1. Introduction
In the food and beverage industry, products tend to foam up unintentionally during processing due to their chemical composition. This phenomenon can be observed in the bottling not only of carbonated beverages but also of non-carbonated fruit juices. Similar to everyday pouring into a jar, in the worst case, the foam increases in volume disproportionately to the actual liquid poured in, takes up the planned space in the vessel, and threatens to overflow. Particularly in the process of hot filling already-pasteurized beverages into glass bottles, the containers are sometimes considerably underfilled, as the headspace fills with foam before the required legal minimum volume has been reached. Below this volume, a bottle of this type may not be sold, which means that the economic loss corresponds with a loss in sales price. The result is lower line efficiency by up to 5%, underfilling of the containers, higher production losses, and greater cleaning efforts for the line. Therefore, it is already of interest to medium-sized companies to increase plant utilization by even 1% through foam prevention and thus save up to €50,000 per year [
1].
In practice, it is only possible to passively reduce foam formation by reducing the filling speed, since a decreased velocity of the free jet carries less gas from the headspace into the liquid. The entrained bubbles rise to the surface and agglomerate to form the foam. The resulting foam height is obtained from the net foam balance, which is the time ratio of foam formation to foam decay. Especially in the short time window of a few seconds for bottle filling, foam decay is dominated by drainage, where films and plateau edges lose liquid and decay below a critical thickness [
2,
3,
4]. Deposited particles, e.g., from pulp, and capillary forces in the plateau areas inhibit drainage; hence, foam persists longer than the time window permits. Naturally fluctuating material properties of the juices, such as the content of proteins, polyphenols, sugars, and soluble solids, etc., constantly change the formation and half-life of the foam, which is why it is constantly necessary to manually adjust the filling speed to avoid over-foaming despite optimized filling tube geometries [
5,
6,
7,
8,
9,
10].
A method of active and adaptive foam destruction during filling is currently not found in literature or in practice. A look at other industrial fields reveals chemical, thermal, and mechanical defoaming methods, but most of these are not applicable to bottling. Chemical methods, such as the use of defoamers, are limited due to strict national food laws and face the disadvantage of more expensive and environmentally hazardous consequences [
11,
12]. Therefore, mechanical foam destruction based on ultrasonic waves is of particular interest.
Ultrasound is noninvasive and propagates as a mechanical wave in gases, liquids, and solids, creating localized pressure differences. It is assumed that ultrasonic waves penetrate the lamellae, atomize the liquid from the lamellae, or hit the resonant frequencies of the bubbles, creating surface waves along the lamellae and enhancing drainage [
13,
14,
15,
16,
17]. Current knowledge on ultrasound-based foam destruction is largely based on laboratory experiments with airborne ultrasound. Ultrasonic foam destruction was initially studied using sirens and Hartman whistles [
18,
19,
20]. The frequency range from audible 0.7 to 29 kHz showed effective defoaming only at sound power levels of at least 145–148 dB [
21]. Additional work on foam control in fermentation vessels showed that defoaming ability increased with higher frequencies from 26 to 34 kHz and rapidly disintegrated foam at high sound intensities of 10 W/cm
2 or 120 dB [
20]. More recent work has investigated airborne sonication of the foams with piezo-acoustic actuators [
14,
15,
16,
22]. Most of the works used a sonotrode with a frequency of 20 kHz and considered the degree of defoaming dependent on the foam and power applied. It was found that ultrasound is more effective with decreasing viscosity and is just suitable for aqueous foams with average bubble diameters of 0.5–5 mm. However, higher foam formation rates and water contents in the case of foams with SDS required stronger amplitudes, since the liquid migrates only to the neighboring plateau areas when the top foam layer is destroyed and the foam thus loses little liquid hold-up [
22,
23]. With increasing liquid hold-up and lamella thickness, the reflection coefficient at the foam interface increase. The acoustic power to be applied is so high that Dedhia et al. [
22] considered pulsed sonication in terms of economy.
However, for an inline application during filling, airborne high-power ultrasound has several significant drawbacks. For very wet foams, defoaming should not occur by cavitation formation and atomization of the liquid in the lamella. On the one hand, the generation of transient cavitation for a decent foam decay requires a sound intensity of about 100 W/cm
2, which is equivalent to a burnout of an heating wire for boiling water. This negatively influences the energy efficiency of the plant, and thermal product degradation could occur. On the other hand, the product is partially atomized, and an aerosol is produced that contaminates the filling system and thereby causes hygienic problems [
24]. At the same time, these methods require ultrasonic actuators with relatively large diameters compared to common bottle dimensions. As a result, due to the limited accessibility to the foam and the small installation space, the sonotrode can only remove the excess foam after filling [
14,
15].
Apart from airborne insonication, Winterburn and Martin [
25] demonstrated that sonication via the liquid also accelerates drainage at 40 kHz and at already 4 W total electrical power, as the impedance between air and water is improved. Although the pressure amplitude was too low to rupture the lamellae in the experiments, as is assumed for airborne ultrasound [
14], the authors detected stronger effects at 40 kHz than at 28 kHz. However, a fundamental understanding of the mechanisms of foam destruction is lacking.
This leads to the research question of an innovative, energy-efficient, and product-friendly alternative in the form of a resonance-based method of foam destruction for beverage filling using ultrasound. By using resonant effects, the acoustic power and thus the energy consumption can be significantly reduced. High temperatures and peak pressures from cavitation events no longer occur; thus, product degradation and aerosol formation may not occur. The lower power requirements allow for a coupling of the ultrasound transducer through the bottle wall and liquid phase, giving plant designers more flexibility for system integration.
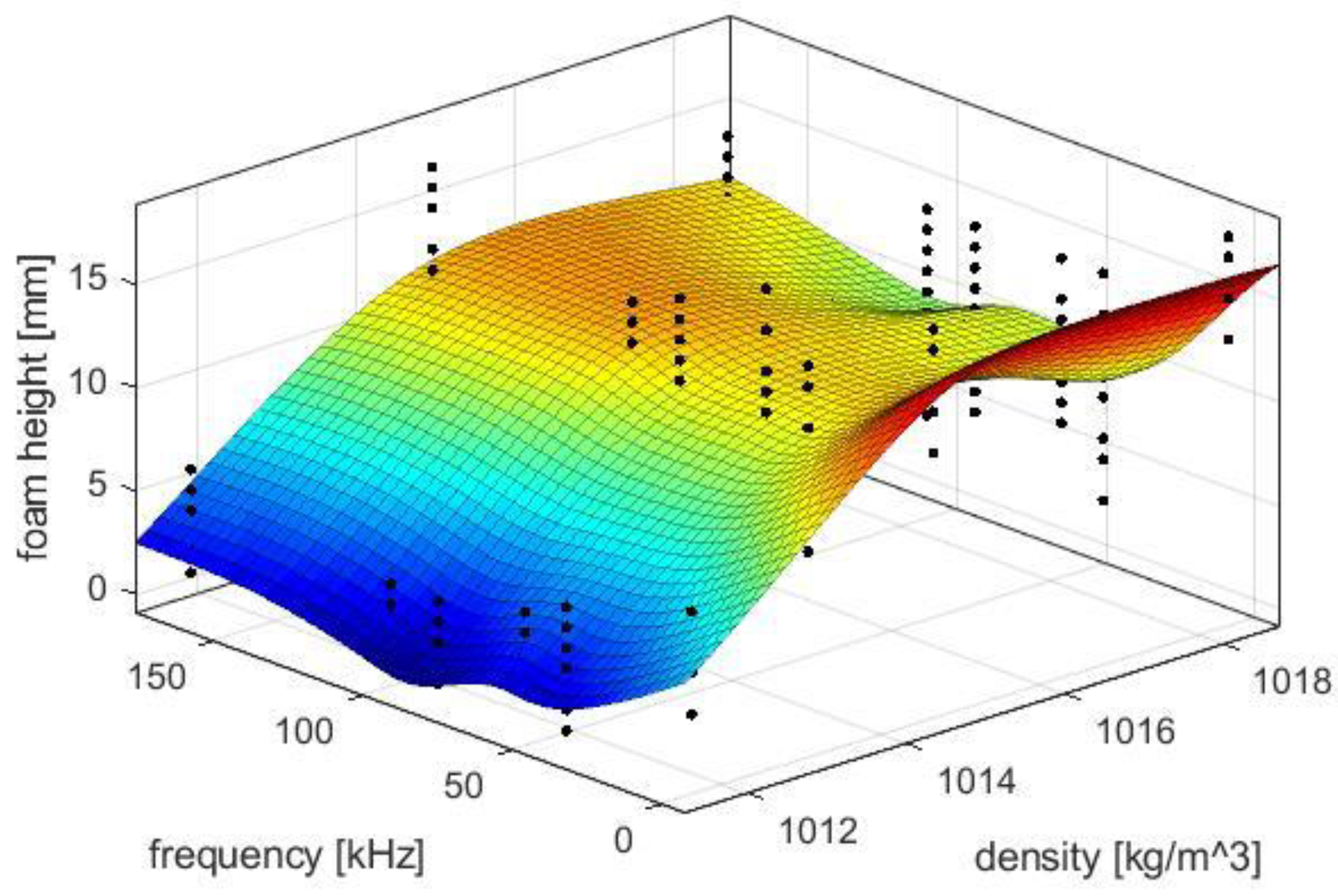
The primary resonance excitation in foam promotes the generation of surface waves and lowers the viscosity of the liquid in the lamellae. Drainage increases, causing the dynamic foam height to be lower. In published literature, ultrasound has been used in food foams only to determine bubble size distribution and gas content, thus providing information on the frequency-specific resonance behaviors of bubbles [
26,
27,
28]. In all work, bubbles are assumed to absorb sound in a frequency-specific manner and oscillate in the process. However, it is not yet fully understood how the resonance effects behave within the foam regarding to enhance defoaming. On the one hand, an increasing gas content in the foam lowers the resonant frequency of a bubble [
28], while the presence of neighboring resonant bubbles at a distance of half a wavelength would increase the frequency [
29]. In the latter case, bubbles with a radius of about 100 µm would resonate at 86 kHz instead of 31 kHz with a gas content of 26%.
The article addresses the following research objectives: (i) discovering which ultrasonic frequencies can minimize the resulting foam height during filling due to resonance effects. Frequencies that are still high enough to keep the potential of transient cavitation low (>40 kHz) and low enough that their wavelengths roughly correspond to the theoretical resonance lengths of the smallest lamellae or bubbles (<200 kHz) are considered. The bubble fractions, which tend to be smaller, are usually located at the bottom of the foam, which is why objective (ii) is whether sonication of the liquid through the wall of the bottle from the side or bottom can achieve the desired effect. This promises no aerosol formation and a lower transmission loss than with the air gap between the transducer and the foam. Finally, for objective (iii), the required ultrasound power and duration of sonication are investigated to deduce the energy requirements of the process. The results are considered in terms of industrial feasibility.
2. Materials and Methods
2.1. Experimental Set-Up
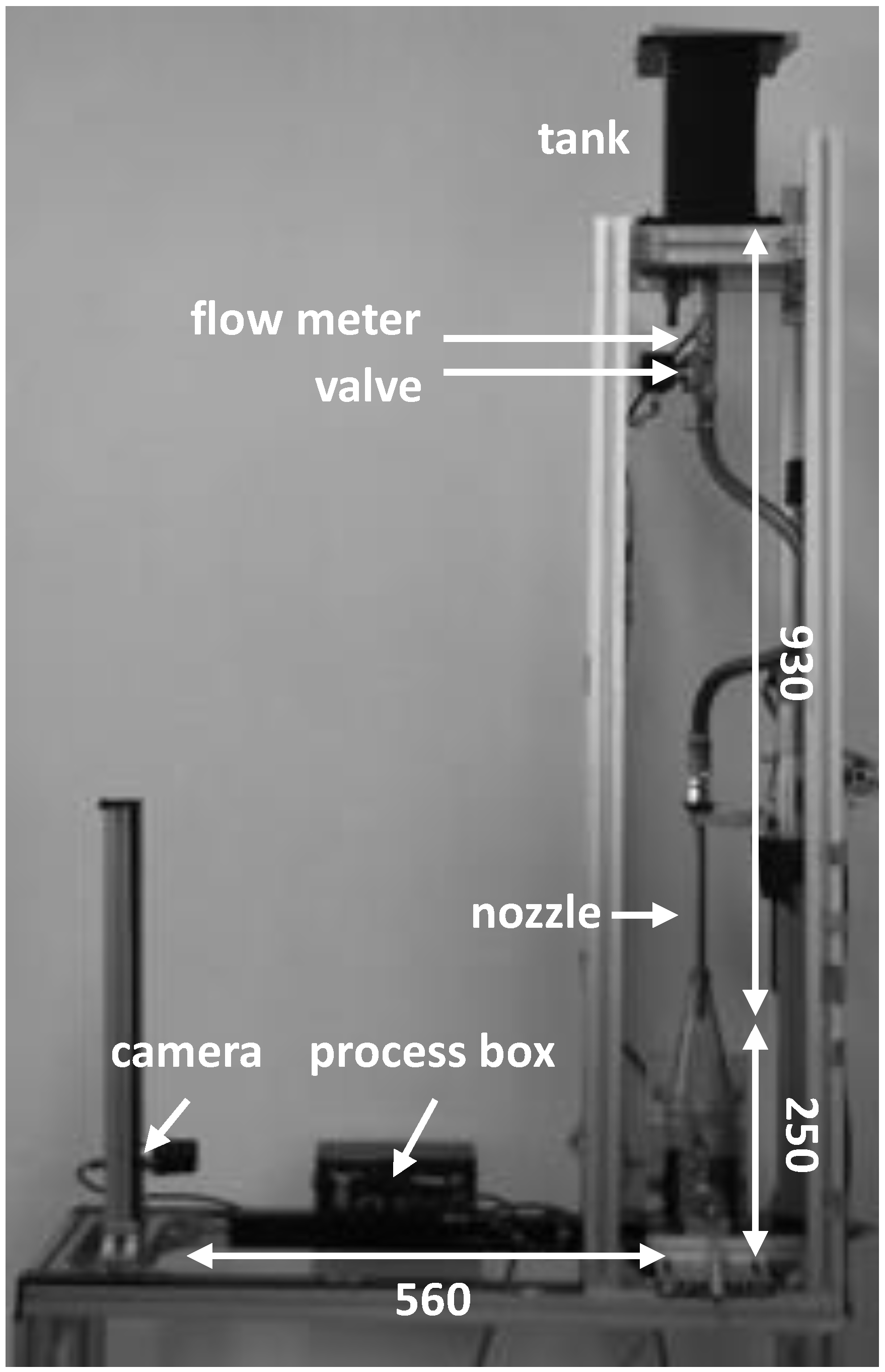
The aim of the tests was to investigate the influence of different frequencies on foam formation during atmospheric filling under realistic conditions. The test conditions were based on the standard filling specifications for fruit juices in refillable glass bottles, in which a maximum filling time of 10 s is specified. The required volume flow was achieved via the height difference between the reservoir tank (100 × 100 × 250 mm) and the filling tube opening of 930 mm (
Figure 1).
Before each filling, 1.2 L of the juice, previously tempered to 70 °C, was taken from a thermobath (3 L) to a reservoir tank. This quantity of juice allowed for the avoidance of additional air intake into the pipe towards the end of the filling.
A magnetic valve and a flow meter below the reservoir tank controlled the filling interval and recorded the flow rate, respectively. The juice entered the bottle via a height-adjustable filling nozzle (length: 250 mm, inner diameter 8 mm). The adjusted height between the nozzle outlet and the bottom of the bottle was 250 mm for the trials with apple juice and currant nectar. For orange juice, the filling nozzle was set at a height of 50 mm. This allowed the foam heights within replicates to be kept as low as possible.
A CCD camera (acA2500-60 uc, Basler GmbH, Ahrensburg, Germany) recorded foam formation at 1 fps during filling. Control of the valve and data recording were done centrally via a mini-PC (Raspberry Pi 4 Model B). The sampling period of all sensors was synchronized to the camera’s recording rate and was 1.03 s between samples.
The individual filling cycles were divided into fixed time periods: initialization (1 s), filling (10 s), and decay (9 s) phases. During the filling phase, the volume flows were kept constant for each of the juices (
Table 1). After the filling phase, the decay phase additionally served to assess the pure foam decay during sonication.
The vessel used was a 300 mm high commercial glass bottle (model: 1 L VdF bottle) consisting of a 149 mm high cylinder and a cone above it. After measuring three different bottles with a caliper, the inner radius
r can be described as a function of the height
h:
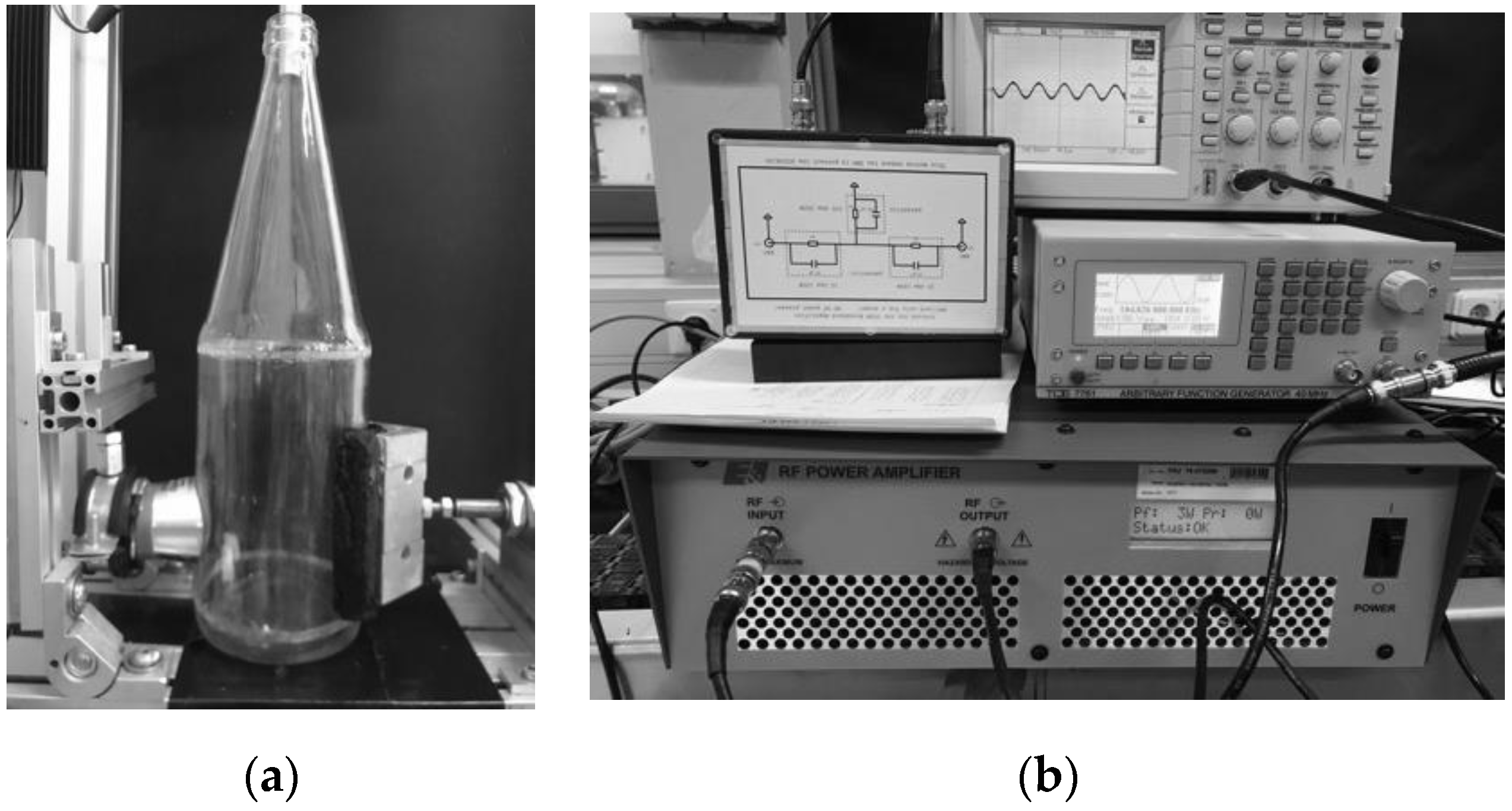
A clamp fixed the bottle to the ultrasonic actuator on the side, which rested against the cylindrical wall of the bottle at a height of 30–70 mm (
Figure 2a).
The ultrasonic transducer was a modified 40 kHz Langevin transducer (Hesentec, Rank E), which had a front face adapted to the curvature of the bottle wall (radius r = 44 mm). This modification changed the resonant frequencies of the transducer to 42, 56, 85, 101, and 168 kHz. A arbitrary function generator (TOE7761, Toellner, Herdecke, Germany) produced a sinusoidal signal of the corresponding frequency, which was amplified by 51 dBV through a voltage amplifier (1040 L, Electronics and Innovation, LTD, Rochester, NY, USA). A parasitic resistor box was connected between the amplifier and the transducer to protect the amplifier from changing impedances of the transducer (
Figure 2b).
Power outputs at 10, 15, and 20 W
el were investigated. The maximum generated pressure amplitudes inside a filled bottle are shown in
Table 2 according to hydrophone measurements.
2.2. Juices
Orange juice (100% fruit content with pulp, Erwin Dietz GmbH, Osterburken, Germany), filtered apple juice (100% fruit content, Erwin Dietz GmbH, Osterburken, Germany), and currant nectar (25% fruit content, Granini, Nieder-Olm, Germany) tempered to 70 °C were used.
Their density, viscosity, and surface tension were triply determined using a hydrometer, a capillary viscometer, and a contact angle meter (Wilhelmy plate method, K100, Krüss, Hamburg, Germany) at 70 °C as shown in
Table 3.
2.3. Statistical Methods
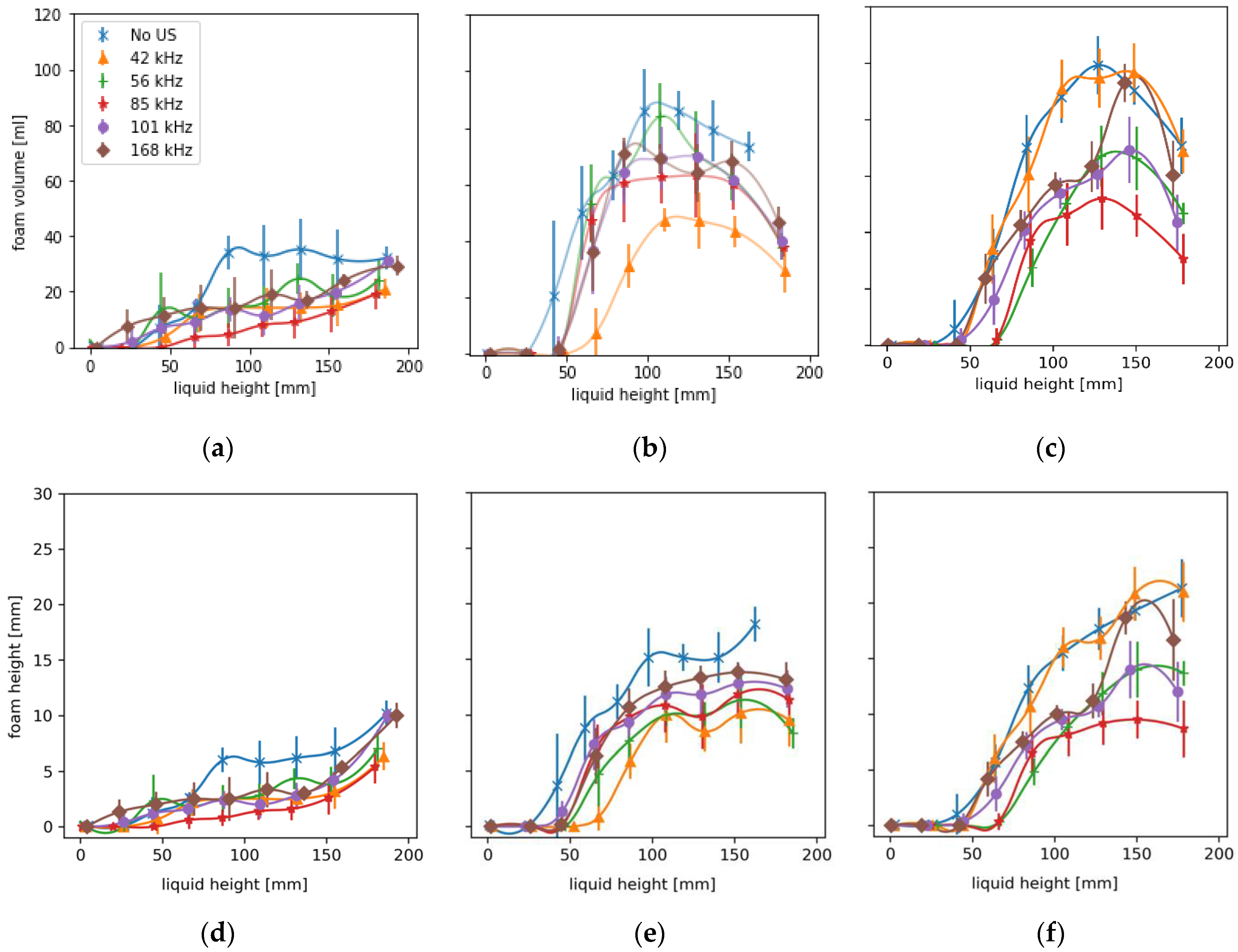
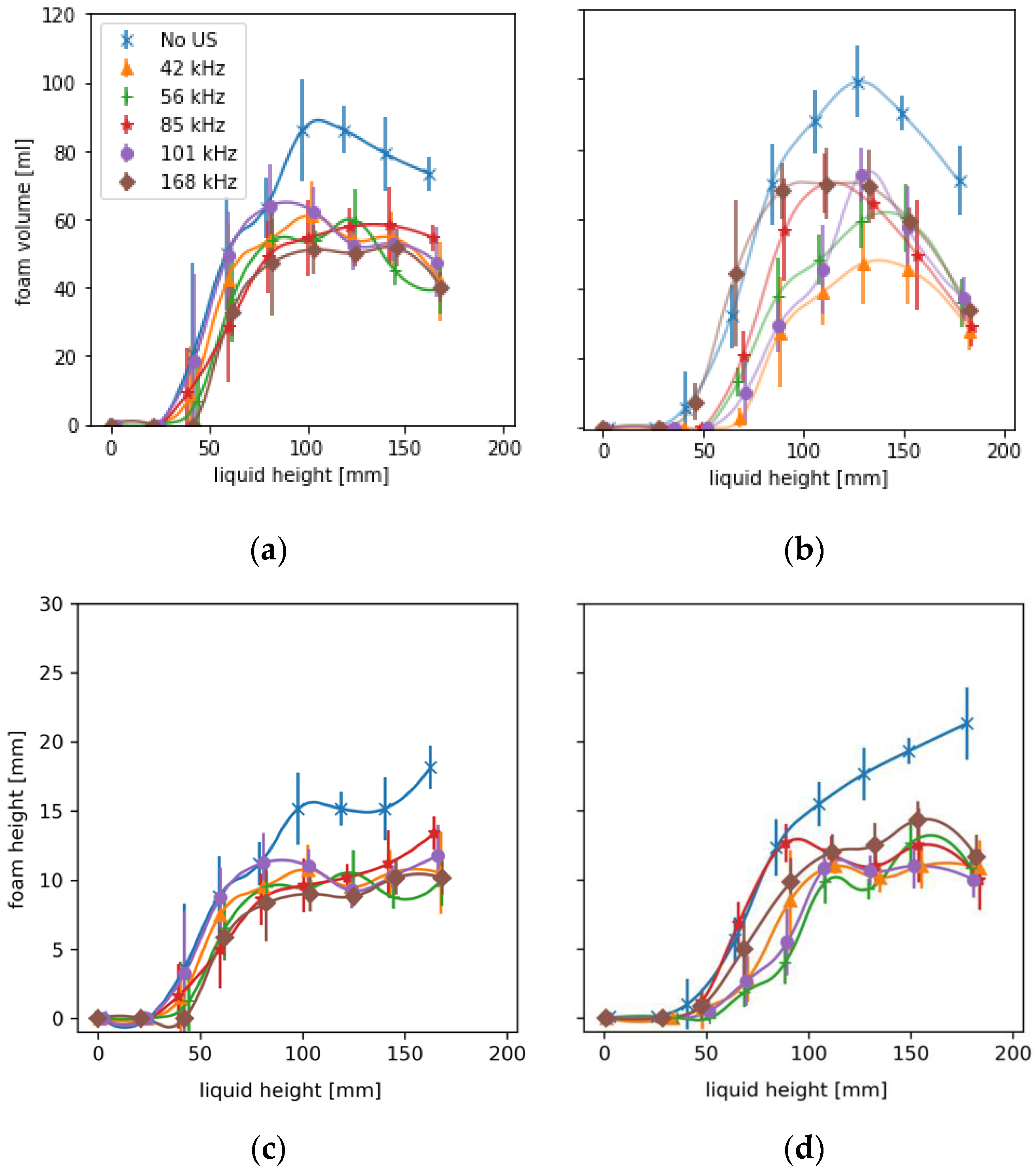
A total of 108 fillings were considered with six replicates per juice, power level and frequency. Results are given as means with standard deviations. For the statistical evaluation with a two-factorial ANOVA and the calculation of the Spearman correlation coefficient in Excel 2016, the foam heights at time t = 8 s were used. At this time, the foam is still in the cylindrical region of the bottles and is not yet affected by the increasing influences during bottle tapering, which would shift the normal distribution to a right-handed one. A two-factor ANOVA (Excel 2016) revealed that trials differed between juices (p-value = 2.51 × 10–42), sonication significantly decreased foam (p-value = 1.86 × 10−11), and interactions between frequencies and juices also occurred (p-value = 1.035 × 10–16).
2.4. Measurement of Foam Heights
The temporal development of foam was recorded via the camera’s image series. Only the pixel lengths in vertical direction were considered. To this end, constant heights of the bottle opening
and bottom
were measured once for each run, and the heights of the top and bottom edges of the foam were determined (
and
) for the individual images. The origin of the coordinate system in the image is in the upper left corner, thus accounting for the y-values increase towards the bottom. The process was performed using a semi-automated script in OpenCV, i.e., foam edges were manually selected and the according y-values were calculated and saved automatically. The resulting foam height
hf and liquid height
hl were calculated by taking the difference of the respective y-values and including the conversion ratio
R of the image.
Finally, the foam volume was calculated from the volume of a truncated cone with the radii according to equation 1 and the foam height. In the region between the cylinder and cone at hl < 149 mm and hl + hf > 149 mm, the foam volume was the sum of a truncated cone of a height h1 = (hl + hf − 149 mm) and a cylinder of h2 = (149 mm − hf).
2.5. Uncertainty Analysis of Measurements
The heights of the bottle, foam, and liquid were dependent on the accuracy of the computer display. At the same time, the edges of the foam in the image were not always horizontal, resulting in measurement uncertainty when measuring the average height.
The single-sample analysis by Moffat calculated the uncertainties separately. Measurements of two filling tests with 20 images each provided the data. Each bottle edge was measured in the respective image. For the changing foam and filling heights, the y-values were determined tenfold in each image. The uncertainties were caused by the repetition and measurement errors by the author and are indicated by the standard deviation σ:
The standard deviations are shown in
Table 4. The relative errors of the foam edges varied depending on the fill level, which is why they are not shown in the table. Instead, the relative errors of the foam height based on them are given later.
The uncertainties contributed by multiple input variables were calculated by a combination of the root-sum-square method:
where the measurement uncertainty
δR, is characterized by the individual uncertainties of the measured variables
. To calculate the uncertainties of the conversion ratio, the foam and the liquid height, the Equation (2) were, respectively, put into (3) and (4) and partially derived according to the individual variables
Xi.
For the conversion coefficient
R, the measurement uncertainty was δR = 0.014 Px/mm and a relative error of 0.31%.
Table 5 shows the absolute measurement uncertainties of the liquid height
δhl and the foam height
δhf, as well as their relative deviations for representative heights, respectively.
Deviations < ±1.37 mm around the mean foam heights shown below are due to measurement uncertainty. Above the value, the deviations are attributable to the system.
5. Conclusions
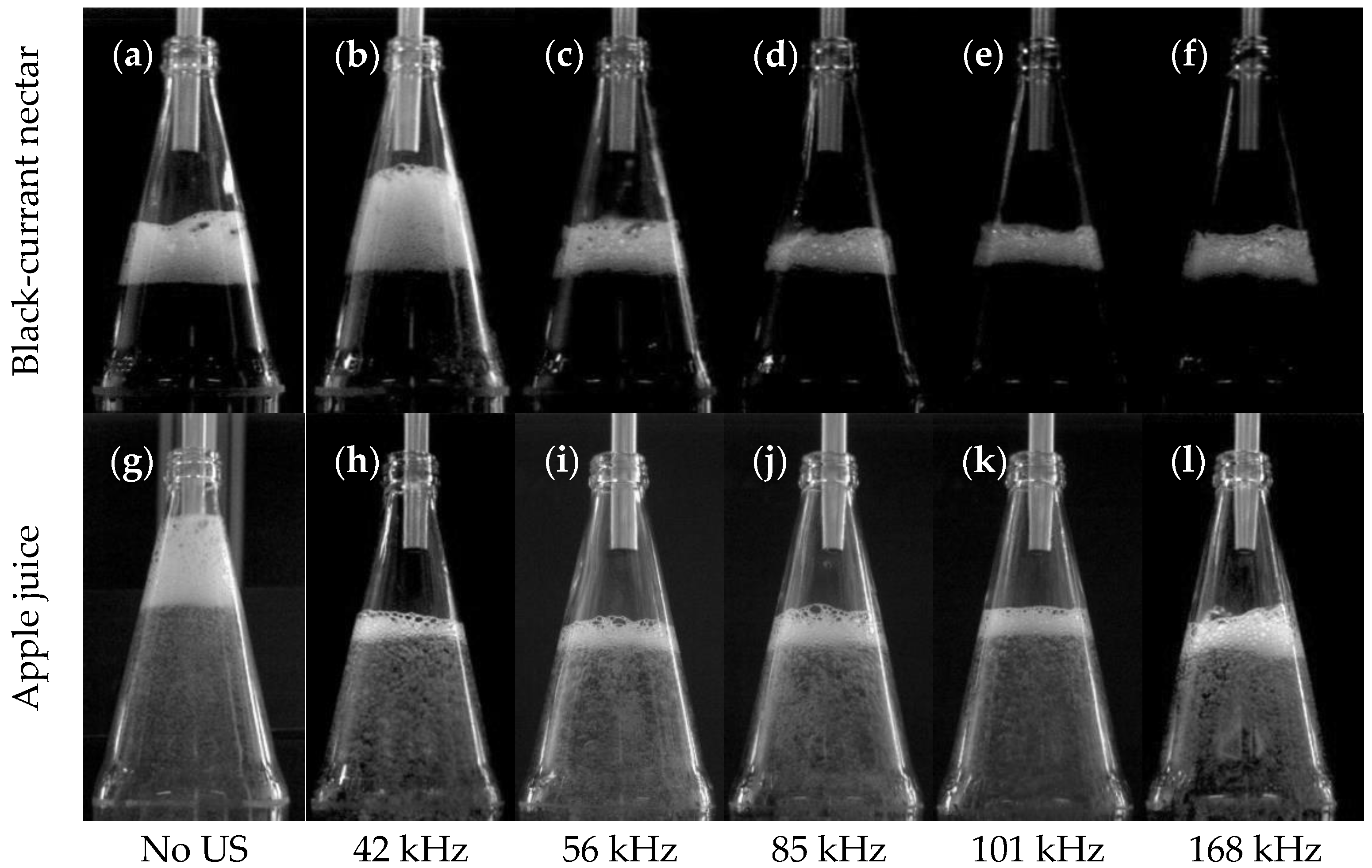
The results show the influence of sonication with ultrasound via the bottle wall on foam development during hot beverage filling at 70 °C. This type of sonication method differs from previous airborne ultrasonic systems due to higher frequencies and modest pressure amplitudes. The resonance effects in the bottom of the foam enhance the drainage and the decay, respectively. By avoiding transient cavitations, the juice is not degraded thermally or mechanically. The comparatively high frequencies between 42–168 kHz have an enhanced effect on wet, dense foams containing a large proportion of small resonant bubbles. A low power of 15 W was sufficient to change the rise of the entrained bubbles and minimize foam development right at the beginning. Compared to industrial airborne ultrasonic defoamers, the proposed method reduces foaming already during filling with 7.7% of the electrical power. Power dependence between 10–20 W and the harmonic relationship of the three most effective frequencies suggests a much stronger role of the natural frequencies of the liquid films or bubbles. The defoaming effects occurred mainly once the liquid level was above the ultrasonic actuator. The advantage of sonication over the liquid is that it more easily dries out, coarsens, and destabilizes wet foams. During filling, foams can be kept low and dry enough in a relatively short time window of a few seconds that little or no liquid can escape from the bottle and contaminate the plant.
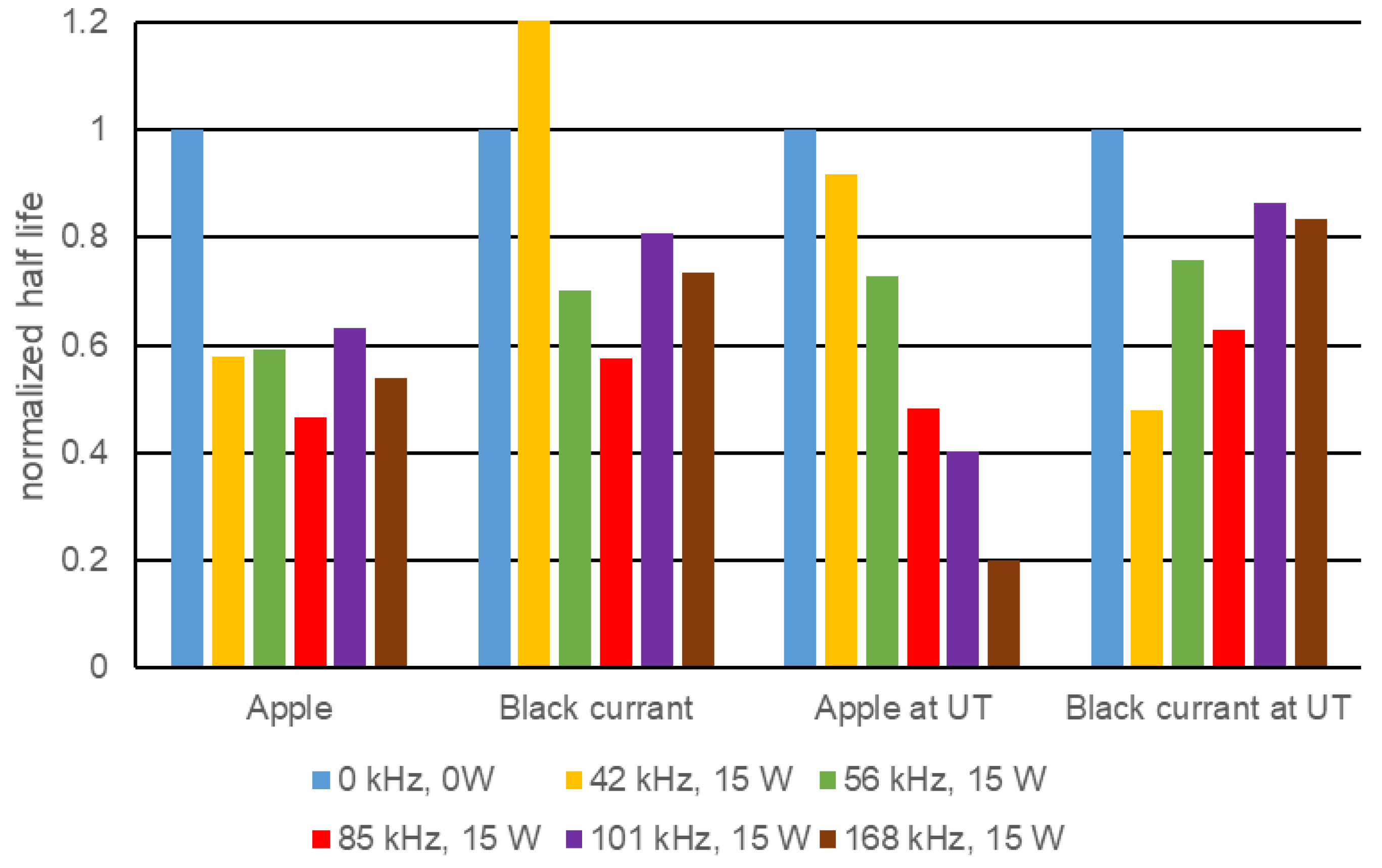
The bottling experiments showed total foam reductions of more than 50% and shorter half-lives of the remaining foam of up to 55% in apple juice and currant nectar. The lowest effects of foam destruction occurred in orange juice, mainly due to the preventive effect pulp particles have on drainage. The experiments have been carried out on a commercial bottle geometry, proving its easy adaptability to industrial processes. However, further experiments on the influence of ultrasound on juices with higher viscosity, e.g., at lower temperature, or on other geometries should follow.