bananas and Their Antioxidant Capacity. Antioxidants 2021, 10, 1521. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, L.; Dorta, E.; Gloria Lobo, M.; González-Mendoza, L.A.; Díaz, C.; González, M. Use of Banana (Musa acuminata Colla AAA) Peel Extract as an Antioxidant Source in Orange Juices. Plant Foods Hum. Nutr. 2017, 72, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Du, K.; Li, J.; Bai, Y.; An, M.; Tan, Z.; Chang, Y. A Green and Efficient Method for the Preconcentration and Determination of Gallic Acid, Bergenin, Quercitrin, and Embelin from Ardisia japonica Using Nononic Surfactant Genapol X-080 as the Extraction Solvent. Int. J. Anal. Chem. 2018, 2018, 1707853. [Google Scholar] [CrossRef] [PubMed]
- Al_Saadi, M.R.; Al-Garawi, Z.S.; Thani, M.Z. Promising Technique, Cloud Point Extraction: Technology & Applications. J. Phys. Conf. Ser. 2021, 1853, 012064. [Google Scholar] [CrossRef]
- Carabias-Martínez, R.; Rodríguez-Gonzalo, E.; Moreno-Cordero, B.; Pérez-Pavón, J.L.; García-Pinto, C.; Fernández Laespada, E. Surfactant Cloud Point Extraction and Preconcentration of Organic Compounds Prior to Chromatography and Capillary Electrophoresis. J. Chromatogr. A 2000, 902, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Mortada, W.I.; Hassanien, M.M.; El-Asmy, A.A. Cloud Point Extraction of Some Precious Metals Using Triton X-114 and a Thioamide Derivative with a Salting-out Effect. Egypt. J. Basic Appl. Sci. 2014, 1, 184–191. [Google Scholar] [CrossRef]
- Pytlakowska, K.; Kozik, V.; Dabioch, M. Complex-Forming Organic Ligands in Cloud-Point Extraction of Metal Ions: A Review. Talanta 2013, 110, 202–228. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.S.; Kaimal, A.M.; Chib, M.; Sonawane, S.K.; Show, P.L. Novel, Energy Efficient and Green Cloud Point Extraction: Technology and Applications in Food Processing. J. Food Sci. Technol. 2019, 56, 524–534. [Google Scholar] [CrossRef]
- Jie, Y.; Chen, F. Progress in the Application of Food-Grade Emulsions. Foods 2022, 11, 2883. [Google Scholar] [CrossRef]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Isolation of Polyphenols from Two Waste Streams of Clingstone Peach Canneries Utilizing the Cloud Point Extraction Method. Biomass 2023, 3, 291–305. [Google Scholar] [CrossRef]
- Mortada, W.I. Recent Developments and Applications of Cloud Point Extraction: A Critical Review. Microchem. J. 2020, 157, 105055. [Google Scholar] [CrossRef]
- Kiai, H.; Raiti, J.; El-Abbassi, A.; Hafidi, A. Recovery of Phenolic Compounds from Table Olive Processing Wastewaters Using Cloud Point Extraction Method. J. Environ. Chem. Eng. 2018, 6, 1569–1575. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A Reproducible, Rapid and Inexpensive Folin–Ciocalteu Micro-Method in Determining Phenolics of Plant Methanol Extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Manousaki, A.; Jancheva, M.; Grigorakis, S.; Makris, D.P. Extraction of Antioxidant Phenolics from Agri-Food Waste Biomass Using a Newly Designed Glycerol-Based Natural Low-Transition Temperature Mixture: A Comparison with Conventional Eco-Friendly Solvents. Recycling 2016, 1, 194–204. [Google Scholar] [CrossRef]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction Optimisation Using Water/Glycerol for the Efficient Recovery of Polyphenolic Antioxidants from Two Artemisia Species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Implementation of Cloud Point Extraction Using Surfactants in the Recovery of Polyphenols from Apricot Cannery Waste. Eng 2023, 4, 1225–1235. [Google Scholar] [CrossRef]
- Alibade, A.; Batra, G.; Bozinou, E.; Salakidou, C.; Lalas, S. Optimization of the Extraction of Antioxidants from Winery Wastes Using Cloud Point Extraction and a Surfactant of Natural Origin (Lecithin). Chem. Pap. 2020, 74, 4517–4524. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Voulgaris, A.; Katsoulis, K.; Lalas, S.I.; Roussis, I.G.; Gortzi, O. Development of Enriched Oil with Polyphenols Extracted from Olive Mill Wastewater. Foods 2023, 12, 497. [Google Scholar] [CrossRef]
- Karadag, A.; Kayacan Cakmakoglu, S.; Metin Yildirim, R.; Karasu, S.; Avci, E.; Ozer, H.; Sagdic, O. Enrichment of Lecithin with Phenolics from Olive Mill Wastewater by Cloud Point Extraction and Its Application in Vegan Salad Dressing. J. Food Process. Preserv. 2022, 46, e16645. [Google Scholar] [CrossRef]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Development of a Cloud Point Extraction Technique Based on Lecithin for the Recovery of Carotenoids from Liquid Tomato Wastewater. Waste 2022, 1, 105–114. [Google Scholar] [CrossRef]
- Zain, N.N.M.; Abu Bakar, N.K.; Mohamad, S.; Saleh, N.M. Optimization of a Greener Method for Removal Phenol Species by Cloud Point Extraction and Spectrophotometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Sosa Ferrera, Z.; Padrón Sanz, C.; Mahugo Santana, C.; Santana Rodríguez, J.J. The Use of Micellar Systems in the Extraction and Pre-Concentration of Organic Pollutants in Environmental Samples. TrAC Trends Anal. Chem. 2004, 23, 469–479. [Google Scholar] [CrossRef]
- Chatzimitakos, T.G.; Pierson, S.A.; Anderson, J.L.; Stalikas, C.D. Enhanced Magnetic Ionic Liquid-Based Dispersive Liquid-Liquid Microextraction of Triazines and Sulfonamides through a One-Pot, pH-Modulated Approach. J. Chromatogr. A 2018, 1571, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Chatzimitakos, T.G.; Stalikas, C.D. Melamine Sponge Decorated with Copper Sheets as a Material with Outstanding Properties for Microextraction of Sulfonamides Prior to Their Determination by High-Performance Liquid Chromatography. J. Chromatogr. A 2018, 1554, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Santalad, A.; Burakham, R.; Srijaranai, S.; Srijaranai, S.; Deming, R.L. Role of Different Salts on Cloud-Point Extraction of Isoprocarb and Promecarb Insecticides Followed by High-Performance Liquid Chromatography. J. Chromatogr. Sci. 2012, 50, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Mori, M.; Itabashi, H. Cloud Point Extraction of Cu(II) Using a Mixture of Triton X-100 and Dithizone with a Salting-out Effect and Its Application to Visual Determination. Talanta 2013, 117, 376–381. [Google Scholar] [CrossRef]
- Sun, F.; Ning, J.; Wang, Y.; Shi, Y.; Li, J.; Li, H.; Li, W. Optimization of Ultrasound-Assisted Cloud Point Extraction of Polyphenols from Pomegranate Peels. J. Biotech Res. 2023, 14, 160–170. [Google Scholar]
- Ji, Y.; Wu, L.; Lv, R.; Wang, H.; Song, S.; Cao, M. Facile Cloud Point Extraction for the Separation and Determination of Phenolic Acids from Dandelion. ACS Omega 2021, 6, 13508–13515. [Google Scholar] [CrossRef]
- Wang, M.; Yan, W.; Zhou, Y.; Fan, L.; Liu, Y.; Li, J. Progress in the Application of Lecithins in Water-in-Oil Emulsions. Trends Food Sci. Technol. 2021, 118, 388–398. [Google Scholar] [CrossRef]
- Toh, P.Y.; Leong, F.S.; Chang, S.K.; Khoo, H.E.; Yim, H.S. Optimization of Extraction Parameters on the Antioxidant Properties of Banana Waste. Acta Sci. Pol. Technol. Aliment. 2016, 15, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, Ö.F.; Nogay, G.; Kafkas, E.; Kafkas, S. Some Fruit Quality Characteristics of ‘Grand Naine’ Banana Fruits during Various Ripening Stages. Int. J. Agric. For. Life Sci. 2022, 6, 24–27. [Google Scholar]
- Rodríguez-Solana, R.; Dantas, M.; Romano, A. Influence of Carob Pod (Ceratonia siliqua L.) Variety and Processing on the Antioxidant Capacity and Total Phenolic Content of Carob Liquors. In Proceedings of the INCREaSE; Mortal, A., Aníbal, J., Monteiro, J., Sequeira, C., Semião, J., Moreira da Silva, M., Oliveira, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 216–226. [Google Scholar]
- Pramote, B.; Waranuch, N.; Kritsunankul, O. Simultaneous Determination of Gallic Acid and Catechins in Banana Peel Extract by Reversed-Phase High Performance Liquid Chromatography. Naresuan Univ. J. Sci. Technol. NUJST 2018, 26, 189–200. [Google Scholar] [CrossRef]
- Zorić, Z.; Markić, J.; Pedisić, S.; Bučević-Popović, V.; Generalić-Mekinić, I.; Grebenar, K.; Kulišić-Bilušić, T. Stability of Rosmarinic Acid in Aqueous Extracts from Different Lamiaceae Species after in Vitro Digestion with Human Gastrointestinal Enzymes. Food Technol. Biotechnol. 2016, 54, 97–102. [Google Scholar] [CrossRef]
- Hernanz, D.; Recamales, Á.F.; González-Miret, M.L.; Gómez-Míguez, M.J.; Vicario, I.M.; Heredia, F.J. Phenolic Composition of White Wines with a Prefermentative Maceration at Experimental and Industrial Scale. J. Food Eng. 2007, 80, 327–335. [Google Scholar] [CrossRef]
- Mascarin, L.G.; Franco, F.W.; Dornelles, R.C.; Figueredo, K.C.; Santos, R.O.; Bauermann, L.d.F.; Emanuelli, T.; Somacal, S.; Sautter, C.K. Effect of Adding Matricaria recutita L., Cymbopogon citratus, or Mentha piperita L. Extracts to Fermented Orange Beverage: Sensory Evaluation, Physicochemical Characterization, and Prediction of Toxic Risks and Biological Activity In Silico. Foods 2023, 12, 243. [Google Scholar] [CrossRef]

| Parameters | Initial Banana Peel Extract | Optimal Total SP |
|---|---|---|
| TPC (mg GAE/L) | 574.86 ± 12.07 a | 541.25 ± 21.19 a |
| TFC (mg RtE/L) | 246.44 ± 16.76 a | 226.38 ± 12.46 a |
| FRAP (mmol AAE/L) | 2.63 ± 0.08 a | 2.52 ± 0.13 a |
| DPPH (mmol AAE/L) | 3.17 ± 0.1 a | 2.91 ± 0.16 a |
| Polyphenolic compounds (mg/L) | ||
| Neochlorogenic acid | 2.12 ± 0.11 a | 1.61 ± 0.07 b |
| Catechin | 387.94 ± 24.05 a | 371.58 ± 13.01 a |
| Chlorogenic acid | 16.44 ± 0.54 a | 9.23 ± 0.37 b |
| Epicatechin | 2.56 ± 0.13 a | 2.42 ± 0.08 a |
| Rutin | 24.01 ± 0.6 a | 17.09 ± 0.68 b |
| Ferulic acid | 12.17 ± 0.88 a | 6.29 ± 0.41 b |
| Quercetin 3-D-galactoside | 17.93 ± 1.27 a | 17.93 ± 1.22 a |
| Narirutin | 9.58 ± 0.24 a | 8.26 ± 0.51 b |
| Rosmarinic acid | 51.08 ± 3.73 a | 48.89 ± 3.08 a |
| Total identified | 523.83 ± 31.55 a | 483.3 ± 19.42 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanasiadis, V.; Chatzimitakos, T.; Mantiniotou, M.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Investigation of the Polyphenol Recovery of Overripe Banana Peel Extract Utilizing Cloud Point Extraction. Eng 2023, 4, 3026-3038. https://doi.org/10.3390/eng4040170
Athanasiadis V, Chatzimitakos T, Mantiniotou M, Kalompatsios D, Bozinou E, Lalas SI. Investigation of the Polyphenol Recovery of Overripe Banana Peel Extract Utilizing Cloud Point Extraction. Eng. 2023; 4(4):3026-3038. https://doi.org/10.3390/eng4040170
Chicago/Turabian StyleAthanasiadis, Vassilis, Theodoros Chatzimitakos, Martha Mantiniotou, Dimitrios Kalompatsios, Eleni Bozinou, and Stavros I. Lalas. 2023. "Investigation of the Polyphenol Recovery of Overripe Banana Peel Extract Utilizing Cloud Point Extraction" Eng 4, no. 4: 3026-3038. https://doi.org/10.3390/eng4040170
APA StyleAthanasiadis, V., Chatzimitakos, T., Mantiniotou, M., Kalompatsios, D., Bozinou, E., & Lalas, S. I. (2023). Investigation of the Polyphenol Recovery of Overripe Banana Peel Extract Utilizing Cloud Point Extraction. Eng, 4(4), 3026-3038. https://doi.org/10.3390/eng4040170











