Abstract
When the coronavirus disease 2019 (COVID-19) pandemic spread globally, wearing face masks in public became one of the most effective protective measures. Subsequently, due to the increasing demand for face masks, researchers explored feasible approaches to optimize their efficiency. The outcome of this research provides insights into the adsorption performance of four commercial face mask filter inserts with activated carbon layers (ACL). The results showed that the ACL of the inserts has very low Brunauer, Emmett, and Teller (BET) surface areas (2–33 m2/g) and low carbon percentages (19–31%). Physical adsorption analysis revealed non-uniform micropore size distributions in all samples. In addition, non-woven structures were obtained through scanning electron microscopy (SEM). The experimental outcomes show that the ACL in the purchased face masks is low-quality adsorbent and not pure carbonaceous material; polymeric components may be predominant.
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has spread across the globe. As of May 2021, the World Health Organization (WHO) has reported that more than 163 million people have been infected, and over 3 million deaths have been caused by the virus [1]. The rapid rise in the number of fatalities has made it essential to practice good hygiene measures such as wearing masks, avoiding large crowds, and regularly washing hands in order to help prevent the spread of this serious illness [2].
The coronavirus is mainly spread through the transmission of respiratory droplets produced by an infected person’s coughs or sneezes. This means that anyone in close contact with an infected individual is at risk of being exposed to these droplets [3,4]. As such, it is recommended by health institutes and relevant guidelines to wear a face mask as a preventive measure [5]. In fact, wearing face masks has become a mandatory action for governments worldwide [6]. N95 face masks are mainly used since they can filter particles down to 0.3 microns [7]. Other masks that are similar to N95 masks include KN95 (China), Face Filtering Piece (FFP) (Europe), and P-2 (Australia and New Zealand) [8]. Due to the shortage of certified face masks, various commercial face mask options have been produced that use different materials to protect against the coronavirus [9]. These include reusable cotton or handmade masks, activated carbon masks, and face shields [10]. However, it is important to investigate the quality of these masks, as Wang et al. found that approximately half of the face masks on the market were invalid [11].
Even though the N95 mask is known to filter viruses, dust, and hazardous gases, masks with an activated carbon layer may improve filtration efficiency [12]. While these masks are generally adequate, they may have limitations in protecting against certain gases, toxic vapors, and very tiny pathogens at higher breathing rates [13]. Sim et al. found that an activated carbon fiber (ACF) filter had an antibacterial efficiency of more than 90% [14]. Additionally, electrostatic attraction forces between the viruses and the carbon structure have been found to cause the adsorption of viruses by the activated carbon (AC) [8]. Generally, AC with a high surface area (>1000 m2/g) is effective at removing viruses, bacteria, VOCs, odors, and other gaseous pollutants from the air [15,16,17]. Matsushita et al. showed that AC with 20–50 nm pores is highly efficient for adsorbing viruses [18], making AC a valuable solution in the fight against COVID-19 [19].
The AC pore structure and carbon percentage are directly related to the adsorption properties and their applications [20]. Consequently, it is essential to choose the appropriate AC pore size for the correct application. For instance, if an AC sample has microporous structures (<2 nm), it will not be able to efficiently adsorb gas molecules of mesoporous size (2–50 nm), such as those of the coronavirus, which are about 60–140 nm [21]. To accurately determine the adsorption capacity of AC, it is necessary to investigate its isotherm plot, surface area, and pore size distribution [22,23].
Some commercial masks have been produced with a filter pocket for a replaceable filter containing an activated carbon layer (ACL) insertion. While some studies have been conducted on the filtration efficiency of face masks and the influence of pore morphology, size, and distribution on the adsorbent materials, there has been limited research done on the surface characteristics of face mask replaceable filter inserts for virus removal [10,17,20,21,22]. Thus, this study seeks to ascertain whether the commercial masks with ACL are a better option than the regular masks by focusing on the physical adsorption efficiency of activated carbon layers purchased from multiple manufacturers in the commercial replaceable filter inserts.
2. Materials and Methods
2.1. Materials
Four commercially available face mask filter inserts (as depicted in Figure 1) featuring an ACL were tested in this study, having been procured from different manufacturers. As a result, filter 1 exhibited a distinct appearance when compared to the other three.
Figure 1.
Four types of face mask inserts with an activated carbon layer were used in this study. Each picture represents a different manufacturer’s filter insert.
Figure 2 shows the ACL of each filter. The inside activated carbon layers of face masks were investigated, and it was found that each filter had only one layer of activated carbon except ACL3, which had two layers.
Figure 2.
Activated carbon layer/layers of each face mask insert.
2.2. Characterization of Physical Adsorption Characteristics of Activated Carbon Layers (ACL)
2.2.1. Surface Characteristics
Analyzing the surface area of an adsorbent is essential since its adsorption capacity largely depends on it [20]. To acquire crucial information about the adsorption process, an N2 adsorption isotherm is commonly used to determine the surface area, pore volume, and pore size distribution of the adsorbent [24,25,26]. It is commonly used for surface analyses [27,28,29,30]. Analyzing the pore size distribution of AC is necessary because the difference in pore size affects the adsorption capacity [31]. To this end, the N2 adsorption isotherm, Brunauer, Emmett, and Teller (BET) surface area, and pore size distribution of the adsorbent were analyzed using the Micromeritics 3 Flex-Accelerated Surface Area and Porosimetry Analyzer (Micromeritics Corp., Norcross, GA, USA) at 77 K. Before the N2 analysis, the dry mass of the sample was calculated by subtracting the weight of the empty sample cell from that of the sample cell. Each sample was regenerated at 120 °C for a duration of more than 12 h, followed by an in-situ degassing process at the same temperature for 1 h, which successfully removed adsorbed contaminants. Subsequently, the pore size distribution of each sample was estimated using density functional theory (DFT), and the analysis was repeated three times (n = 3) for each sample.
2.2.2. Proximate Analysis
Proximate analysis was used to determine the materials’ moisture, volatile matter, ash, and fixed carbon content based on their respective weights [32]. If the moisture content is high, the adsorption of AC will be low [33]. Volatile matter content indicates the organic compounds, and ash content shows the amount of inorganic substituents in the AC [34]. High ash content is undesirable due to its associated low adsorption capacity. Activated carbon with low ash and moisture content is thus more desirable for adsorption studies. Activated carbon with low ash and moisture content is an attractive candidate for adsorption studies. Moisture, ash, and volatile matter content were measured by ASTM D2867-14, ASTM D2866-94, and ASTM D5832-98, respectively [35,36,37]. Fixed carbon refers to carbon content and is reported by subtracting the moisture content, volatile matter content, and ash content from 100% [32].
2.2.3. Scanning Electron Microscope (SEM) Analysis
The surface morphology of each ACL was investigated using an FEI Quanta 250. The SEM analysis was operated at an accelerating potential of 10 kV. Before the analysis, the AC samples were mounted onto an aluminum stub using carbon tape.
3. Results and Discussion
3.1. Surface Characteristics
3.1.1. Adsorption Isotherms
The adsorption isotherms of ACL have been investigated using a nitrogen (N2) adsorption analysis technique, a standard method [38]. Figure 3 represents the N2 adsorption isotherms at 77 K of the ACL, which were found to be Type I isotherms according to the International Union of Pure and Applied Chemistry (IUPAC) criteria [39]. Georgin et al. have also reported that their AC samples are Type I isotherms by using the IUPAC consideration [40].
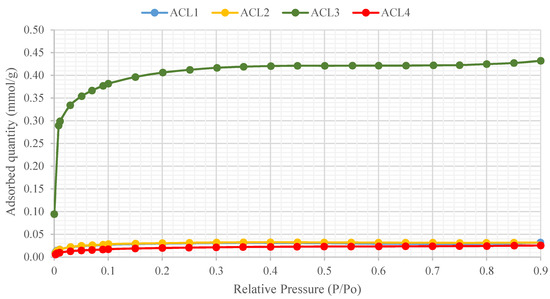
Figure 3.
N2 adsorption isotherms of ACL.
The rapid rise of N2 adsorption over a low range of relative pressure verifies the presence of microspores for all the samples, as illustrated in Figure 3. It is apparent that ACL4 has the lowest N2 adsorption, whereas the adsorption isotherms of ACL1 and ACL2 overlap and ACL3 has the highest N2 adsorption. This variability in data may be attributed to the different precursor materials used. Moreover, except for ACL3, most of the pore volumes of the ACLs are filled below P/Po ≈ 0.1. The pore volumes are filled below P/Po ≈ 0.3 for ACL3. After an initial increase to 0.1, the isotherms gradually bend, indicating smaller increments in further adsorption. As the relative pressure increases, the adsorption of all samples becomes almost horizontal, which indicates that saturation has been reached.
3.1.2. Surface Area, Pore Area, and Pore Size
The Brunauer–Emmett–Teller (BET) method was used to determine surface areas [41]. The BET surface area, pore area, and pore size of each ACL are presented in Table 1 (n = 3).

Table 1.
Physical adsorption properties of ACL.
The results indicate that ACL4 has the lowest surface area (1.91 ± 0.1 m2/g), ACL1 and ACL2 have similar surface areas, and ACL3 has the highest surface area (33.27 ± 0.3 m2/g). Georgin et al. investigated the adsorption performance of AC for coronaviruses and found that their AC samples had surface areas of around 800 m2/g [40]. These outcomes are consistent with the isotherm plots, which suggest that the greater the surface area, the higher the quantity of nitrogen absorbed for each ACL form. It has been observed that high surface areas (>1000 m2/g) are essential for activated carbon applications; however, the experimental results indicate that all the samples have weak adsorption capacities due to their low surface areas [42].
For all the samples, the micropore area increases as the surface area increases, but this trend is not observed for the micropore area percentage. This indicates that additional factors other than surface area may be influencing the porosity of the materials, such as the precursors used for manufacturing the activated carbon layers and the activation process, resulting in different pore structures. Moreover, N2 gas is being used to determine the surface characteristics; however, Krypton (Kr) would provide a more sensitive analysis since the surface areas are very low for all the samples [43]. The pore diameters found in the present study are similar to each other. The pore diameters determined in this study show that the average pore sizes are similar, ranging from 1.88–2.1 nm. Canh et al. observed that pores larger than the virus contribute more to virus removal than micropores (<2 nm) and mesopores (2–50 nm) [44]. Furthermore, ACL1, ACL2, and ACL4 have relatively close micropore area percentages (46–47%), and ACL3 has the highest micropore area percentage (65%). Considering coronavirus diameter sizes of 60–140 nm, these commercial ACL are not effective adsorbents for adsorbing coronaviruses.
3.1.3. Pore Size Distribution
The pore size distribution is another important parameter since it affects the AC adsorption capacity [45]. Thus, analyzing the entire pore size distribution of the adsorbent is essential. It can also be used to characterize the structural heterogeneity of porous materials [46]. The DFT method is often used to investigate the pore size distribution. As shown in Figure 4, the pore size of all the ACLs is not uniform and is less than 3 nm. Their pore size distributions overlap for ACL1, ACL2, and ACL3, with most of the micropores having a diameter of around 1.3 nm. ACL3 has the highest volume of micropores and mesopores and a second, smaller peak between 1.6 and 2.2 nm, indicating an increasing volume of micropores as the surface area increases.
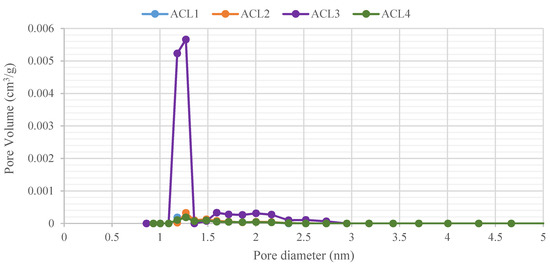
Figure 4.
Pore size distributions of different activated carbon layers. Each color represents a different insert’s activated carbon layer.
3.1.4. Proximate Analysis
The carbon surface captures the adsorbate molecules, and a higher fixed carbon content indicates a larger carbon surface area. Therefore, fixed carbon content is the main factor in the proximate analysis. It is desirable for a typical commercial AC to have low ash content (2–10%), low moisture (5–8%), and high carbon content (85–90%) [42,47,48]. Although there are some standards for the volatile content of the AC in powder (25%) and particle (15%) forms, no criteria are available in the literature [49]. Nevertheless, high volatile content is not desired as the ash and volatile matter fill up the AC pores, resulting in a reduction of the surface area and consequential adverse effects on the AC adsorption properties [50]. The results of the proximate analysis are presented in Table 2. Nam et al. studied the effect of granular AC adsorption and found that the fixed carbon content was 86.25%, the moisture content was 6.08%, and the ash content was 2.67% [51]. They observed that AC with a high fixed carbon percentage provides a high adsorption capacity.

Table 2.
Proximate Analysis of ACL.
The proximate analysis revealed a low moisture content (2–3.7%), ash content (4–5.7%), and fixed carbon content (18.9–31.3%), with a high volatile content (62.7–72.9%). These results are in line with the expected trends from the adsorption isotherm and surface area characteristics [52].
3.1.5. Scanning Electron Microscopy (SEM) Analysis
SEM has been used to closely examine the morphology of samples. At the lowest magnification (100×), it has been demonstrated that the inter-fiber structures of the ACL are composed of non-woven, randomly distributed fibers. This provides an insight into the intricate structure of the ACL (Figure 5).
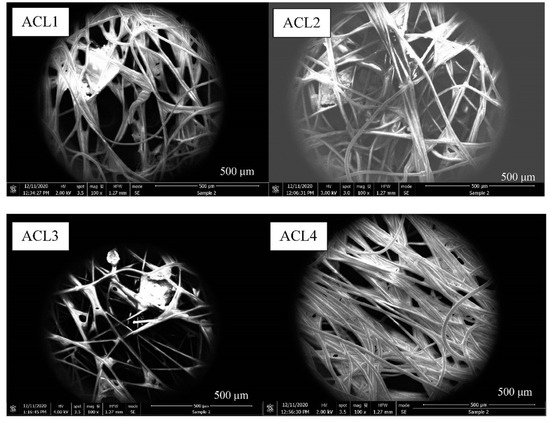
Figure 5.
SEM images of ACL at 100× magnification.
As shown in Figure 5, impurities have been identified in the images. ACL3 has the least amount of impurities, which may explain why it has the highest surface area, whereas ACL4 has the most impurities and the lowest surface area.
At 1000× magnification, individual fibers are clearly visible, allowing for the observation of their cylindrical shape and smooth surfaces (Figure 6). Avval et al. investigated coronavirus filtration by developing an adsorbent with AC nanomaterials, and the surface morphology and porosity analysis results showed that this type of filtration is very effective for coronavirus protection [53]. Moreover, Ibrahim et al. studied AC derived from biomass and observed irregular and nonuniform tubular structures through SEM investigations [54]. Nacke et al. state that these types of structures are good candidates for high adsorption capacities [55].
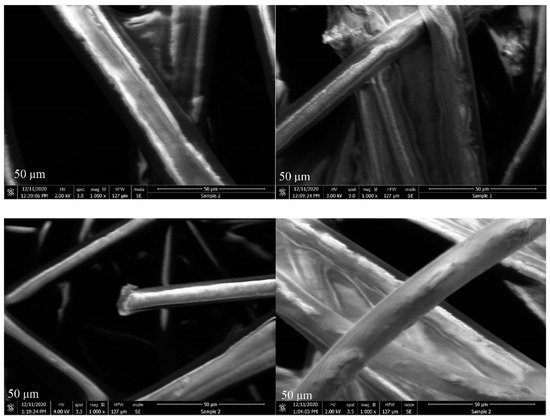
Figure 6.
SEM images of ACL at 1000× magnification.
The porosity of samples was not observed in this study, likely due to the samples’ low surface area, which made them difficult to detect. Additionally, the ACL was not purely made of carbon; the carbon layers were covered or sprayed with other materials, as seen in the images. This may have further contributed to the low surface areas and, thus, the lack of observation of porosity.
4. Conclusions
The ACL of all samples has similar physical adsorption properties, with ACL3 having a slightly better BET surface area (33.27 m2/g) compared to the other samples (BET surface areas between 1.91 and 2.64 m2/g) and a higher carbon percentage of 31.3% in comparison to the other samples (18.9–26.4%). However, despite the physical properties, all samples have inferior adsorption characteristics. ACL3 has two AC layers, though it is not necessarily effective due to its small surface area. For efficient coronavirus filtration, an ACL with a high surface area (>1000), a high carbon percentage (>80%), and a pore size of 0.1 microns is recommended. Non-woven surface morphology has been observed for all the samples. This study focuses only on the ACL of commercial face mask filter inserts and concludes that inserts with ACL do not perform better than inserts without ACL in adsorption. Furthermore, it was revealed that the inserts are not pure carbonaceous materials and that polymeric components may be predominant. Future studies should focus on the inserts’ performance by examining the filter media’s efficacy in aerosol testing through breakthrough testing in order to determine its effectiveness.
Funding
This work was supported by the University of South Alabama (USA) Support Fund.
Conflicts of Interest
The author declares no conflict of interest.
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 21 May 2021).
- Coronavirus Disease (COVID-19) Advice for the Public: When and How to Use Masks. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/when-and-how-to-use-masks (accessed on 15 December 2020).
- Water, Sanitation, Hygiene, and Waste Management for SARS-CoV-2, the Virus That Causes COVID-19. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-IPC-WASH-2020.4 (accessed on 10 July 2020).
- Singhal, T. A review of Coronavirus disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; You, Y.; Zhou, X.; Zong, Z.; Hauang, H.; Zhang, H.; Yong, X.; Cheng, Y.; Yang, L.; Guo, Q.; et al. Selection of homemade mask materials for preventing transmission of COVID-19: A laboratory study. PLoS ONE 2020, 15, e0240285. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Fan, X.; Yan, H. RetinaMask: A face mask detector. arXiv 2020, arXiv:2005.03950. [Google Scholar]
- Jotz, G.; Bittencourt, A. Why we need to use and which mask types are effective against the novel Coronavirus (COVID-19)? Int. Arch. Otorhinolaryngol. 2020, 24, e255–e257. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.; Hasan, A.; Ahmed, A.; Afroze, S.; Bakar, M.; Islam, S.; Azad, A. COVID-19 prevention: Role of activated carbon. J. Eng. Technol. Sci. 2021, 53, 1–12. [Google Scholar] [CrossRef]
- Hill, W.; Hull, M.; MacCuspie, R. Testing of commercial masks and respirators and cotton mask insert materials using SARS-CoV-2 virion-sized particulates: Comparison of ideal aerosol filtration efficiency versus fitted filtration efficiency. Nano Lett. 2020, 20, 7642–7647. [Google Scholar] [CrossRef]
- Deng, W.; Sun, Y.; Yao, X.; Subramanian, K.; Ling, C.; Wang, H.; Chopra, S.; Xu, B.; Wang, J.-X.; Chen, J.-F.; et al. Masks for COVID-19. Adv. Sci. 2021, 9, 2102189. [Google Scholar] [CrossRef]
- Wang, W.; Chen, T.; Li, Z.; Tan, Q.; Meng, Z.; Qiu, H.; Liu, X.; Zheng, J. Comparison of filtration efficiency and respiratory resistance of COVID-19. Am. J. Infect. Control 2022, 50, 516–524. [Google Scholar] [CrossRef]
- Reza, M.S.; Hasan, A.; Afroze, S.; Bakar, M.; Taweekun, J.; Azad, A.K. Analysis on preparation, application, and recycling of activated carbon to aid in COVID-19 protection. Int. J. Integr. Eng. 2020, 12, 233–244. [Google Scholar] [CrossRef]
- Bałazy, A.; Toivola, M.; Adhikari, A.; Sivasubramani, S.; Reponen, T.; Grinshpun, S. Do N95 respirators provide 95% protection level? Am. J. Infect. Control 2006, 34, 51–57. [Google Scholar] [CrossRef]
- Sim, K.M.; Kim, K.; Hwang, G.; Seo, S.; Bae, G.-N.; Jung, J.H. Development and evaluation of antimicrobial activated carbon fiber filters using Sophora flavescens nanoparticles. Sci. Total Environ. 2014, 493, 291–297. [Google Scholar] [CrossRef]
- Dizbay-Onat, M.; Vaidya, U.; Lungu, C. Preperation of industrial sisal fiber waste derived activated carbon by chemical activation and effects of carbonization parameters on surface characteristics. Ind. Crops Prod. 2017, 95, 583–590. [Google Scholar] [CrossRef]
- Kim, S.; Yoon, Y.; Kim, K. Performance of activated carbon-impregnated cellulose filters for indoor VOCs and dust control. Int. J. Environ. Sci. Technol. 2016, 13, 2189–2198. [Google Scholar] [CrossRef]
- Activated Carbon Face. Available online: https://healingvip.com/product/n95-activated-carbon-face-mask (accessed on 15 May 2020).
- Matsushita, T.; Suzuki, H.; Shirasaki, N.; Matsui, Y.; Ohno, K. Adsorptive virus removal with super-powdered activated carbon. Sep. Purif. Technol. 2013, 107, 79–84. [Google Scholar] [CrossRef]
- Tang, C. Challenges and opportunities in the post COVID-19 world. Environ. Geotech. 2020, 8, 172–192. [Google Scholar] [CrossRef]
- Li, W.; Yang, K.; Zhang, L.; Guo, S.; Xia, H. Effects of carbonization temperatures on charateristics of porosity in coconut shell chars and activated carbons derived from carbonized coconut shell chars. Ind. Crops Prod. 2008, 28, 190–198. [Google Scholar] [CrossRef]
- Tay, M.; Poh, C.; Rénia, L.; MacAry, P.; Ng, L. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Serafin, J.; Sreńscek-Nazzal, J.; Kamińska, A.; Paszkiewicz, O.; Michalkiewicz, B. Management of surgical mask waste to activated carbons for CO2 capture. J. CO2 Util. 2022, 59, 101970. [Google Scholar] [CrossRef]
- Ng, C.; Wong, L.; Bashir, M.; Ng, S. Development of hybrid polymeric polyerthersulfone (PES) membrane incorporated with powdered activated carbon (PAC) for palm oil mill effluent (POME) treatment. Int. J. Integr. Eng. 2018, 10, 137–141. [Google Scholar] [CrossRef]
- Bansal, R.; Goyal, M. Activated Carbon Adsorption; Taylor and Francis Group: Abingdon, UK, 2005. [Google Scholar]
- Deshmukh, N. Comparison of Adsorption Capacities of Nanoadsorbents wih Conventional Activated Carbon for Volatile Organic Compounds. Master’s Thesis, The University of Texas at Arlington, Ann Arbor, MI, USA, 2005. [Google Scholar]
- Balanay, J. Adsorption Characteristics of Activated Carbon Fibers (ACFs) for Toluene. Ph.D. Thesis, The University of Alabama at Birmingham, Ann Arbor, MI, USA, 2011. [Google Scholar]
- Carrott, P.; Roberts, R.; Sing, K. Standard nitrogen adsorption data for nonporous carbons. Carbon 1987, 25, 769–770. [Google Scholar] [CrossRef]
- Mikhail, R.S.; Brunauer, S.; Bodor, E.E. Investigations of a complete pore structure analysis of micropore. J. Colloid Interface Sci. 1968, 26, 45–53. [Google Scholar] [CrossRef]
- Harkins, W.; Jura, G. An adsorption method for determination of the area of a solid without the assumption of a molecular area and the area occupied by nitrogen molecules on the surfaces of solids. J. Chem. Phys. 1943, 11, 431. [Google Scholar] [CrossRef]
- Aranovich, G.; Donohue, M. Analysis of adsorption isotherms: Latis theory predictions, classification of isotherms for gas-solid equilibria, and similarities in gas and liquid adsorption behaivour. J. Colloid Interface Sci. 1998, 200, 273–290. [Google Scholar] [CrossRef]
- Dinesh, S. Development and Characterization of Pellet Activated Carbon from New Precursor; IB.Eng Project; National Institute of Technology: Rourkela, India, 2011. [Google Scholar]
- Goswami, D. The CRC Handbook of Mechanical Engineering; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Silgado, K.; Marrugo, G.; Puello, J. Adsorption of Chromium (VI) by activated carbon produced from oil palm. Endocarp Chem. Eng. Trans. 2014, 37, 721–726. [Google Scholar]
- Baseri, J.P.P.; Sivakumar, P. Preparation and characterization of activated carbon from thevetia peruviana for the removal of dyes from textile waste water. Adv. Appl. Sci. Res. 2012, 3, 377–383. [Google Scholar]
- ASTM D2867-14; Standard Test Methods for Moisture in Activated Carbon. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D2866-11; Standard Test Method for Total Ash Content of Activated Carbon. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM D5832-08; Standard Test Method for Volatile Matter Content of Activated Carbon Samples. ASTM International: West Conshohocken, PA, USA, 2008.
- XWang; Li, D.; Li, W.; Peng, J.; Xia, H.; Zhang, L.; Guo, S.; Guo, C. Optimization of mesoporous activated carbon from coconut shells by chemical activation with phosphoric acid. Bioresources 2013, 8, 6184–6195. [Google Scholar]
- Hu, Z.; Srinivasan, M.; Ni, Y. Preparation of mesoporous high-surface-area activated carbon. Adv. Mater. 2000, 12, 62–65. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.; Netto, M.; Manzar, M.; Meili, M.; Piccilli, D.; Silva, L. Adsorption of the first-line Covid treatment analgesic onto activated carbon from residual pods of erythrina ppeciosa. Environ. Manag. 2022, 1–14. [Google Scholar] [CrossRef]
- Senthilkumar, T.; Raghuraman, R.; Miranda, L. Parameter optimization of activated carbon production from Agave Sisalana and Punica Granatum peel: Adsorbents for C.I. reactive orange 4 removal from aqueous solution. Clean–Soil Air Water 2013, 41, 797–807. [Google Scholar] [CrossRef]
- Dizbay-Onat, M.; Vaidya, U.K.; Balanay, J.A.G.; Lungu, C.T. Preparation and characterization of flax, hemp, and sisal fiber-derived mesoporous activated carbon adsorbents. Adsorpt. Sci. Technol. 2018, 36, 441–457. [Google Scholar] [CrossRef]
- Gas Adsorption for Surface and Pore Size Analysis, Anton Paar. Available online: https://wiki.anton-paar.com/en/gas-adsorption-for-surface-area-and-pore-size-analysis/ (accessed on 15 May 2021).
- Canh, V.; Tabata, S.; Yamanoi, S.; Onaka, Y.; Yokoi, T.; Furumai, H.; Katayama, H. Evaluation of porous carbon adsorbents made from rice Husks for virus removal in water. Water 2021, 13, 1280. [Google Scholar] [CrossRef]
- Ilomuanya, M.; Nashiru, B.; Ifudu, N.; Igwilo, C. Effect of pore size and morphology of activated charcoal prepared from midribs of Elaeis guineensis on adsorption of poisons using metronidazole and Escherichia coli O157: H7 as a case study. J. Microsc. Ultrastruct. 2017, 5, 32–38. [Google Scholar] [CrossRef]
- Sudaryanto, Y.; Hartono, S.; Irawaty, W.; Hindarso, H.; Ismadji, S. High surface area activated carbon prepared from cassava peel by chemical activation. Bioresour. Technol. 2005, 97, 734–739. [Google Scholar] [CrossRef]
- Jabit, N.B. The Production and Characterization of Activated Carbon Using Local Agricultural Waste through Chemical Activation Process. Master’s Thesis, School of Material and Mineral Engineering, Universiti Sains Malaysia, Penang, Malaysia, 2017. [Google Scholar]
- Devi, B.; Jahagirdar, A.; Ahmed, M. Adsorption of chromium on activated carbon prepared from coconut shell. Int. J. Eng. Res. Appl. 2012, 2, 364–370. [Google Scholar]
- Hanum, F.; Bani, O.; Wirani, L. Characterization of activated carbon from rice husk by HCl activation and its application for Lead (Pb) removal in car battery wastewater. In Proceedings of the 1st Annual Applied Science and Engineering Conference, Bandung, Indonesia, 24 August 2017; Volume 180. [Google Scholar]
- Lua, A.; Yang, T. Characteristics of activated carbon prepared from pistachio-nut shell by zinc chloride activation under nitrogen and vacuum conditions. J. Colloid Interface Sci. 2005, 290, 505–513. [Google Scholar] [CrossRef]
- Nam, J.Y.; Lee, T.R.; Tokmurzin, D.; Park, S.J.; Ra, H.W.; Yoon, S.J.; Mun, T.-Y.; Yoon, S.M.; Moon, J.H.; Lee, J.G.; et al. Hydrogen-rich gas production from disposable COVID-19 mask by steam gasification. Fuel 2023, 331, 125720. [Google Scholar] [CrossRef]
- Cuhadar, C. Production and Characterization Activated Carbon from Hazelnut Shell and Hazelnut Husk. Master’s Thesis, Middle East Technical University, Ankara, Turkey, 2005. [Google Scholar]
- Avval, Y.F.; Pour, G.; Aram, M.M. Fabrication of high efficiency coronavirus filter using activated carbon nanoparticles. Int. Nano Lett. 2022, 12, 421–426. [Google Scholar] [CrossRef]
- Ibrahim, W.; Hassan, A.; Azab, Y. Biosorption of toxic heavy metals from aqueous solution by Ulva lactuca activated carbon. Egypt. J. Basic Appl. Sci. 2016, 3, 241–249. [Google Scholar] [CrossRef]
- Nacke, H.; Gonçalves, A.C.; Coelho, G.F.; Schwantes, D.; Campagnolo, M.A.; Leismann, E.A.V.; Junior, C.; Miola, A.J. Removal of Cd (II) from water using the waste of jatropha fruit (Jatropha curcas L.). Appl. Water Sci. 2016, 7, 3207–3222. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).